ABSTRACT
Objectives: Fanconi anaemia (FA) is a rare inherited bone marrow failure and autosomal recessive blood disorder. FA patients have a higher risk of cancer, including acute myeloid leukaemia and squamous cell carcinoma. Maximum, but not all, affected individuals have one or more somatic abnormalities, including skin, skeletal, genitourinary, gastrointestinal, cardiac and neurological anomalies, etc. Positive stress cytogenetics has immense implications for the treatment and management of FA. The aim of our study was to find out the incidence of FA in the population of phenotypically normal aplastic anaemia (AA) patients in West Bengal.
Methods: Ethical clearances were obtained from the corresponding institutional committees. A total of 117 AA cases was selected. Stress cytogenetics was performed from peripheral venous blood (PVB) samples of 63 AA patients (age ≤ 50 years) and 63 age- and sex-matched healthy individual (control) using Mitomycin C (MMC).
Results: Out of 63 AA patients, 6 (9.25%) cases showed positive stress cytogenetics suggestive of FA, which is statistically significant (p-value – 0.000532), analysed by chi-square test.
Discussion: A considerable percentage of patients showing sensitivity towards MMC, even if they are phenotypically normal and did not have any distinguishable features which are generally found in FA.
Conclusion: This observation may indicate that stress cytogenetics analysis of phenotypically normal AA patients (≤50 years) is essential for the improvement of the treatment procedure.
1. Introduction
The Fanconi anaemia (FA) is defined by genes elucidating a novel DNA repair mechanism required for maintaining genomic stability and preventing cancer [Citation1]. Mutation in these genes can cause a specific clinically recognizable entity with a unifying cellular feature of hypersensitivity to cross-linking agents like Mitomycin C (MMC) or di-epoxy butane (DEB) [Citation2]. FA is the mostly found inherited/congenital aplastic anaemia (AA), while others being Dyskeratosis congenita, Shwachman diamond syndrome, Blackfan diamond syndrome, etc … Another category is acquired AA, which have relation with some definite aetiological agents. FA is a rare genetic disorder with an incidence of 1 in 3,60,000 births [Citation3,Citation4]. The patients with FA have an increased risk of solid tumours and leukaemia [Citation5]. Thirteen complementation groups, defined by somatic cell hybridization, are associated with the development of FA [Citation6]. The complementation groups have been designated as FANCA, B, C, D1, D2, E, F, G, I, J, L, M and N. The most frequent mutations are found in FANCA, FANCC or FANCG[Citation7]. It has been proposed that the A and C genes produce cytoplasmic proteins forming an ‘FA core complex’, whereas the products of genes B, E, F, G, L and M form adaptors or phosphorylators [Citation8,Citation9]. The complex translocates to the nucleus and ubiquitylates FANCD2 and protects the cell from DNA cross-linking agents, thereby participating in the DNA repair mechanism. DNA damage triggers the activation of the FA/BRCA pathway and ubiquitylation of FANCD2 which in turn activates the DNA repair mechanism by the help of DNA repair proteins like BRCA1, FANCD1/BRCA2, FANCN/PALB2 and RAD51 [Citation9]. In the presence of a mutant protein, the normal function is hampered, leading to the damage of haematopoietic stem cells (HSC) and bone marrow failure. Previous studies reported that FA patients face some typical abnormalities, which are summarized in , with their probable percentage of occurrence.
Table 1. The typical features associated with FA and their percentage of occurrence.
It is known that the carrier frequency of FA is 1:181 in the US and 1:93 in Israel [Citation12]. But the exact incidence of FA in India is not known due to lack of properly designed trials. This study is one of the pioneering researches with FA patients selecting the population of West Bengal. Although phenotypically normal FA patients are found but in India very few research works have been made in this sector. Our study was aimed to find out the incidence of FA cases in phenotypically normal AA patients.
2. Materials and methods
2.1. Ethics
Ethical clearances were obtained from the ethics committee of Nil Ratan Sircar Medical College & Hospital, Kolkata and Vivekananda Institute of Medical Sciences, Kolkata.
