 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives: Iron deficiency is common in obese children although the underlying mechanism is unclear. The aim of this study was to investigate the associations between iron parameters, leptin, hepcidin and adiponectin levels in obese children.
Methods: A total of 237 children, ranging in age from 5 to 18 years, 180 with primary obesity and 57 healthy children and adolescents, were enrolled. Complete blood count, serum iron levels, iron-binding capacity, ferritin levels, leptin, hepcidin and adiponectin levels were studied.
Results: White blood cell and platelet count, iron-binding capacity, high-sensitive C-reactive protein, leptin and hepcidin values in the obese group were higher than those of the control group (p < 0.001, p = 0.002, p < 0.001, p < 0.001, p < 0.001 and p < 0.001, respectively). However, mean corpuscular volume, adiponectin and transferrin saturation values in the obese group were lower than in the control group (p = 0.026, p = 0.003, and p < 0.001, respectively). No significant differences were found in terms of hemoglobin, serum ferritin, iron and IL-6 levels.
Conclusions: Our study suggests that hepcidin levels do not contribute to the development of iron deficiency anemia in pediatric obese individuals.
KEYWORDS:
Introduction
The prevalence of obesity in children is increasing similarly to that in adults [Citation1]. The World Health Organization describes obesity as a chronic disease and one of the main threats to public health [Citation2]. The association between obesity and iron deficiency was first revealed by Wenzel et al. in 1962 [Citation3]. Wenzel’s research findings were supported by those of Seltzer and Mayer [Citation4]. The rapid growth in obesity and the problems it causes have also led to an increase in research involving obesity and iron deficiency. In addition to obesity by itself and the increasing mortality and morbidity it causes, iron deficiency and the additional problems increase the importance of and interest in the subject.
Various hypotheses have been proposed for the association between obesity and iron deficiency. These include iron deficiency developing due to imbalanced nutrition in obese subjects [Citation5], an increase in iron requirements due to increased blood volume [Citation6], a decrease in myoglobin that binds iron in the muscles due to a decrease in physical activity [Citation4] and genetic predisposition [Citation7]. However, iron intake through diet does not differ between the obese and the non-obese subjects [Citation8].
Various proinflammatory cytokines known as adipokines are released from adipose tissue. The inflammation caused by these is thought to be capable of playing a role in the development of obesity-related comorbid conditions, such as iron deficiency [Citation9]. Hepcidin, being one of these adipokines, regulates iron absorption from the gut and iron release from macrophages and is, therefore, an important regulator for erythropoiesis. A high concentration of hepcidin has been found in the obese despite iron deficiency. This suggests that iron deficiency observed in the obese might arise from a hepcidin-related mechanism [Citation10–13]. However, it is unclear how the regulation of iron metabolism in the obese occurs through hepcidin regulation. The first hypothesis on this subject is that adiponectin such as interleukin 6 or leptin secreted from white adipose tissue stimulate hepcidin secretion [Citation10,Citation11,Citation14]. However, studies on this subject have reported inconsistent findings [Citation10,Citation11]. Another finding is that hepcidin expression in adipose tissue in the obese increases independently of metabolic syndrome and diabetes [Citation15]. In addition, publications have reported more significant levels of hepcidin synthesis in the liver compared to adipose tissue in the obese [Citation12].
Therefore, studies are needed to clarify iron deficiency in the obese and the role of hepcidin and its association with leptin in this process. To the best of our knowledge, no previous pediatric study has investigated interleukin 6, hepcidin, leptin and adiponectin together with inflammation markers such as CRP in terms of pubertal stage.
Materials and methods
Study design and subjects
One hundred and eighteen obese and 57 healthy subjects aged 5–18 years who were admitted to the Gazi University School of Medicine Department of Pediatric Endocrinology were included in this case–control study. The presented study was approved by Gazi University Ethical Committee with the number 27.06.2012/302.
A detailed medical history, physical examination and anthropometric evaluations were performed in all subjects. A 0.5-cm spaced stadiometer was used for height measurements and a 0.1 kg precision scale for weight measurements. Body mass index (BMI) (kg/m2) greater than 95th percentile based on age and sex was used as a criterion of obesity. The control group included healthy subjects with BMI <85th percentile [Citation16]. Patients with diabetes mellitus, Cushing syndrome, growth hormone deficiency, hypertension, familial hypercholesterolemia, chronic liver disease, a history of corticosteroid therapy, receiving iron therapy within the previous 6 months, with infection or an inflammatory disease, at high risk for iron deficiency anemia or with red blood cell function impairment and chronic disease were excluded. Pubertal status was evaluated based on Tanner criteria [Citation17].
