ABSTRACT
Background: Acute myelogenous leukemia (AML) may be cured in a substantial number of patients using intensive chemotherapeutic regimens leading to temporary severe myelosuppression. Patients belonging to the denomination of Jehovah's Witnesses (JW), however, are bound by their religious convictions not to accept blood products and are therefore at higher risk for life-threatening events. Reports how to handle this challenge are mainly anecdotal.
Material and methods: We here report in much more detail about our experience with nine patients belonging to the denomination of JW who were treated for AML in our department from 1998 to 2007 and who explicitly wished to receive chemotherapy without blood transfusions.
Results: Reduced dose induction chemotherapy administered by several treatment cycles to prevent sustained myelosuppression still led to complete remissions in three out of nine of JW patients but was associated with a high rate of relapse. No durable remission was achieved. The overall hazard ratio for death was 12.1 compared to a matched control group treated with full transfusion support. The predominant cause of non-AML mortality was severe anemia (four out of five early deaths) and uncontrollable bleeding (n = 1).
Conclusion: Reduced dose chemotherapy without transfusion support in JW suffering from AML is associated with a lower rate of remission, high mortality by severe anemia and very low chances for long-term remissions. Less hematotoxic treatment options including hypomethylating agents or molecular targeted therapies with intensive consolidation after improvement of bone marrow function are promising for these patients but need further investigation.
Background
During the last decades, the outcome for systemic treatment of acute myelogenous leukemia (AML) has improved markedly. Treatment with single agents was followed by dose-intense schedules of combination chemotherapy with enhanced responses and survival. However, more intensive chemotherapy also led to increased toxicity, particularly neutropenia, anemia and thrombocytopenia [Citation1]. Since the introduction of tailored blood products, neutropenic infection has become the most hazardous treatment-related toxicity.
Jehovah’s Witnesses (JW) belong to a religious denomination with more than 8.2 million members worldwide [Citation2]. Many practicing JW patients will not accept transfusions of whole blood or its major components such as packed red blood cells, platelet concentrates or plasma derivatives. According to their belief, transfusion of blood is equivalent to oral ingestion and therefore prohibited by the bible (‘But flesh with the life thereof, which is the blood thereof, shall ye not eat.’ Genesis, Ch.9 v.4) [Citation3]. Some JW refuse blood transfusions but nevertheless do accept an autologous or even allogeneic stem cell transplantation.
Several strategies have been implemented in the management of JW undergoing surgery [Citation4] but guidance for systemic chemotherapy in patients with AML is very scarce. Only a few case reports and small case series have been published so far without giving detailed information on the clinical course [Citation5–8].
The British writer Ian McEwan has recently raised a keen public debate on judicial problems with this issue. In his novel The Children Act, the hematologist called before the court to give medical evidence that offers a dreadful description of risks and complications [Citation9].
We here report our experience with 11 JW patients suffering from AML who explicitly wished to be treated using antineoplastic chemotherapy without any blood transfusions. To the best of our knowledge, this cohort collected from 1998 to 2007 represents the largest published retrospective case series of AML patients treated at a single institution so far.
Materials and methods
Patients
We retrospectively searched the electronic database of our institution for JW patients suffering from AML. This database contains complete data on any antineoplastic chemotherapy and results of all laboratory tests in detail performed in our department. All patients have routinely been asked for their religious affiliation at admission but this information was not compulsory. All JW patients did explicitly confirm their refusal to accept any transfusion of blood or derivatives in a formal written and signed statement that also exempted the hospital and its physicians from liability as to any damage incurred by not transfusing blood products. German law clearly states that a patient's will (voluntas aegroti) should take precedence over his medical well-being (salus aegroti) as judged by medical professionals. Thus, utmost care was taken at all times to reconcile physicians’ duty to fully inform patients about imminent risks of their refusal and the respect for self-determination.
The diagnosis of AML was based on cytological evaluation according to FAB classification and risk categories were used according to cytogenetic analysis.
