ABSTRACT
Objective: Myeloid/lymphoid neoplasms with fibroblast growth factor receptor-1 (FGFR1) rearrangement are hematopoietic stem cell disorders with a poor prognosis, but no established standard therapy. Methods: We experienced a patient with T-lymphoblastic lymphoma (LBL) associated with FGFR1 rearrangement who underwent cord blood transplantation, but died of pulmonary complication. We collected the clinical data of patients with FGFR1 rearrangement from the medical literature and analyzed 45 patients, including our patient. Results: The primary diagnoses were myeloproliferative neoplasm (MPN) or myelodysplastic syndromes (MDS) in 14 and acute leukemia or LBL in 31. In MPN and MDS patients, the cumulative incidence of transformation to blast phase (BP) at 12 months was 46.2%. The 1-year overall survival (OS) from diagnosis in all cases was 43.1%. With regard to the impact of treatment response on survival, the achievement of complete response with a landmark at 2 months after diagnosis of BP was associated with a superior OS (40.0% vs. 26.0% P = 0.011 for 1-year OS from BP). Allogeneic hematopoietic stem cell transplantation (HSCT) was performed in 13 patients, and the 1-year OS from allogeneic HSCT was 61.5%. The hazard ratio for mortality was 0.34 (95% CI, 0.08–1.51, P = 0.15) for allogeneic HSCT treated as a time-dependent covariate, which suggests that allogeneic HSCT may confer a clinical benefit. Conclusion: The further accumulation of clinical data is needed to determine the optimal therapeutic approach for these neoplasms.
Introduction
Myeloid/lymphoid neoplasms with fibroblast growth factor receptor-1 (FGFR1) rearrangement are rare hematological malignancies associated with chromosomal rearrangement of the short arm of chromosome 8 [Citation1]. This category of neoplasms was listed in the 2008 World Health Organization classification of tumors of hematopoietic and lymphoid tissues. Approximately 15 cases of FGFR1 translocation partner genes have been identified, and the most common gene partner is ZNF198 on chromosome 13q12 [Citation2]. The term 8p11 myeloproliferative syndrome (EMS) was coined to explain this different entity.
Hematopoietic neoplasms with rearrangement of FGFR1 are most commonly diagnosed as myeloproliferative neoplasms (MPN). Its clinical course varies widely, and rapid transformation, most often to lymphoblastic lymphoma (LBL), acute lymphoblastic leukemia (ALL), or acute myeloid leukemia (AML), occurs within several months after diagnosis [Citation3]. The prognosis is very poor despite intensive chemotherapy. Several reports have indicated that allogeneic hematopoietic stem cell transplantation (HSCT) achieved cytogenetic remission and is the only curative treatment option [Citation4–11].
In a literature review of 65 cases of EMS [Citation3], although various therapeutic regimens were used, the complete clinical remission rate was 27% and overall survival (OS) was 15 months. The median survival time after transformation for patients who received stem cell transplantation was 24 months. In this review, chromosome 8q11 abnormality was evaluated by conventional chromosomal analysis and FGFR1 rearrangement was not necessarily detected by a molecular analysis in all cases. An analysis limited to cases with confirmed FGFR1 rearrangement has not yet been reported.
Here we describe a patient with T-LBL associated with FGFR1 rearrangement who underwent allogeneic HSCT and achieved cytogenetic remission. Furthermore, we collected a case series by reviewing the literature to evaluate the clinical outcomes and therapeutic strategies, including allogeneic HSCT, for patients with hematologic neoplasms associated with confirmed FGFR1.
Patients and methods
Case presentation
A 34-year-old female visited another hospital for leukocytosis discovered during a medical checkup in July 2015. She had no subjective symptoms. Bone marrow (BM) aspirate and biopsy showed hypercellularity with hypereosinophilia, but no increase in the number of blasts. A G-banding chromosomal analysis revealed 46,XX,t(8:13)(p12;q12) karyotype in all 20 BM-derived metaphases. Reverse transcription-polymerase chain reaction (RT-PCR) detected a gene rearrangement of ZNF198-FGFR1. Therefore, she was diagnosed with MPN associated with FGFR1 rearrangement.