2.2. Questionnaire and consent
Patients and control cases were thoroughly informed about the research work. Written consent was taken before the collection of PVB samples. A detailed questionnaire was administered to record their data.
2.3. Study design
A total of 72,800 patients with different type of hematological disorders were screened in the Department of Hematology, Nil Ratan Sircar Medical College & Hospital, Kolkata from January 2015 to December 2016. Among these, 520 cases were newly diagnosed pancytopenia patients. These 520 pancytopenia patients were undergone through bone marrow aspiration and biopsy examination to confirm AA. A total of 117 phenotypically normal yet confirmed AA patients participated in this study. Detailed clinical information, including history of consanguinity was recorded and their physical examination was done by the respective clinicians. According to the BCSH (British Committee for Standards in Haematology) guideline chromosomal breakage analysis from PVB should be done to exclude FA, if the patient is <50 years old. In case the age of the patient is ≥50 years, the disease is considered to be acquired aplastic anaemia.
In our study, among 117 AA cases, only 63 were <50 years old and hence were subjects of stress cytogenetics test for the screening of FA. Echo-cardiogram and ultrasonography were done on these 63 patients to ensure if there was any cardiac or renal abnormality, although the results showed normal. PVB samples were collected from these 63 patients and stress cytogenetics was done using Mitomycin C (MMC). PVB samples were also collected from 63 age- and sex-matched healthy individuals without pancytopenia (Negative control). For the standardization of the stress cytogenetics procedure, PVB samples were collected from three confirmed FA patients (Positive control). Stress cytogenetics study of these cases and controls (positive & negative) were performed in the Department of Genetics, Vivekananda Institute of Medical Sciences, Kolkata.
2.4. Materials and methodology
A volume of the 500 μl PVB sample was inoculated to the culture media containing RPMI 1640 [Gibco] (4 ml), Foetal bovine serum (FBS) [Gibco] (1 ml) and Phytohaemagglutinin (PHA) [Gibco] (200 μl). In this complex media, RPMI 1640 helps in serum-free expansion and FBS acts as a serum-supplement for human lymphocytes, respectively. PHA stimulates the selective proliferation of T lymphocytes, which ensure a good amount of cell pellet. A good number as well as good quality of metaphase must be obtained to analyse the break and radial structures. Cultures were incubated at 37°C for 72 hours in O2 incubator. MMC was introduced to the culture after 24 hours of inoculation. Three cultures, each for cases and controls (0 ng MMC/ml culture, 50 ng MMC/ml culture, 100 ng MMC/ml culture) were set. A volume of 300 μl of working Colcimid [Karyomax] solution (1 μg/ml) was added. After centrifugation at 1200 rpm for 10 minutes harvesting was started with the addition of 10 ml KCl solution (0.075 M) to the cell pellet and kept in 37°C water bath for 10 minutes. The cells are pre-fixed by the addition of 1–2 ml fixative solution (methanol: glacial acetic acid = 3:1). The culture was centrifuged again and 10 ml of fixative solution was added. Washing step by fixative was repeated at least for 4–5 times till white pellet of leukocyte was obtained. Keeping the slide in slanting condition, 20 μl of this pellet was poured on the slide. The smear was washed thoroughly by fixative solution. Giemsa stain [Gibco] was used to stain the chromosomes. GTG banding is not needed to visualize the break and radial structures. A set of 60 metaphases each from cases and control (positive & negative) (20 metaphases from each culture) was observed under microscope (Zeiss Axioskop Microscope) 100× magnification for detecting the presence of gap, break and radial structure (bi-radial, tri-radial, higher order radial structures) suggestive for FA. The standard formula was used to analyse the sensitivity of MMC [% of cells with tri-radials + (1.6 × Total number of radials in 50 & 100 ng MMC/ml Culture)]; the cut-off for the sensitivity of MMC is >40. The bi-radial, tri-radial and higher order radial structures all are counted as tri-radial, whereas the gap and break structures are not considered in the formula.
3. Results
3.1. Results of the clinical manifestation of stress cytogenetics positive cases
FA patients suffer from some typical birth defects (listed in ), which generally help the clinicians to diagnose the disease. But in our study, six patients who are found to be positive for stress cytogenetics had no such clinical features. The clinical data and history of family pedigree of the stress cytogenetics positive cases are summarized in .
