ABSTRACT
Background and aims: To explore the relationship between FLT3 (encoding Fms related tyrosine kinase 3) internal tandem duplication (ITD) mutations with the prognosis of acute promyelocytic leukemia. The PubMed database, the Cochrane Library, conference proceedings, the EMBASE databases, and references of published trials and review articles were searched. Two reviewers independently assessed the quality of the trials and extracted the data. Odd ratios (ORs) for complete remission (CR) rate after induction therapy, 5-year overall survival (OS), and 5-year disease free survival (DFS) were pooled using the STATA package.
Main results: Seventeen trials involving 2252 patients were ultimately analyzed. The pooled OR showed that the FLT3 ITD mutation group had a poor prognosis in terms of CR rate (OR = 0.53, 95% confidence interval (CI), 0.30–0.95, P = 0.03), 5-year OS (OR = 0.47, 95% CI, 0.29–0.75, P = 0.002), and as 5-year DFS (OR = 0.48, 95% CI, 0.29–0.78; p = 0.003).
Conclusions: The results suggested that FLT3 ITD mutations could become an indicator of poor prognosis of APL, and these patients should receive more intensive therapy according to current guidelines.
Acute promyelocytic leukemia (APL) is a discrete subtype of acute myeloid leukemia characterized by a t(15;17) translocation, rearrangement of the PML and RARA genes, and the formation of an abnormal chimeric retinoic acid receptor transcription factor (PML-RARα) [Citation1]. APL accounts for 8–10% of de novo cases of acute myeloid leukemia and in general, has a favorable prognosis [Citation2]. According to the National Comprehensive cancer Network (NCCN) guidelines, treatment regimens for APL should be based on the prognostic factors of the patients, and treatment intensification should be different in patients with different prognoses. Thus, prognostic stratification of APL at the beginning of the disease is important. Several factors contribute to the prognosis of APL: A high white blood cell (WBC) count (>10 × 109/L) is supposed to be the main factor associated with relapse. The Sanz score subdivides APL patients according to peripheral blood counts into three risk groups: low (WBC ≤10 × 109/L and platelet count >40 × 109/L), intermediate (WBC ≤10 × 109/L and platelet count ≤40 × 109/L), and high (WBC >10 × 109/L) [Citation3]. Another known predictive marker for negative outcome is the M3v subtype [Citation4]. Furthermore, a high PML–RARA expression level at diagnosis correlates with a worse outcome [Citation5].
The FLT3 gene, which is located on chromosome 13q12 in humans, encodes Fms related tyrosine kinase 3, a class III tyrosine kinase receptor (RTK). FLT3 plays an important role in the differentiation of hematopoietic stem cells. FLT3 mutations have been noted, including internal tandem duplications (ITDs) in the juxtamembrane domain, which frequently involve exon 14 and eventually intron 14 or exon 15 [Citation6]. Various studies have shown that ITDs are detectable in approximately 20% of de novo acute myelocytic leukemias (AMLs), thus representing the most frequent genetic aberration currently known in this disease. Interestingly, these alterations are more commonly detected in certain subsets, such as APL, and in AMLs with a normal karyotype. Moreover, in non-APL AMLs, the presence of ITDs was associated in most reported series with an unfavorable outcome [Citation6,Citation7–10]. In APL, the incidence of FLT3-ITD mutations ranged from 12 to 38% [Citation11]. The role of FLT3-ITDs in APL as a prognostic factor for long-term outcome has not yet been clarified and the significance of these genetic alterations remains controversial. Several studies of APL have reported an association between FLT3-ITDs and various characteristics, including elevated WBC count, BCR3 (breakpoint cluster region pseudogene 3) isoforms, and microgranular morphology (M3v) [Citation12,Citation13]. However, other studies have found that FLT3-ITDs do not have a significant impact on clinical outcome; there were statistically non-significant trends towards disease-free survival (DFS) and higher relapse rates in patients with an FLT3 mutation [Citation13–15]. In a recent study of 82 APL patients, FLT3-ITDs had a significant negative impact on overall survival (OS), and there was a significant association between FLT3-ITDs and leukocytosis, indicating that the presence of FLT3-ITDs enhances biological proliferation [Citation16]. Nevertheless, the clinical significance of FLT3-ITDs remains unidentified. The aim of the present study was to explore the effect of FLT3-ITDs on the prognosis of adult APL via a systematic review and meta-analysis.