In order to compare the number of transfusions and survival, for each of the nine JW with AML, 2 patients with AML, matched for age and cytogenetic risk group were retrospectively selected as case controls. All 18 patients in the control group underwent standard induction chemotherapy (including blood products as necessary).
Statistical analysis
Survival data were analyzed using the Kaplan-Meier method and Cox proportional regression analysis. Overall survival was calculated from the first day of treatment in our institution until the patient's death. Patients alive or lost to follow-up were censored at the time of the last visit.
All statistical tests were two-sided with a level of significance of α < .05 and were performed using the SAS software package version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Treatment and outcome
Our database search identified 12 individuals as JW patients treated for AML. Three patients suffered from acute promyelocytic leukemia (APL) and have been excluded from this analysis, since targeted non-myelotoxic treatments, e.g. ATRA or arsenic trioxide, were available. Thus, nine patients with AML receiving conventional antineoplastic chemotherapy without blood product support have been included in the detailed analysis. These patients have been treated between 1998 and 2007.
We treated four out of nine AML patients with first-line induction chemotherapy containing cytosine-arabinoside (araC) and idarubicine. Due to expected prolonged myelosuppression not to be salvaged by transfusions, araC and idarubicin were administered in several cycles (mean number of cycles 1.5, range 1–3) with reduced dose intensity (mean araC dose per cycle 50% of standard dose, range 42–73%, mean idarubicin dose 37% of standard dose, range 29–57%). The remaining five patients received individual chemotherapy regimens including etoposide/MTX, araC/thioguanine, araC or gemtuzumab ozogamicin. Patients were informed about smaller chances of remission induction when dose reductions were planned. All patients received erythropoietin during the treatment phase. Thrombopoietin analogs were not available at the time of treatment.
Three (33%) cytological complete responses (CR) and four substantial reductions of the blast count were achieved. Two patients did not respond. Two out of three patients achieving a CR received further cycles of the initial therapy for consolidation. Two patients achieving CR subsequently relapsed (after 243 days and 317 days); one patient was lost to follow-up early. Thus, no long-term survival was observed. Chemotherapies, laboratory data and outcomes are summarized in .
Table 1. Characteristics, treatment and hemoglobin levels.
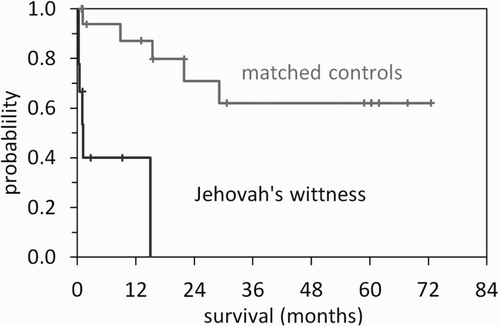
The hazard ratio for death in our series of JW patients with AML compared to a control group of patients with AML (not refusing transfusions, treated between 1997 and 2008), matched for age and cytogenetic risk group and treated with full-dose induction chemotherapy containing araC and idarubicin was 12.1 (95% confidence interval 2.3–63.8). A Kaplan-Meier plot of overall survival is shown in .
Anemia
Predominant causes of non-AML mortality were severe anemia (four out of five early deaths) and overt fatal bleeding (1 case).
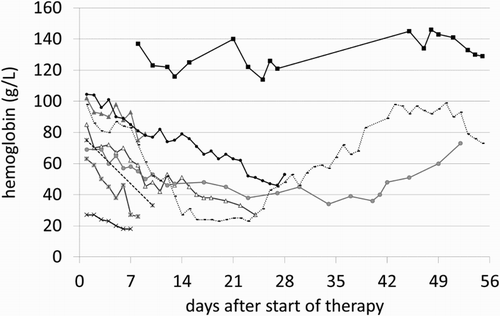
We determined the daily decline of hemoglobin concentrations in JW patients during the initial 14 days of induction chemotherapy for AML. Regression analysis including all JW patients with AML yielded an estimated linear decline of hemoglobin concentration of 2.8 g/L per day (95% confidence interval 2.2–3.3 g/L per day). The median initial hemoglobin level was 87 g/L (range 27–137 g/L). Notably, haptoglobin was not reduced in any patient ruling out treatment-induced hemolysis. In our matched control group, median of 7 (range 2–16) stored blood units and 5 (range 1–14) platelet concentrates were transfused during the first cycle of induction chemotherapy. All matched control patients were in need of some transfusion at one point of treatment. The course of hemoglobin levels of each JW patient during the first 8 weeks of treatment is depicted in .