Two months later, she presented with bilateral cervical lymphadenopathy and was referred to our hospital for further management. Physical examinations showed diffuse cervical and axial lymphadenopathy. A computed tomography scan detected tonsillar swelling, systemic lymphadenopathy, and mild hepatosplenomegaly. Complete blood count showed a hemoglobin level of 13.5 g/dL, platelet count of 108 × 109/L, and white blood cell count of 65.9 × 109/L. Differential counts showed 0% blasts, 5.6% myelocytes, 3.8% metamyelocytes, 58.0% neutrophils, 5.0% lymphocytes, 7.2% monocytes, 18.0% eosinophils, and 2.4% basophils. There were no increased blasts in the BM. We performed a biopsy of the cervical lymph node and the pathology showed the infiltration of lymphoblastic cells. Chromosome analysis of the lymph node cells revealed the 46,XX,t(8:13)(p12;q12) karyotype in 20/20 cells. Immunohistochemical stains and flow cytometric analysis demonstrated that the lymphoma cells were positive for CD3, CD4, CD5, CD7, and terminal deoxyribonucleotidyl transferase. Based on the above findings, the patient was diagnosed with transformation to T-LBL.
The patient underwent intensive chemotherapy according to the ALL regimen. The induction regimen consisted of prednisolone, daunorubicin, vincristine, cyclophosphamide, and L-asparaginase. After induction therapy, 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) showed a complete response (CR); however, BM cells still showed the 46,XX,t(8:13)(p12;q12) karyotype. The patient then received consolidation therapy, including dexamethasone, etoposide, cytarabine, and L-asparaginase, and maintained CR. She planned to undergo allogeneic HSCT, but, unfortunately, a healthy family donor was not available. During coordination to identify an unrelated donor, she relapsed in January 2016 with bilateral cervical lymphadenopathy. She received reinduction chemotherapy consisting of dexamethasone, daunorubicin, cytarabine, cyclophosphamide, mercaptopurine, and L-asparaginase, but failed to achieve CR. She received a cord blood transplantation (CBT) in March 2016 after a conditioning regimen that included cyclophosphamide (60 mg/kg) for 2 days and total body irradiation (TBI) of 2 Gy twice daily for 3 days. However, neutrophil recovery was not achieved at 28 days after transplantation, and a chimerism analysis of BM cells at that time showed that all cells were of recipient origin and FGFR1 rearrangement was detected by fluorescent in situ hybridization (FISH) analysis. She was diagnosed as primary graft failure and a second CBT was carried out on day 43 after the first CBT, after a conditioning regimen consisting of fludarabine (30 mg/kg) for 4 days, cyclophosphamide (30 mg/kg), and TBI 2 Gy. She developed stage 1 acute cutaneous graft-vs-host disease (GVHD) on day 21 after the second CBT and was treated with topical corticosteroids. The neutrophil count surpassed 500/μL on day 28 after the second CBT, and a chimerism analysis of BM showed that 99.7% of cells were derived from the second cord blood donor. Furthermore, FISH analysis detected no FGFR1 rearrangement in the BM cells, which confirmed the achievement of cytogenetic remission. However, she died in July 2016 on day 73 after the second CBT due to the acute exacerbation of interstitial lung disease.
Literature selection
We collected cases from a computerized search of the medical literature using PubMed. We searched with words (#1: FGFR1 OR 8p11 and #2: leukemia OR lymphoma OR myeloproliferative), and extracted literature that satisfied both #1 AND #2 on January 2017. The following clinical data were obtained from the literature. This study was conducted in approach with the Declaration of Helsinki.
Statistical methods
Survival time was calculated by the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were performed with EZR version 1.30 (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.2.2). More precisely, it is a modified version of R commander (version 2.2–0) that includes statistical functions that are frequently used in biostatistics [Citation12].
Results
Review of the literature
We obtained 356 articles after a primary search of the title and abstract. We selected cases that provided sufficient information on survival times from diagnosis or allogeneic HSCT. Cases without a confirmation of FGFR1 abnormalities by FISH or RT-PCR, even if clinically diagnosed as EMS, were excluded. As a result, we identified 39 reports (44 cases) with hematological neoplasms associated with FGFR1 abnormalities [Citation4–43]. Finally, 45 cases including our patient were included in the following analyses.
Patient characteristics
The clinical characteristics of the patients are summarized in . The median age at diagnosis was 46 years (range, 0–87), and 13 patients (28.9%) were older than 60 years. The primary diagnosis was MPN (n = 13), AML (n = 12), T-ALL/LBL (n = 10), B-ALL/LBL (n = 4), acute leukemia (AL) of ambiguous lineage (n = 5), and myelodysplastic syndrome (MDS) (n = 1). In a RT-PCR analysis, ZNF198 (n = 12) was the most frequently detected FGFR1 translocation partner gene.
Table 1. Patient characteristics (n = 45).