Blood sampling and serum preparation
After 12-h fasting, complete blood count, iron, iron-binding capacity and ferritin values were analyzed in all patients using auto-analyzers. Transferrin saturation was also calculated for all subjects, including the control group, using the formula iron/total iron-binding capacity (TIBC) × 100. Three blood-containing tubes were centrifuged at 4000 rpm, and the sera were separated and stored at −80°C for further measurement of hepcidin, leptin, interleukin-6 (IL-6), high-sensitive C-reactive protein (hs-CRP) and adiponectin values. With commercially available ELISA kits according to the manufacturer’s instructions. IL-6, hs-CRP and leptin ELISA kits were purchased from DIAsource Immunoassays, and hepcidin and adiponectin ELISA kits were obtained from Cloud-Clone Corp and BioVendor, respectively.
Statistical analysis
The data were analyzed using SPSS (Statistical Package for Social Sciences) version 15 software. Categoric data were expressed as number and percentage and were analyzed using the chi-square test. Quantitative variables were expressed as mean, standard deviation, median, minimum, maximum or interquartile range (IQR) values. Normal distribution of variables was assessed using the Kolmogorov–Smirnov or Shapiro–Wilk tests. The independent sample t-test was used to assess the significance of differences between variables among normally distributed data, while the Mann–Whitney U-test was used for non-normally distributed data.
Relations between variables were analyzed using Pearson correlation analysis for normally distributed data and using Spearman correlation analysis for non-normally distributed data. p < 0.05 was regarded as statistically significant for all analyses.
Results
Mean hemoglobin values were below mean −2 standard deviation for age and sex in seven individuals in the obese group and five in the control group [Citation18], and these were excluded from the study. Data obtained following the exclusion of these individuals from their respective groups are shown in . There were no significant differences between the groups in terms of age, gender distribution or height (p > 0.05). However, a statistically significant difference was observed in BMI between the obese and control groups (p < 0.001). White blood cell and platelet count, iron-binding capacity, hs-CRP, leptin and hepcidin values in the obese group were higher than those of the control group (p < 0.001, p = 0.002, p < 0.001, p < 0.001, p < 0.001 and p < 0.001, respectively). Mean corpuscular volume, adiponectin and transferrin saturation values in the obese group were lower than in the control group (p = 0.026, p = 0.003, and p < 0.001, respectively). No significant differences were found in terms of hemoglobin, ferritin, serum iron or IL-6 levels.
Table 1. General characteristics of study population.
Evaluation based on pubertal status
Findings obtained when the obese group was subdivided on the basis of pubertal status are shown in . Leptin and adiponectin values varied between the pubertal and non-pubertal groups (p = 0.001, p = 0.016). However, no difference was observed between pubertal and non-pubertal subjects in the control groups with the exception of age, body weight, height and body mass. Results of the obese and control groups in terms of pubertal status are given in . The difference observed in IL-6 values before puberty disappeared when the obese and control groups were compared during puberty. In addition, the level of subjects with low ferritin values [Citation19] in the control group was higher in the pubertal group (34.4%) compared to the non-pubertal group (8%) (p = 0.019). However, no such difference was observed among the obese subjects.
Table 2. General features of obese group according to puberty.
Table 3. Comparison of obese and healthy group according to puberty.
Evaluation based on gender
Leptin values were significantly higher in females than in males in both the obese group and the control group (p < 0.001, p = 0.007). No difference in terms of gender was found for any other parameter in the obese or control groups.
Evaluation in terms of iron parameters
When the obese and control groups were subdivided according to transferrin saturation, ferritin values in subjects with low transferrin saturation (≤15%) were 36.3 ± 25.4 ng/mL (8.6–102.4 ng/mL), compared to 24.2 ± 20.1 ng/mL (9.5–63.0 ng/mL) in the control group. The difference between the two groups was not statistically significant (p = 0.112). When the obese group was evaluated from this perspective, ferritin values in subjects with low (≤15%) or normal (>15%) transferrin saturation were 36.3 ± 25.4 ng/mL (8.6–102.4 ng/mL) and 36.2 ± 20.1 ng/mL (5.0–107.5 ng/mL), respectively, and the difference between them was also not significant (p = 0.529). However, ferritin values in the subjects with low transferrin saturation in the control group [24.2 ± 20.1 ng/mL (9.5–63.0 ng/mL)] were significantly lower than those of the group with normal transferrin saturation [40.4 ± 18.6 ng/mL (15.0–79.0 ng/mL)] (p = 0.007).