Thrombocytopenia
Seven of our JW patients suffered from severe thrombocytopenia (defined as <10 × 109/L) for at least one day (maximum 33 days in one patient). Overall, there were 93 cumulative days of severe thrombocytopenia. There were no signs of overt gastrointestinal bleeding or massive petechial hemorrhage. One female patient with AML died from sudden uncontrolled vaginal bleeding after spending 33 days with blood platelets below 10 × 109/L despite use of antifibrinolytic agents.
Discussion
Myelosuppression is one of the major side effects of dose intensive antineoplastic chemotherapy used to treat patients with AML.
Of nine JW patients suffering from AML (all younger than 60 years) treated with reduced dose chemotherapy only three patients achieved CR and five suffered early death. Two patients achieving CR subsequently relapsed; one patient was lost to follow-up after 2.8 months. Only two patients survived more than six months but no durable remission was achieved.
Only few AML cases treated without blood product support have been reported so far. These case reports indicate that some patients did survive intensive chemotherapy and did obtain long-term complete remission, but mortality from anemia and severe bleeding was substantial [Citation3,Citation8,Citation10–12]. As a consequence, the dose reduction of induction therapy repeatedly administered in several cycles has been favored in our institution. However, this dose reduction resulted in a decreased CR rate, an increased rate of relapse and an overall HR for death of 12.1 compared to a matched control group treated with full transfusion support. As a consequence, we now would consider chemotherapy for patients refusing transfusions only if initial hemoglobin levels were sufficient.
Recent progress in treatment of AML will most likely improve survival in patients refusing transfusion. JW patients with APL have been treated successfully by others and us with ATRA and/or arsenic trioxide [Citation13]. Hypomethylating agents (HMA) for the treatment of AML are less myelotoxic but also less specific and without curative potential when used only as monotherapy. Nevertheless, successful use of HMA in combination with allogeneic stem cell transplantation without blood transfusions has been reported [Citation8]. Molecular profiling of AML has led to more targeted therapies often associated with less hematological toxicity. Thus, JW with AML and FLT3-ITD or FLT3-TKD mutation or IDH1 or -2 mutations may be treated with specific inhibitors [Citation14,Citation15] opening new options to obtain a remission of the AML followed by intensive consolidation treatment including autologous or allogeneic stem cell transplantation when bone marrow function has been improved. Stem cell transplantations without blood transfusions have been performed successfully [Citation8,Citation16–18].
The predominant cause of death in our series of JW patients with AML was progressive anemia leading to four early deaths but no lethal infections. In JW patients refusing blood transfusions while undergoing surgery, postoperative 30-day mortality was 0% with hemoglobin levels from 71 to 80 g/L but increased markedly to 34.4% with hemoglobin levels from 41 to 50 g/L [Citation19]. In our series, four patients died with a hemoglobin concentration of 33, 27, 26 and 18 g/L, respectively (). Initial hemoglobin levels of 69 and 98 g/L of two other JW patients fell down to 34 and 23 g/L but both patients surprisingly survived induction therapy, achieved CR and hemoglobin concentration later recovered to 79 and 144 g/L.
After induction chemotherapy and subsequent myelosuppression, an average decline of hemoglobin concentration by 2.8 g/L per day was observed despite intensive use of erythropoietin in the JW patient group.
Assuming a baseline hemoglobin concentration of 120 g/L for healthy persons and an erythrocyte survival time of 120 days, a complete stop of red cell production should lead to a daily decline of approximately 1 g/L. The daily decline in our JW patients was greater, pointing to additional mechanisms of blood loss since there was no evidence of hemolysis. Religious belief precluded any detailed studies of blood loss such a reinjection of marked erythrocytes. The use of antifibrinolytic agents in patients with severe thrombocytopenia may have helped to reduce bleeding.