Experimental procedures
Study inclusion criteria
Our inclusion criteria limited the main comparison to trials comparing clinical outcome of FLT3-ITD-positive and FLT3-ITD-negative expression (or FLT3-ITD WT) in adult promyelocytic leukemia (age >18 years). Prospective cohort studies were included. We did not set a minimum number of patients (sample size) to be considered. However, studies were excluded if no survival data was mentioned in the full text. Full-text publications in English were considered. We set no limits on the year of publication or year of treatment.
Search strategy
MEDLINE (to 2016), EMBASE (to 2016), and The Cochrane Library (to 2016) were searched without restrictions on study design, publication year, and language. A final search was conducted in PubMed using the MeSH terms ‘FLT3’ and ‘APL’ or ‘AML-M3’. Reference lists of all included original articles were searched manually. Information on the American Society of Hematology (ASH) conferences was also searched online using the search terms ‘FLT3’ and ‘APL’ or ‘AML-M3’.
Study selection
To be included in the present systematic review, we required that a study focused on the relationship between FLT3-ITD and the complete remission (CR) rate, the 5-year DFS rate or the 5-year OS rate of adult APL. All the data were extracted from the articles. In the first step, articles were excluded if the title and/or the abstract clearly referred to other markers of APL. In the second step, articles were excluded if information such as the CR rate, DFS, or OS were not clear or could not be extracted. In the third step, articles were also excluded if the more than 15% of the patients’ information was lost at the end point of the study. A flowchart of the search process is shown in . Two independent reviewers performed all the literature screening process steps. Any disagreements were resolved by discussion.
Data extraction
The following data were extracted from original articles, if available: (1) Basic characteristics of the FLT3-ITD-positive group and FLT3-WT group, such as age, sex, WBC count at the beginning of diagnosis; (2) the last name of the first author and the year of publication; (3) the therapy applied (induction chemotherapy, consolidation chemotherapy, and maintenance chemotherapy); (4) the CR rate, (5) survival time (data extraction was finalized using Engauge Digitizer software (Fujitsu Company, Japan)). The CR rate was extracted from the manuscript. Survival estimates were extracted directly from the text or deduced from the survival curve of the publication. We extracted the number of indicated patients for the 5-year DFS and 5-year OS. If we could not obtain the results from the reported studies directly, we used the Engauge Digitizer to extract the data from the survival curve.
Statistical analysis
The analyses were tested by pairwise comparisons of the FLT3-ITD-positive type-based arms with the FLT3-ITD-negative type-based arms. The odds ratio (OR) was used for the CR rate, 5-year DFS, and 5-year OS. All the analyses were performed using the STATA SE 10.1 package (StataCorp, College Station, TX, USA). A statistical test with a p value less than 0.05 was considered significant. OR scores of less than 1 reflected less CR rate or poorer DFS or OS in the FLT3-ITD-positive group. To investigate the statistical heterogeneity between trials, the standard χ2 Q test was applied (meaningful differences between studies were indicated by p < 0.10). The results were generated using a random effects model when there was evidence of significant statistical heterogeneity, which generated a more conservative estimate. All p values were two-sided. All CIs had a two-sided probability coverage of 95%.
Results
Trial flow
One hundred and fifty seven reports were retrieved originally after electronic searching, and 24 reports were identified after scanning the titles and abstracts. Seven reports were excluded for following reasons: Three were not clinical trials of FLT3-ITD mutations and APL [Citation17–19]. Twenty-one studies concerned the relationship between FLT3-ITD and the prognosis of APL. Some studies did not contain adequate information about the CR, 5-year OS, and 5-year DFS. Thus, seventeen trials were ultimately analyzed. Thirteen studies contained 1875 patients and were analyzed for the CR rate of the FLT3-ITD-positive and FLT3-ITD-negative groups; 17 studies contained 2252 patients for 5-year OS of the FLT3-ITD-positive and FLT3-ITD-negative groups; and 15 studies contained 1931 patients for 5-year DFS of the FLT3-ITD-positive and FLT3-ITD-negative groups ().