Table 2. The data of clinical manifestation and family history of six stress cytogenetics positive patients.
Out of six stress cytogenetics positive cases, four patients (66.66%) born out of consanguineous marriage. Among these 4 patients 3 (75%) were female, 1 (25%) was male.
3.2. Results of the clinical findings and haematological profile
Out of 63 cases, 46 (73.01%) were male and 17 (26.98%) were female; the median age being 22 years and age ranging from 3 to 50 years. Only six (9.52%) patients were found to be positive for stress cytogenetics which is suggestive for FA. Other 57 AA patients who are not sensitive to MMC can be assigned under acquired AA category. The median age of patients positive for stress cytogenetics was 6 years. The mean neutrophil count, mean haemoglobin (Hb) count, mean reticulocyte count and mean platelet count was 0.16 ± 0.05 (×109/l), 56.83 ± 19.84 (g/l), 14.3 ± 0.14 (×109/l) and 11.16 ± 3.53 (×109/l), respectively, in MMC-sensitive cases. In stress cytogenetics positive cases, 6.35% (4 of 63) were categorized under very severe aplastic anaemia group, 3.17% (2 of 63) were categorized under severe aplastic anaemia group, but no patient of non-severe aplastic anaemia category was found. The data of clinical findings and haematological profile are tabulated in . The graphical representations of the haematological data are presented in .
Figure 1. The graphical representation of the mean and standard deviation of the (a) neutrophil, (b) haemoglobin, (c) reticulocyte and (d) platelet, which are responsible for pancytopenia. The data of stress cytogenetics positive cases and positive control are almost consistent with each other. The counts of other 57 AA cases, which are not sensitive to MMC are moderately higher than MMC-sensitive and positive control groups. The counts of the age- and sex-matched negative controls are significantly higher than the other three groups.
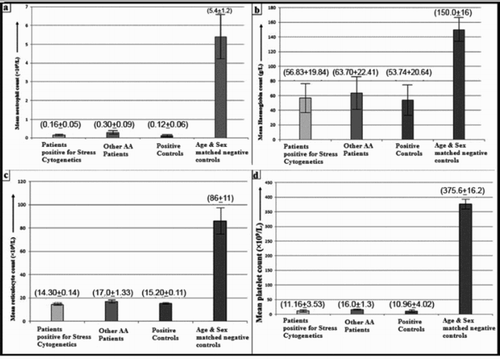
Table 3. The clinical findings and haematological profile of patients positive for stress cytogenetics, other AA patients, positive and negative controls.
3.3. Results of the stress cytogenetics using MMC
Break, gap and radial structures were observed under the microscope (100× magnification). In the positive controls as well as in every stress cytogenetics positive case, high sensitivity index of MMC (>40) was found calculated by the above-mentioned formula. Other 57 AA cases as well 63 negative controls were found to be not sensitive to MMC. Hence, the characteristic chromosomal structures were not observed ().
Figure 2. The representative picture of the metaphases obtained from the stress cytogenetics culture; (a) metaphase cells from the negative control cases, where virtually no break or radial structure was found, (b) metaphase cell from the positive control cases, where very high number of break, radial, bi-radial, and tri-radial structures are observed, (c) metaphases from the six stress cytogenetics positive patients, having a high number of break, radial, bi-radial, and tri-radial structures like positive controls. The picture of the metaphases found from other 57 AA cases who are not positive for stress cytogenetics was like negative controls. MMC acts as a potent DNA cross-linker. Chromosome breakage and radial formation are nothing but Interstrand cross-links (ICLs). ICLs can arise in the pre and post state of DNA replication between homologous regions of sister chromatid. In case of post-replication state ICLs, there is two ways, either homologous repair between sister chromatids produce an error-free repair or mitosis proceeds normally and one of the daughter cells inherits an un-repaired ICL. In case of pre-replication state ICLs, where a homologous sister chromatid is not available for re-combinational repair, the mechanism is completed by three ways, (1) non-homologous end joining, (2) excision repair and/or lesion bypass and (3) homologous repairs between homologous chromosomes. Whereas first two mechanisms are error-prone, the third one is error-free. A mandatory-break is generated by un-repaired ICLs during mitosis. The red, green and blue arrows are indicating the radial structures, chromosomal break and gap respectively.