For prevention of anemia under antineoplastic chemotherapy of solid tumors, erythropoietic agents (ESA) were thought to offer an alternative to red blood cell transfusions [Citation20]. However, for chemotherapy of acute leukemia or of aggressive lymphoma, blood transfusions are required early since erythropoietin will not act sufficiently fast to rescue patients [Citation21]: after 4 weeks of ESA treatment, a hemoglobin increase of almost 10 g/L and after 8 weeks of 20 g/L may be expected in patients undergoing chemotherapy for solid tumors and hematological malignancies [Citation22,Citation23]. Of late, the use of ESA has become controversial in patients with AML [Citation24] and is now considered to be of no substantial benefit in acute anemic states. Artificial red blood cells or cell-free hemoglobin substitutes are still at an investigative stage. Case reports including even a JW patient after allogeneic stem cell transplantation did show some feasibility but formal approval of these products has not been obtained yet [Citation25–29].
We recorded a total of 93 cumulative patient days with blood platelets below 10 × 109/L for our JW patients with AML, but only one death from bleeding after spending 33 days with platelets below 10 × 109/L. The relatively low risk of bleeding in patients with very low platelet count after receiving intensive chemotherapy is in accordance with recent studies showing that a low transfusion threshold of <10 or even <5 × 109/L is acceptable [Citation30].
Due to the strong religious conviction and the intensive support from fellow church members, acceptance of transfusion in the course of treatment has been very rare for JW patients. The high rate of patients in this report standing by their conviction not to accept blood transfusions should be seen in the light of their transfer to our institution after being denied active treatment in the referring hospitals. Still, JW patients must be fully and repeatedly counseled to all available options and results to be expected. It has been helpful then to illustrate reduced survival to patients by telling them that transfusional support would more than double the chances of survival and that only rare patients could survive without use of blood products. Our present analysis as of now, however, illustrates a risk of death more than 12 times.
Religious beliefs of patients deserve most respect, despite interfering with the physician's intent of helping and healing. German law clearly qualifies our attitude to set the patient's will higher than his or her well-being as presumed by medical science. The bioethics of refusal of medical treatment has been discussed elsewhere including the question if JW can truly decide autonomously regarding this issue [Citation9,Citation31–34].
In summary, reduced dose induction chemotherapy administered in several cycles to patients with AML refusing blood products was associated with reduced CR rates of only 33%, an increased rate of relapse. None of our AML patients achieved a durable remission. Less myelotoxic treatment options with molecular targeted therapies (ATRA, etc.) or HMA followed by intensive consolidation after improvement of bone marrow function and/or stem cell transplantations have already been shown to be promising for these patients but need further investigation.
Acknowledgements
SW and RO designed the study, SW performed statistical analyses, SW and RO interpreted data, wrote and approved the final manuscript. We thank Prof. T.H. Brummendorf for helpful discussions and critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Atallah E, Cortes J, O’Brien S, et al. Establishment of baseline toxicity expectations with standard frontline chemotherapy in acute myelogenous leukemia. Blood. 2007;110(10):3547–3551. DOI: 10.1182/blood-2007-06-095844. PubMed PMID: 17673605.
- About Jehovah’s Witnesses, Internet. [cited 2016 Nov 28]. Available from: https://www.jw.org/en/jehovahs-witnesses/
- Marsh JC, Bevan DH. Haematological care of the Jehovah’s Witness patient. Br J Haematol. 2002;119(1):25–37. PubMed PMID: 12358900. doi: 10.1046/j.1365-2141.2002.03639.x
- Lawson T, Ralph C. Perioperative Jehovah’s Witnesses: a review. Br J Anaesth. 2015;115(5):676–687. DOI: 10.1093/bja/aev161. PubMed PMID: 26068896.