Characteristic of the trials
Seventeen trials [Citation13,Citation18,Citation20–34] meeting the inclusion criteria were identified. The baseline characteristics of all the trials are listed in . The treatment regimens and principles of all the trials were similar to each other. All the patients could be divided into an FLT3-ITD-positive group and FLT3-ITD-negative group. FLT3 ITDs were identified from patients’ bone marrow samples. The studies showed no significant differences in terms of gender, age, and cytogenetics; however, the FLT3-ITD-positive group had a higher WBC count at diagnosis. The treatment regimens in the subgroups were comparable, which reduced the confounding bias.To explore the indicative effect of FLT3 ITDs in adult APL, we calculated the OR for the CR rate after induction therapy, the 5-year OS, and 5-year DFS.
Table 1. The characteristics of each study included in the meta-analysis.
The CR rate
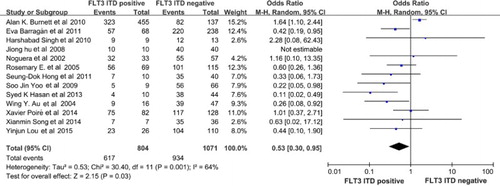
Thirteen studies compared the CR rate after standard induction therapy between the FLT3-ITD-positive and FLT3-ITD-WT (negative) group among the seventeen studies. The CR rate was available for 1875 patients in the trials. As a result, the FLT3-ITD-positive group had a poorer CR rate compared with the FLT3-ITD-WT group (OR = 0.53, 95% CI, 0.30–0.95, P = 0.03, ).
5-year OS
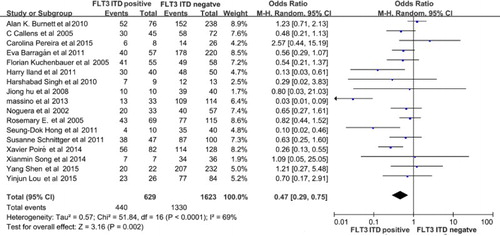
All the patients involved in the studies received chemotherapy. Although the medicine was slightly different in each clinical trial, it basically contained induction therapy, consolidation therapy, maintenance therapy, and central nervous system propagation. Seventeen studies compared the 5-year OS between the FLT3-ITD-positive and FLT3-ITD-WT (negative) group. All the data were acquired or extracted from the manuscripts; the 5-year OS data were available for 2252 patients in the trials. As a result, the FLT3-ITD-positive group had a poorer 5-year OS compared with the FLT3-ITD-WT group (OR = 0.47, 95% CI, 0.29–0.75, P = 0.002, ).
5-year DFS
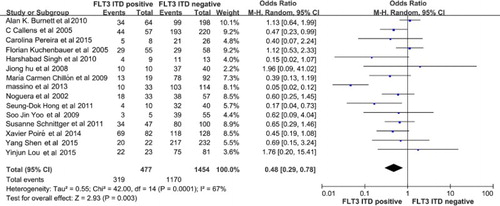
The 5-year DFS data and p values were available for 1931 patients in fifteen trials. As a result, the 5-year DFS for the FLT3-ITD-positive group was poorer than that of the FLT3-ITD-negative group (OR = 0.48, 95% CI, 0.29–0.78; p = 0.003, ).
Figure 4. The pooled OR showing that the FLT3-ITD mutated group have a significant difference with the FLT3-ITD WT group (p = 0.003) in 5-year DFS. FLT3, Fms related tyrosine kinase 3, ITD, internal tandem duplication; WT, wild-type; OR, odds ratio; DFS, disease-free survival.
Thus, FLT3-ITD positivity was likely to be a predictor of poor prognosis of APL.
Discussion
APL is a subtype of AML. Following the clarification of pathology and molecular characteristics, and treatment with drugs such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), the prognosis of APL improves significantly. Nowadays, risk stratification of APL is very important, and treatment regimens are different for low and high-risk groups. However, the effects of FLT3 ITD mutations remain controversial. Some studies, such as Massino et al. [Citation18] and Hong et al. [Citation29] indicated that FLT3 ITD mutations represent a poor marker of adult APL; whereas other studies indicated that the FLT3-ITD group did not show a significant difference compared with the FLT3-ITD WT group [Citation20–28]. In the present systematic review and meta-analysis, FLT3 ITD mutations were likely to predict a poor outcome in adult APL, in terms of the CR rate after induction therapy, the 5-year OS rate, and 5-year DFS rate.