Discussion
Obesity is an energy metabolism disorder resulting in the excessive storage of fat and that may also lead to physical and psychological problems [Citation20,Citation21]. Since the 1980s, obesity has drastically increased across all ages and socio-economic groups worldwide. Although various studies over the years have shown an association between obesity and iron deficiency, some elements of this are still unclear. This study investigated markers concerning iron metabolism while also considering the pubertal status and sex of obese children. In addition, we also investigated the relation between these parameters and leptin, hepcidin and adiponectin.
Serum ferritin, accepted to be a positive acute-phase protein, may be markedly elevated during states of inflammation, most probably due to elevated cytokine levels [Citation22]. Acute inflammation may be caused by infection or injury, whereas chronic inflammation may result from metabolic disturbances. Both types of inflammation can affect the iron homeostasis by affecting the regulation and synthesis of certain hepatic acute-phase proteins, such as ferritin, transferrin, haptoglobin and hepcidin. These acute-phase proteins, once induced by the acute-phase response, may affect the distribution of iron to cells throughout the body [Citation23]. As a result, the levels of most iron indicators will be directly affected by inflammation [Citation24]. In addition, inflammation may affect iron balance negatively by decreasing both food intake and intestinal absorption [Citation25].
The relationship between obesity and iron metabolism has been compared to a ‘Janus-faced’-like clinical picture by some researchers. This is because a dysmetabolic iron overload syndrome exists with a ferritin elevation despite concurrent iron deficiency [Citation26]. Findings compatible with this were also obtained in our study. The rate of subjects with a transferrin saturation below 15% was 39.8% in obese children, compared to 22.8% in the control group. However, no difference was observed in terms of ferritin values between subjects with low or normal transferrin levels in the obese group. Besides, no difference was found between serum ferritin values in the obese and control groups. Moreover, no difference was found in ferritin values in the obese group according to the gender and pubertal status. However, a previous study reported higher ferritin values in male obese adolescents due to the effect of testosterone [Citation27]. Additionally, although there was no difference in terms of ferritin values between pubertal and non-pubertal subjects in the control group, the number of individuals with low ferritin values was higher in pubertal subjects (). This may be attributed to rapid growth during puberty, menstruation in girls or dietary habits. Sanad et al. [Citation28] reported lower ferritin values in non-obese subjects with iron deficiency than in obese individuals with iron deficiency. However, in that study, ferritin values in the obese group did not differ from those of the healthy control group despite the presence of iron deficiency. Indeed, ferritin values in obese patients still displayed no significant difference after iron deficiency anemia had been treated. This reveals various similarities to the present study. Ferritin values of obese individuals in our study were also not different from those of the control group despite the high level of iron deficiency in the obese. What might be anticipated in iron deficiency is an analogy between low transferrin saturation and low ferritin values. However, there are studies suggesting that this association is compromised in obese subjects and that ferritin values are, therefore, not a good parameter for monitoring iron deficiency. It has been also suggested that this picture is caused by inflammation arising from white adipose tissue [Citation29]. The insufficiency of ferritin for evaluating iron metabolism in the obese has led to efforts to use other markers for this purpose. The first of these is the transferrin receptor. Transferrin receptor is a marker that is not affected by inflammation so that it can be used to diagnose iron deficiency in patients with accompanying inflammation. Nevertheless, the sTfR assay is not used universally due to variability among reported ranges from different manufacturers [Citation29].
Figure 1. (A) Correlation between transferrin saturation and hemoglobin in obese group, (B) correlation between transferrin saturation and mean corpuscular volume in obese group, (C) correlation between hepcidin and leptin in obese group, (D) correlation between hepcidin and ferritin in control group.
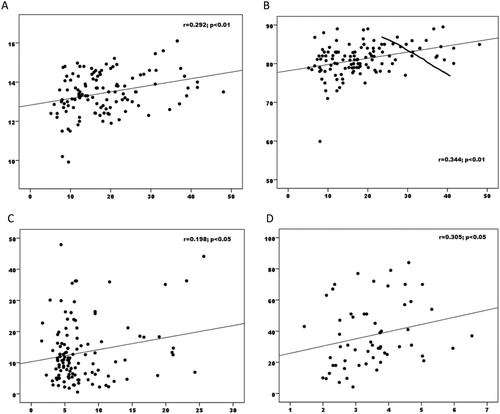
Obesity is known to lead to hypoferremia. A similar picture was observed in our study. In their meta-analysis, Zhao et al. showed that serum iron and transferrin are the best markers of iron metabolism in the obese. Similar findings were obtained in our study as well, and a significant correlation was observed particularly between transferrin saturation, hemoglobin and MCV in the obese group ((a,b)). In addition, transferrin saturation was significantly lower in the obese group compared to the control group (p < 0.001). It should be kept in mind that ferritin-based tests could misdiagnose iron deficiency in obese patients.