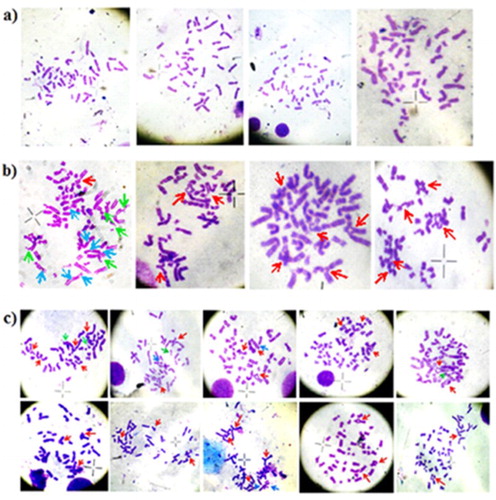
All the cultures were done in PHA-stimulated condition. Compared to the negative control and 57 AA cases, which are not sensitive to MMC, a significantly high percentage of the radial structure was found in the positive control and stress cytogenetics positive patients. The comprehensive data are listed in .
Table 4. The quantitation of characteristic chromosomal structure in the stress cytogenetics positive patients, controls (positive & negative) and other AA cases.
3.4. Results of statistical analysis
In our study, the calculated value of χ2 was 12, which is greater than the chi-square table value, i.e. 9.21 at 1% confidence level (degrees of freedom 1). The two-tailed p-value is 0.000532. The result is significant at p < 0.01. This statistical analysis is supporting the fact that stress cytogenetics in phenotypically normal AA patients (<50 years) is essential in ruling out FA.
4. Discussion
FA is an autosomal recessive chromosomal instability syndrome characterized by congenital abnormalities, progressive bone marrow failure and cancer predisposition. It has already been documented in various literatures that the gold standard diagnostic test for FA consists of cytogenetic quantitation of chromosomal breakage in response to DNA cross-linking agents like diepoxybutane (DEB) or MMC [Citation13–22].
We have already mentioned the detailed protocol of stress cytogenetics in Section 2. In this context, one thing should be mentioned, that MMC needs to stay 48 hours in culture for introducing the characteristic chromosomal structures, i.e. radial. Bone marrow (BM) contains HSC, which have the rapid proliferation capability that the PVB sample is devoid off. If PHA stimulation is used in BM sample, after 72 hours, it will reach to nutrient deficient status and the cell will virtually die. In case of cytogenetic analysis, PHA may help in the proliferation of only normal cells, thus can suppress the growth of cells with an abnormal chromosomal profile which should be properly screened for the diagnosis of the acquired or malignant diseases. For these reasons, ideally, BM sample should be used in case of malignant or acquired disorders in the unstimulated condition to screen the cytogenetic abnormality but not in constitutional diseases. If PVB is the sample for cytogenetic study in case of acquired or malignant chromosomal abnormality detection, the culture must be done in unstimulated condition and the study protocol has to be standardized accordingly.
PHA is used in the PVB culture as stimulation and it is perfectly co-ordinated with the incubation period and helps to get sufficient amount of cell pellet. The major clinical manifestation of FA patients is pancytopenia. Thereby very few cells are present in the PVB sample. In such a condition, unstimulated PVB culture (without PHA) is unable to produce adequate amount of cell. Hence, the stress cytogenetics test must be done from PVB in PHA-stimulated condition, not from unstimulated or BM sample as FA is a constitutional/ inherited disease.