- Brown NM, Keck G, Ford PA. Acute myeloid leukemia in Jehovah Witnesses. Leuk Lymphoma. 2008;49(4):817–820. DOI: 10.1080/10428190801911670. PubMed PMID: 18398752.
- Cardenas-Araujo D, Gonzalez-Lopez EE, Gonzalez-Leal XJ, et al. A clinical challenge: treatment of acute myeloid leukemia in a Jehovah’s Witness. Revista brasileira de hematologia e hemoterapia. 2016;38(4):358–360. DOI: 10.1016/j.bjhh.2016.05.003. PubMed PMID: 27863765; PubMed Central PMCID: PMC5119660.
- Dalal S, Boddapati M, Lowery MN, et al. Treatment of acute myeloid leukemia in a Jehovah’s Witness. Ann Hematol. 2006;85(6):407–408. DOI: 10.1007/s00277-006-0087-3. PubMed PMID: 16518601.
- Garelius H, Grund S, Stockelberg D. Induction with azacytidine followed by allogeneic hematopoietic stem cell transplantation in a Jehovah’s Witness with acute monocytic leukemia. Clin Case Rep. 2015;3(5):287–290. DOI: 10.1002/ccr3.212. PubMed PMID: 25984306; PubMed Central PMCID: PMC4427369.
- McEwan I. The Children Act. London: Nan A. Talese; 2014.
- Broccia G. Long-term continuous complete remission of acute myeloid leukemia in a Jehovah’s Witness treated without blood support. Haematologica. 1994;79(2):180–181. PubMed PMID: 8063269.
- Cullis JO, Duncombe AS, Dudley JM, et al. Acute leukaemia in Jehovah’s Witnesses. Br J Haematol. 1998;100(4):664–668. PubMed PMID: 9531331. doi: 10.1046/j.1365-2141.1998.00634.x
- Menendez A, Svarch E, Martinez G, et al. Successful treatment of acute promyelocytic leukemia using all-trans retinoic acid and erythropoietin in a Jehovah’s Witness boy. Ann Hematol. 1998;76(1):43–44. PubMed PMID: 9486924. doi: 10.1007/s002770050359
- Keane C, Mollee P, Marlton P, et al. Treatment of acute promyelocytic leukaemia in the Jehovah’s Witness population. Ann Hematol. 2011;90(3):359–360. DOI: 10.1007/s00277-010-1023-0. PubMed PMID: 20607537.
- Medeiros BC, Fathi AT, DiNardo CD, et al. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;31(2):272–281. DOI: 10.1038/leu.2016.275. PubMed PMID: 27721426.
- Hospital MA, Green AS, Maciel TT, et al. FLT3 inhibitors: clinical potential in acute myeloid leukemia. OncoTargets Ther. 2017;10:607–615. DOI: 10.2147/OTT.S103790. PubMed PMID: 28223820; PubMed Central PMCID: PMC5304990.
- Ford PA, Grant SJ, Mick R, et al. Autologous stem-cell transplantation without hematopoietic support for the treatment of hematologic malignancies in Jehovah’s witnesses. J Clin Oncol. 2015;33(15):1674–1679. DOI: 10.1200/JCO.2014.57.9912. PubMed PMID: 25870085; PubMed Central PMCID: PMC4559609.
- Al-Nawakil C, Quarre MC, Heshmati F, et al. Autologous stem cell transplantation in patients who object to a blood transfusion: contribution of new pharmacological haematopoiesis support. Br J Haematol. 2013;161(5):738–740. DOI: 10.1111/bjh.12284. PubMed PMID: 23480574.
- Sloan JM, Ballen K. SCT in Jehovah’s Witnesses: the bloodless transplant. Bone Marrow Transplant. 2008;41(10):837–844. DOI: 10.1038/bmt.2008.5. PubMed PMID: 18246110.