Several hypotheses exist to explain the occurrence of iron deficiency in the obese. Excessive adiposity is characterized by low-grade chronic inflammation. This leads to the production of certain inflammatory cytokines, such as IL-6 and tumor necrosis factor-alpha. IL-6 is well known to stimulate hepatic hepcidin production [Citation30–32]. As hepcidin reduces the export of iron from macrophages, hepatocytes and enterocytes [Citation33,Citation34], which result in reduced iron absorption, and sequestration of iron within splenic and hepatic macrophages, less iron is released and more iron is stored, explaining the reduced iron use in the obese. In addition, it is likely that the adipose tissue produces minor levels of hepcidin, which further contributes to the iron deficiency state observed in obese [Citation35].
Hepcidin functions as the ‘orchestral conductor’ of iron metabolism in the body [Citation28]. Hepcidin synthesis is thought to increase under stimulation by adipokines such as IL-6 and/or leptin released by white adipose tissue [Citation11]. Sanad et al. reported higher hepcidin values in a group of obese individuals with iron deficiency compared to a control group with iron deficiency. However, initially elevated hepcidin levels remained unchanged in the obese patients when iron deficiency was treated [Citation28]. We also found significantly higher hepcidin values in obese children compared to the control group (p < 0.001). However, IL-6 levels did not differ between the obese and control groups. As anticipated, in our study, correlation was observed between IL-6, CRP and hepcidin, the best markers of inflammation, in the control group (p = 0.005), while no correlation was observed between hepcidin and IL-6, CRP or adiponectin in the obese group. A positive correlation was only observed with leptin ((c)). A hepcidin-related decrease in iron absorption from the gut and the obstruction of passage of iron from macrophages into serum might be the mechanism involved in obesity-associated iron deficiency. However, no correlation was observed between hepcidin and either ferritin or Transferrin Saturation Index (TSI) in our study. Yet, the anticipated correlation between hepcidin and ferritin was observed in the control group ((d)). The absence of any correlation with inflammatory markers despite an increase in hepcidin in obese subjects together with the absence of a correlation with a marker showing iron deficiency in the obese, such as TSI, and, as shown by Sanad et al., hepcidin values remaining high despite amelioration of iron deficiency raises questions concerning the role of hepcidin in iron deficiency in obese individuals. The question now arises whether this should be considered from a different perspective.
A significant correlation between iron deficiency and obesity was again observed in the present study. Our search of the literature revealed no previous studies performed in the pediatric age group considering the relation between iron and other adipokines in terms also of gender and pubertal status. Our findings revealed no relation between hepcidin and iron deficiency in the obese patients. Although the correlation in the control group could not be observed in the obese subjects, it is at least clear that it is impaired. Additionally, the anticipated correlation with natural stimulators was not observed. The fact that a relation was only determined with leptin may be due to the fact that both derive from adipose tissue. This causes doubt on many previous assumptions and raises two questions. The first of these is whether hepcidin synthesized from adipose tissue performs a different function. Our second question is whether hepcidin regulation in obese children is mediated by IL-10, as suggested by Chang et al. [Citation36]. The answer may be so simple like the greater iron requirement during adolescence as Zhao et al. [Citation29] suggested. In addition, owing to the different relation between iron and hepcidin in obese than in the non-obese in our study, we think that larger studies are needed to answer these questions.
Compliance with Ethical Standards
All procedures followed were in accordance with the ethical standards of the Helsinki Declaration. The study does not contain any studies with animal subjects performed by any of the authors and was approved by Gazi University Ethical Committee (number: 27.06.2012/302).
Acknowledgements
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747
- World Health Organization. WHO physical status: the use and interpretation of anthropometry. Expert Committee on Physical Status World Health Organization, Geneva, 1995. Available from: http://www.who.int/childgrowth/publications/physical_status/en. [Last accessed 2016 Oct 24].