Many international researches have been made on the FA patients using DNA cross-linking agents. A study showed that FA patients have high susceptibility to DNA cross-linking agents [Citation13]. Another study was conducted in Southern Brazil on 17 patients has found seven confirmed FA cases [Citation4]. A research was made on FA patients with atypical phenotypes which is very much frequent in FA. They used DEB test for the detection of the disease [Citation17]. Again a large cohort study applied flow-based MMC sensitivity test to evaluate FA phenotype in fibroblasts [Citation20]. A study in Serbia was performed on FA-affected children and found the percentage of DNA cross-linker-induced aberrant cells was increased more than 26 times in FA patients compared to non-FA patients [Citation22]. This study also supports this observation of DNA cross-linker-induced chromosomal breakage is very high in FA patients. A case report published from Bangladesh showed genetic study should be done if possible in all the cases of suspected FA, siblings, parents and close blood relatives. Also said that the screening of the FANCA gene for mutations supports the clinical diagnosis of FA [Citation23]. Our study also justifies the fact of the high percentage of consanguinity is found among FA patients.
Few literatures have been documented from the northern India. A study on 94 AA patients of all age groups, showed 13.8% patients with FA [Citation24], whereas another study, showed 11.3% positive for stress cytogenetics [Citation25]. However, a larger study carried out on 300 Indian AA patients, found 9.20% patients with FA [Citation26]. Another study revealed 7 (14%) out of 50 examined patients to have an FA cellular phenotype with increased MMC-induced chromosome fragility [Citation27]. Again a study conducted in Delhi on 488 patients found that 64 (13.1%) patients with a significant increase in the number of breaks in comparison to their control [Citation28]. A 14-year-old boy was presented with clinical features of AA, chromosomal breakage test using clastogenic agent MMC showed 88% stress induced chromosomal/chromatid breaks, gaps and rearrangements revealing an underlying FA [Citation29]. But from the southern part of India, there is no such documentation. Although from the western part, there are few studies [Citation30–32]. From eastern India, one case study has been documented on FA with the incidental haemoglobin E trait [Citation33]. The differences in the percentage affected by FA may be due to the different number of cases participated in these studies. Above-mentioned studies considered both kinds of AA patients, irrespective of their phenotypical condition for stress cytogenetics. But in our study, we tried to find the frequency of FA in phenotypically normal AA patients in West Bengal. This is the unique perspective of our study.
The absence of typical physical abnormalities sometimes makes the clinicians confuse. But through this study, we have tried to emphasize on the fact that phenotypically normal AA patients can be positive for stress cytogenetics which is suggestive for FA. In our study, we have done stress cytogenetics from 63 patients who are phenotypically normal and 9.52% cases were found to be sensitive to MMC. In future FANCD2 western blotting, immunobloting [Citation34–35] and next-generation sequencing [Citation22] will be effective in the more robust diagnosis of FA. Nonetheless, the study of larger sample size is required for the establishment of this finding.
4.1. Importance
Many syndromes are associated with the FA and the clinical features overlap in one or more diseases. Early diagnosis of FA is important due to understanding the complex nature of the disease and its clinical manifestation. Hence the role of stress cytogenetics is immense. The huge phenotypic diversity in FA critically demands for a diagnostic laboratory test to be sure in diagnosis. FA inflicts many other disorders also. Stress cytogenetics also helps in the exclusion of other diseases and prevents inappropriate treatment of disease like acquired AA, myelodysplastic syndrome, acute leukaemia, etc. Surgical interventions might be accelerated in FA patients before the development of cytopenias or bleeding tendencies.
Prior diagnosis of FA helps clinicians in the management of specific cancer surveillance. However, it is also helpful for them in counsels the patients about the probable propagation of the disease in future. Genetic counselling is also vital, because of the 25% risk, for FA carrier couple in each subsequent pregnancy.
Over and above stress cytogenetics allows proper consideration of stem cell transplant, androgens, haematopoietic growth factors or supportive care. FA patients require a modified pre-transplantation conditioning regimen, with a lower dose of cyclophosphamide or chemotherapeutic agents as they are hypersensitive to all DNA cross-linking agents.
4.2. Limitation
Other information except consanguineous marriage about the parents (who are the carrier of FA) of stress cytogenetic positive patients should also be considered for a better understanding of the disease.
After diagnosis of the disease, the adequate follow up was not made in our study. It might give us additional information about the disease.