Why did some studies come to different conclusions? Perhaps the treatment protocols affected the results. Although the treatment principle in all the trials was the same, drugs such as ATO added to induction therapy might improve survival [Citation27]. In addition, induction therapy containing ATRA and ATO might have improved the prognosis of patients with APL; however, some studies, such as Poiré et al. [Citation31], concluded that the addition of ATO consolidation improved the outcome regardless of the FLT3 mutation type or level and initial WBC count. The number of patients in each trial also influenced the results. Although the clinicians obtained almost enough patients to acquire accurate results, even in our meta-analysis, the number of patients with APL might not have been sufficient. Thus, it is possible that the outcomes of other ongoing trials could change the results of our meta-analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Wenfang Zhuang http://orcid.org/0000-0002-2068-381X
References
- Wang Z-Y, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. doi: 10.1182/blood-2007-07-102798
- Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.V100.1.59
- Sanz MA, Lo Coco F, Martin G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–1253.
- Lengfelder E, Reichert A, Schoch C, et al. Double induction strategy including high dose cytarabine in combination with all-trans retinoic acid: effects in patients with newly diagnosed acute promyelocytic leukemia. German AML Cooperative Group. Leukemia. 2000;14:1362–1370. doi: 10.1038/sj.leu.2401843
- Schnittger S, Weisser M, Schoch C, et al. New score predicting for prognosis in PML-RARA+, AML1-ETO+, or CBFBMYH11+ acute myeloid leukemia based on quantification of fusion transcripts. Blood. 2003;102:2746–2755. doi: 10.1182/blood-2003-03-0880
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.V98.6.1752
- Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080.
- Kondo M, Horibe K, Takahashi Y, et al. Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med Pediatr Oncol. 1999;33:525–529. doi: 10.1002/(SICI)1096-911X(199912)33:6<525::AID-MPO1>3.0.CO;2-8
- Abu-Duhier FM, Goodeve AC, Wilson GA, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111:190–195. doi: 10.1046/j.1365-2141.2000.02317.x
- Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.V97.1.89
- Beitinjaneh A, Jang S, Roukoz H, et al. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations in acute promyelocytic leukemia: a systematic review. Leuk Res. 2010;34:831–836. doi: 10.1016/j.leukres.2010.01.001
- Minami S, Kuriyama K. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia study group of the ministry of health and welfare (Kohseisho). Leukemia. 1997;11(10):1447–1452.
- Noguera NI, Breccia M, Divona M, et al. Alterations of the FLT3 gene in acute promyelocytic leukemia: association with diagnostic characteristics and analysis of clinical outcome in patients treated with the Italian AIDA protocol. Leukemia. 2002;16(11):2185–2189. doi: 10.1038/sj.leu.2402723
- Kiyoi H, Naoe T, Yokota S, et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia study group of the ministry of health and welfare (Kohseisho). Leukemia. 1997;11:1447–1452. doi: 10.1038/sj.leu.2400756
- Shih L-Y, Kuo M-C, Liang D-C, et al. Internal tandem duplication and Asp835 mutations of the FMS-like tyrosine kinase 3 (FLT3) gene in acute promyelocytic leukemia. Cancer. 2003;98:1206–1216. doi: 10.1002/cncr.11636
- Au WY, Fung A, Chim CS, et al. FLT-3 aberrations in acute promyelocytic leukaemia: clinicopathological associations and prognostic impact. Br J Haematol. 2004;125:463–469. doi: 10.1111/j.1365-2141.2004.04935.x
- Molica M, Breccia M. FLT3-ITD in acute promyelocytic leukemia: clinical distinct profile but still controversial prognosis. Leuk Res. 2015;39:397–439. doi: 10.1016/j.leukres.2015.01.004