- Wenzel BJ, Stults HB, Mayer J. Hypoferraemia in obese adolescents. Lancet. 1962;2:327–328. doi: 10.1016/S0140-6736(62)90110-1
- Seltzer CC, Mayer J. Serum iron and iron-binding capacity in adolescents. II. Comparison of Obese and Nonobese Subjects. Am J Clin Nutr. 1963;13:354–361. doi: 10.1093/ajcn/13.6.354
- Pinhas-Hamiel O, Newfield RS, Koren I, et al. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27:416–418. doi: 10.1038/sj.ijo.0802224
- Failla ML, Kennedy ML, Chen ML. Iron metabolism in genetically obese(ob/ob) mice. J Nutr. 1988;118:46–51. doi: 10.1093/jn/118.1.46
- Nead KG, Halterman JS, Kaezorowski JM, et al. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114:104–108. doi: 10.1542/peds.114.1.104
- Menzie CM, Yanoff LB, Denkinger BI, et al. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J Am Diet Assoc. 2008;108:145–148. doi: 10.1016/j.jada.2007.10.034
- Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;1:485–492.
- Aeberli I, Hurrell RF, Zimmermann MB. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes. 2009;33:1111–1117. doi: 10.1038/ijo.2009.146
- Del Giudice EM, Santoro N, Amato A, et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab. 2009;94:5102–5107. doi: 10.1210/jc.2009-1361
- Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity. 2010;18:1449–1456. doi: 10.1038/oby.2009.319
- Hamza RT, Hamed AI, Kharshoum RR. Iron homeostasis and serum hepcidin-25 levels in obese children and adolescents: relation to body mass index. Horm Res Paediatr. 2013;80:11–17. doi: 10.1159/000351941
- Cheng HL, Bryant C, Cook R, et al. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev. 2012;13:150–161. doi: 10.1111/j.1467-789X.2011.00938.x
- Bekri S, Gual P, Anty R, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–796. doi: 10.1053/j.gastro.2006.07.007
- Neyzi O, Günöz H, Furman A, et al. Türk çocuklarında vücut ağırlığı, boy uzunluğu, baş çevresi ve vücut kitle indeksi referans değerleri. Çocuk Sağlığı ve Hastalıkları Dergisi. 2008;51:1–14.
- Tanner JM. Education and physical growth for educational theory and practice. 4th ed. Blackwell; 1978.
- Lanzkowsky P. Hematologic reference values. In: Lanzkowsky P, editor. Manual of pediatric hematology and oncology. 5rd ed. London: Academic Press; 2011. p. 971–975.
- Camaschella C. Iron deficiency: new insights into diagnosis and treatment. Hematology. 2015;2015:8–13. doi: 10.1182/asheducation-2015.1.8
- Kakinami L, Henderson M, Delvin EE, et al. Association between different growth curve definitions of overweight and obesity and cardiometabolic risk in children. CMAJ. 2012;184(10):E539–E550. doi: 10.1503/cmaj.110797
- McCarthy HD. Measuring growth and obesity across childhood and adolescence. Proc Nutr Soc. 2014;73(2):210–227. doi: 10.1017/S0029665113003868
- Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response–lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45:18–32. doi: 10.1258/acb.2007.007167
- Ross AC. Impact of chronic and acute inflammation on extra- and intracellular iron homeostasis. Am J Clin Nutr. 2017;106:1581S–1587S. doi: 10.3945/ajcn.117.155838
- Raiten DJ, Sakr Ashour FA, Ross AC, et al. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr. 2015;145:1039S–1108S. doi: 10.3945/jn.114.194571
- Bresnahan KA, Tanumihardjo SA. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr. 2014;5:702–711. doi: 10.3945/an.114.006361
- Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients. 2014;6:3587–3590. doi: 10.3390/nu6093587
- Jeon YJ, Jung IA, Kim SH, et al. Serum ferritin level is higher in male adolescents with obesity: results from the Korean National Health and Nutrition Examination Survey 2010. Ann Pediatr Endocrinol Metab. 2013;13:141–147. doi: 10.6065/apem.2013.18.3.141
- Sanad M, Osman M, Gharib A. Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: A case control study. Ital J Pediatr. 2011;37:34–39. doi: 10.1186/1824-7288-37-34
- Zhao L, Zhang X, Shen Y, et al. Obesity and iron deficiency: a quantitative meta-analysis. Obesity Reviews. 2015;16:1081–1093. doi: 10.1111/obr.12323
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013
- Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209.
- Verga Falzacappa MV, Vujic Spasic M, Kessler R, et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358.
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303
- Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86. doi: 10.1159/000243791
- Hutchinson C. A review of iron studies in overweight and obese children and adolescents: a double burden in the young? Eur J Nutr. 2016;55(7):2179–2197. doi: 10.1007/s00394-016-1155-7
- Chang JS, Li YL, Lu CH, et al. Interleukin-10 as a potential regulator of hepcidin homeostasis in overweight and obese children: A cross-sectional study in Taiwan. Nutrition. 2014;30:1165–1170. doi: 10.1016/j.nut.2014.02.021