The PVB samples were collected only from Nil Ratan Sircar Medical College & Hospital as it is the nodal centre for haematological diseases in Eastern India. Patients from rural parts of Eastern India are unable to avail the government medical facility. Sample collection from more than one centre could increase the sample size.
In this study, all the stress cytogenetic positive cases are found to be phenotypically normal. So, any statistics of phenotypically normal vs. abnormal FA cases cannot be established through this study.
This study again emphasizes on the high percentage of history of consanguinity among FA patients. But does not express whether the phenotypical manifestation of FA is dependent on consanguinity.
The significantly disproportional male–female ratio in our sample would not allow determining any gender biases associated with FA.
Most of the patients participated in this study belong to low socioeconomic status and their lack of affordability make it even more difficult to diagnose such patients.
FA awareness is very much poor in West Bengal, India. Lack of awareness and exposure about this rare disorder makes it problematic to estimate the real prevalence of this disease. Therefore, in many cases remain undiagnosed.
In West Bengal, there is a severe shortage of FA testing centres. However, in the rural areas of the state, clinical centres are not in proximity. Thus, in many cases remain undetected.
Acknowledgements
The authors are indebted to, Swami Satyadevananda, Secretary, Ramakrishna Mission Seva Pratishthan, Vivekananda Institute of Medical Sciences, Kolkata and Department of Science of Technology (WB) for giving us inventory support and financial support. The authors are also obliged to Department of Haematology, N.R.S Medical College & Hospital, Kolkata for providing samples and other kind of assistances.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Younghoon K, D’Andrea1 AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 2010;24:1680–1694. doi: 10.1101/gad.1955310
- Ketan J, Patel A, Joenjeb H. Fanconi anemia and DNA replication repair. Genes Dev. 2007;6:885–890.
- Sagaseta IM, Molina J, Lezáun I, et al. Anemia de Fanconi. Consideraciones actuals. Anales Sis San Navarra. 2003;26:63–78.
- Zen PRG, de Moraes FN, Rosa RFM, et al. Clinical characteristics of patients with Fanconi anemia. Rev Paul Pediatr. 2011;29:392–399. doi: 10.1590/S0103-05822011000300014
- Katzenellenbogen RA, Carter JJ, Stern JE, et al. Skin and mucosal human papillomavirus sero prevalence in persons with Fanconi anemia. Clin Vaccine Immunol. 2015;22:413–420. doi: 10.1128/CVI.00665-14
- Dokal I, Vulliamy T. Inherited aplastic anaemias/bone marrow failure syndromes. Blood Rev. 2008;22:141–153. doi: 10.1016/j.blre.2007.11.003
- Jacquemont C, Taniguchi T. The Fanconi anemia pathway and ubiquitin. BMC Biochem. 2007;8:S10. doi: 10.1186/1471-2091-8-S1-S10
- Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenital. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880
- Shukla P, Ghosh K, Vundinti BR. Current and emerging therapeutic strategies for Fanconi anemia. Hugo J. 2012;6:1. doi: 10.1186/1877-6566-6-1
- Eiler ME, Frohnmayer D, Frohnmayer L, et al. Fanconi anemia: guidelines for diagnosis and management. 4th ed. Eugene (OR): Fanconi Anemia Research Fund, Inc; 2014.
- Tischkowitz MD, Hodgson SV. Fanconi anaemia. J Med Genet. 2003;40:1–10. doi: 10.1136/jmg.40.1.1
- Rosenberg PS, Tamary H, Alter BP. How high are carrier frequencies of rare recessive syndromes? Contemporary estimates for Fanconi anemia in the United States and Israel. Am J Med Genet. 2011;155:1877–1883. doi: 10.1002/ajmg.a.34087
- Sasaki MS, Tonomura A. A high susceptibility of Fanconi’s anemia to chromosome breakage by DNA cross-linking agents. Cancer Res. 1973;33:1829–1836.
- Auerbach AD, Rogatko A, Schroeder-Kurth TM. International Fanconi anemia registry: relation of clinical symptoms to diepoxybutane sensitivity. Blood. 1989;73:391.
- Auerbach AD. Fanconi anemia diagnosis and the diepoxybutane (DEB) test. Exp Hematol. 1993;21:731–733.