- Breccia M, Loglisci G, Loglisci MG, et al. FLT3-ITD confers poor prognosis in patients with acute promyelocytic leukemia treated with AIDA protocols: long-term follow-up analysis. Haematologica. 2013;98(12):e161–e163. doi: 10.3324/haematol.2013.095380
- Li W, Zhang L, Huang L, et al. Meta-analysis for the potential application of FLT3-TKD mutations as prognostic indicator in non-promyelocytic AML. Leuk Res. 2012;36:186–191. doi: 10.1016/j.leukres.2011.08.014
- Burnett AK, Hills RK, Green C, et al. The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and CEBPA. Blood. 2010;115:948–956. doi: 10.1182/blood-2009-08-236588
- Callens C, Chevret S, Cayuela J-M, et al. Prognostic implication of FLT3 and Ras gene mutations in patients with acute promyelocytic leukemia (APL): a retrospective study from the European APL Group. Leukemia. 2005;19:1153–1160. doi: 10.1038/sj.leu.2403790
- Souza Melo CP, Campos B, Dutra ÁP, et al. Correlation between FLT3–ITD status and clinical, cellular and molecular profiles in promyelocytic acute leukemias. Leuk Res. 2015;39:131–137. doi: 10.1016/j.leukres.2014.11.010
- Barragán E, Montesinos P, Camos M, et al. Prognostic value of FLT3 mutations in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy. Haematologica. 2011;96(10):1470–1477. doi: 10.3324/haematol.2011.044933
- Kuchenbauer F, Schoch C, Kern W, et al. Impact of FLT3 mutations and promyelocytic leukaemia-breakpoint on clinical characteristics and prognosis in acute promyelocytic leukaemia. Br J Haematol. 2005;130:196–202. doi: 10.1111/j.1365-2141.2005.05595.x
- Iland H, Bradstock K, Seymour J, et al. Results of the APML3 trial incorporating all-trans-retinoic acid and idarubicin in both induction and consolidation as initial therapy for patients with acute promyelocytic leukemia. Haematologica. 2012;97(2):227–234. doi: 10.3324/haematol.2011.047506
- Singh H, Werner L, DeAngelo D, et al. Clinical outcome of patients with acute promyelocytic leukemia and FLT3 mutations. Am J Hematol. 2010;85(12):956–957. doi: 10.1002/ajh.21867
- Hu J, Liu Y-F, Wu C-F, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci. 2009;106(9):3342–3347. doi: 10.1073/pnas.0813280106
- Gale RE, Hills R, Pizzey AR, et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood. 2005;106:3768–3776. doi: 10.1182/blood-2005-04-1746
- Hong S-D, Kim Y-K, Kim H-N, et al. Treatment outcome of all-trans retinoic acid/anthracycline combination chemotherapy and the prognostic impact of FLT3 /ITD mutation in acute promyelocytic leukemia patients. Korean J Hematol. 2011;46:24–30. doi: 10.5045/kjh.2011.46.1.24
- Schnittger S, Bacher U, Haferlach C, et al. Clinical impact of FLT3 mutation load in acute promyelocytic leukemia with t(15;17)/PML-RARA. Haematologica. 2011;96(12):1799–1807. doi: 10.3324/haematol.2011.049007
- Poiré X, Moser BK, Gallagher RE, et al. Arsenic trioxide in front-line therapy of acute promyelocytic leukemia (C9710): prognostic significance of FLT3 mutations and complex karyotype. Leuk Lymphoma. 2014;55(7):1523–1532. doi: 10.3109/10428194.2013.842985
- Song X, Hu X, Lu S, et al. Incorporation of arsenic trioxide in induction therapy improves survival of patients with newly diagnosed acute promyelocytic leukaemia. Eur J Haematol. 2014;93:54–62. doi: 10.1111/ejh.12301
- Shen Y, Fu Y-K, Zhu Y-M, et al. Mutations of epigenetic modifier genes as a poor prognostic factor in acute promyelocytic leukemia under treatment with all-trans retinoic acid and arsenic trioxide. EBioMedicine. 2015;2:563–571. doi: 10.1016/j.ebiom.2015.04.006
- Lou Y, Ma Y, Suo S, et al. Prognostic factors of patients with newly diagnosed acute promyelocytic leukemia treated with arsenic trioxide-based frontline therapy. Leuk Res. 2015;39:938–944. doi: 10.1016/j.leukres.2015.05.016