- Carson JL, Noveck H, Berlin JA, et al. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42(7):812–818. PubMed PMID: 12375651. doi: 10.1046/j.1537-2995.2002.00123.x
- Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406–3417. PubMed PMID: 19188662. doi: 10.1182/blood-2008-10-167643
- Heh-Foster AM, Naber M, Pai MP, et al. Epoetin in the ‘untransfusable’ anaemic patient: a retrospective case series and systematic analysis of literature case reports. Transfus Med. 2014;24(4):204–208. DOI: 10.1111/tme.12120. PubMed PMID: 24697987.
- Oberhoff C. Speed of haemoglobin response in patients with cancer: a review of the erythropoietic proteins. Support Care Cancer. 2007;15(6):603–611. PubMed PMID: 17277926. doi: 10.1007/s00520-006-0191-x
- Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357(10):965–976. PubMed PMID: 17804841. doi: 10.1056/NEJMoa071533
- Michallet M, Goldet K, Sobh M, et al. Prospective study of erythropoietin use on quality of life and cost effectiveness in acute myeloid leukemia and allogeneic hematopoietic stem cell transplantation patients. Cancer. 2013;119(1):107–114. DOI: 10.1002/cncr.27686. PubMed PMID: 22744794.
- Allison G, Feeney C. Successful use of a polymerized hemoglobin blood substitute in a critically anemic Jehovah’s Witness. South Med J. 2004;97(12):1257–1258. PubMed PMID: 15646766. doi: 10.1097/01.SMJ.0000140857.11967.2D
- Torres Filho IP, Spiess BD, Barbee RW, et al. Systemic responses to hemodilution after transfusion with stored blood and with a hemoglobin-based oxygen carrier. Anesth Analg. 2005;100(4):912–920. PubMed PMID: 15781498. doi: 10.1213/01.ANE.0000146960.79532.DB
- Marelli TR. Use of a hemoglobin substitute in the anemic Jehovah’s Witness patient. Crit Care Nurse. 1994;14(1):31–38. PubMed PMID: 8194324.
- Lanzinger MJ, Niklason LE, Shannon M, et al. Use of hemoglobin raffimer for postoperative life-threatening anemia in a Jehovah’s Witness. Can J Anaesth. 2005;52(4):369–373. PubMed PMID: 15814750. doi: 10.1007/BF03016278
- Smith SE, Toor A, Rodriguez T, et al. The administration of polymerized human hemoglobin (Pyridoxylated) to a Jehovah’s Witness after submyeloablative stem cell transplantation complicated by delayed graft failure. Compr Ther. 2006;32(3):172–175. PubMed PMID: 17435270. doi: 10.1007/s12019-006-0008-3
- Estcourt LJ, Desborough M, Hopewell S, et al. Comparison of different platelet transfusion thresholds prior to insertion of central lines in patients with thrombocytopenia. Cochrane Database Syst Rev. 2015;2015(6). DOI: 10.1002/14651858.CD011771. PubMed PMID: 26814707; PubMed Central PMCID: PMC4699253.
- Muramoto O. Bioethics of the refusal of blood by Jehovah’s Witnesses: Part 2. A novel approach based on rational non-interventional paternalism. J Med Ethics. 1998;24(5):295–301. PubMed PMID: 9800583; PubMed Central PMCID: PMC1377601. doi: 10.1136/jme.24.5.295
- Muramoto O. Bioethics of the refusal of blood by Jehovah’s Witnesses: Part 1. Should bioethical deliberation consider dissidents’ views?. J Med Ethics. 1998;24(4):223–230. PubMed PMID: 9752623; PubMed Central PMCID: PMC1377670. doi: 10.1136/jme.24.4.223
- Muramoto O. Bioethics of the refusal of blood by Jehovah’s Witnesses: Part 3. A proposal for a don’t-ask-don’t-tell policy. J Med Ethics. 1999;25(6):463–468. PubMed PMID: 10635499; PubMed Central PMCID: PMC479294. doi: 10.1136/jme.25.6.463
- Rajtar M. Bioethics and religious bodies: refusal of blood transfusions in Germany. Soc Sci Med. 2013;98:271–277. DOI: 10.1016/j.socscimed.2013.02.043. PubMed PMID: 23538204.