- Meng CY, Noor PJ, Ismail A, et al. Chromosomal breakage test in the diagnosis of Fanconi anema in patients with aplastic anemia. Curr Res Med Med Sci. 2014;4:3–6.
- Esmer C, Sánchez S, Ramos S, et al. Deb test for Fanconi anemia detection in patients with atypical phenotypes. Am J Med Genet. 2004;124A:35–39. doi: 10.1002/ajmg.a.20327
- Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2016;85:8.7.1–8.7.17.
- Pinto F O, Leblanc T, Chamousset D, et al. Diagnosis of Fanconi anemia in patients with bone marrow failure. Haematologica. 2009;94:487–495. doi: 10.3324/haematol.13592
- Auerbach AD, Warburton D, Bloom AD, et al. Prenatal detection of the Fanconi anemia gene by cytogenetic methods. Am JHum Genet. 1979;31:77–81.
- Oostra AB, Nieuwint AWM, Joenje H, et al. Diagnosis of Fanconi anemia: chromosomal breakage analysis. Hindawi Publishing Corporation. 2012;9.
- Cirkovic S, Guc-Scekic M, Vujic D, et al. Diagnosis of Fanconi’s anemia by diepoxybutane analysis in children from Serbia. BJMG. 2011;14:65–70.
- Aziz A, Mesba Chowdhury U, Khan R, et al. Fanconi anaemia – a rare case report. Bangladesh Med Res Counc Bull. 2016;42:1–5.
- Varma N, Varma S, Marwaha RK, et al. Multiple constitutional aetiological factors in bone marrow failure syndrome (BMFS) patients from North India. Indian J Med Res. 2006;124:51–56.
- Gupta V, Tripathi S, Singh TB, et al. A study of bone marrow failure syndrome in children. Indian J Med Sci. 2008;62:13–18. doi: 10.4103/0019-5359.38917
- Jain D, Raina V, Fauzdar A, et al. Chromosomal breakage study in aplastic anemia patients in India. Asian J Med Sci. 2010;2:227–232.
- Malla TM, Zargar MH, Dar FA, et al. Clastogen-induced chromosomal breakage analysis of suspected Fanconi’s anemia cases of Kashmir, North India. J Genet Syndr Gene. 2016;7:1–4. doi: 10.4172/2157-7412.1000295
- Chowdhry M, Makroo RN, Srivastava P, et al. Clinicohematological correlation and chromosomal breakage analysis in suspected Fanconi anemia patients of India. Indian J Med Paediatr Oncol. 2014;35:21–25. doi: 10.4103/0971-5851.133706
- Sinha S, Bhargava M. Fanconi anemia presenting as an “evolving” acute leukemia diagnostic challenges. Indian J Med Paediatr Oncol. 2013;34:305–308. doi: 10.4103/0971-5851.125251
- Vijay Kumar BA, Biradar SG, Patil V, et al. Clinicohaematological profile of aplastic anaemia in BRIMS, teaching hospital, Bidar. IJBAR. 2014;5:10.
- Athate UH, Rao SR, Kadam PR, et al. Fanconi’s anemia: a clinico-hematological and cytogenetic study. Indian Paediatrics. 1991;28:1003–1011.
- Korgaonkar S, Ghosh K, Vundinti BR. Clinical, genetic and cytogenetic study of Fanconi anemia in an Indian population. Hematology. 2010;15:58–62. doi: 10.1179/102453310X12583347009531
- Chakrabarti I, Saha A, Guha Mallick Sinha M, et al. Fanconi anemia with incidental haemoglobin E trait: the first reported case in literature. Indian J Hematol Blood Transfus. 2014;30:S111–S114. doi: 10.1007/s12288-013-0279-7
- Shimamura A, de Oca RM, Svenson JL, et al. A novel diagnostic screen for defects in the Fanconi anemia pathway. Blood. 2002;100:4649–4654. doi: 10.1182/blood-2002-05-1399
- Miglierina R, Coniat ML, Berger R. A simple diagnostic test for Fanconi anemia by flow cytometry. Anal Cell Pathol. 1991;3:111–118.