Survival analysis
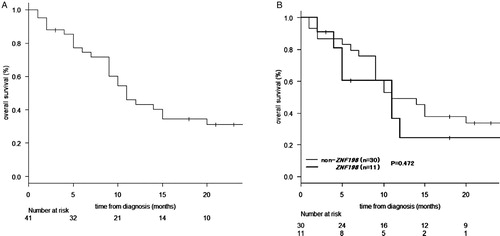
The OS data from diagnosis were available for 41 patients. The median follow-up time was 10 months (range, 1–66 months). The 1-year OS from diagnosis was 43.1% (95% confidence interval [CI], 26.8–58.4%) (A). There was no statistically significant difference in OS between patients with (n = 11) and without (n = 30) ZNF198-FGFR1 translocation (P = 0.472) (B).
Patients with MPN and MDS
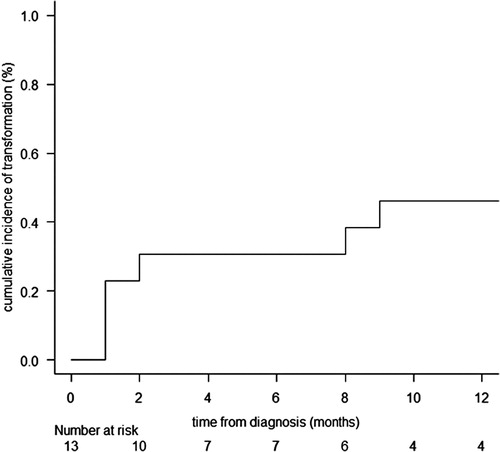
Fourteen patients with MPN (n = 13) or MDS (n = 1) were treated with hydroxyurea (HU) (n = 7), HU and prednisolone (n = 1), HU and mercaptopurine (n = 1), or imatinib (n = 1). The remaining 4 were observed without treatments. Of the 13 patients with clinical data regarding the duration to blast phase (BP), transformation to BP, including AML (n = 5), B-ALL (n = 1), and T-LBL (n = 1), was observed in seven patients with a cumulative incidence of transformation to BP at 1 year of 46.2% (95% CI, 17.6–70.9%), considering death (n = 2) or allogeneic HSCT (n = 2) as competing risks (). The median OS from the diagnosis was only 9.0 months (range, 2–66 months) in patients who did not receive allogeneic HSCT before the transformation to BP, whereas two of the three patients who received allogeneic HSCT before the transformation to BP achieved long-term remission of 30 and 57 months, respectively, after diagnosis.
Patients with BP
Next, we analyzed 38 patients including patients with BP at diagnosis (primary BP, n = 31) and those with BP transformation from MPN or MDS (n = 7). The diseases included ALL/LBL (n = 15), AML (n = 18), and AL of ambiguous lineage (n = 5).
Induction therapy data for BP were available for 32 patients. CR was achieved in 15 of 32 patients (46.9%), including 11 (84.6%) of 13 with ALL/LBL, 2 of 14 (14.3%) with AML, and 2 of 5 (40%) with AL of ambiguous lineage. There were no significant differences in CR rates between patients with primary BP (n = 27, 51.9%) and those with transformed BP (n = 5, 20%) (P = 0.338).
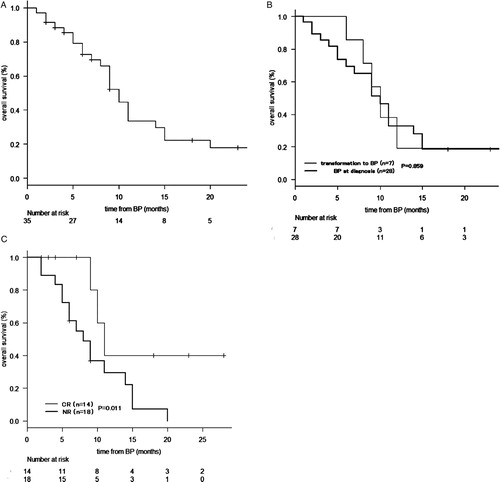
Survival data for BP were available for 35 patients, including 28 with primary BP and seven with transformed BP. The 1-year OS from BP was 29.8% (95% CI, 14.4–46.8%) (A). There were no significant differences in 1-year OS from the diagnosis of BP between patients with primary BP and those with transformed BP (P = 0.859) (B). With regard to the impact of treatment response on survival, the achievement of CR with a landmark at 2 months after diagnosis of BP was associated with a superior OS (40.0% [95% CI, 12.3–67.0%] vs. 26.0% [95% CI, 7.3–50.1%], P = 0.011 for 1-year OS from BP) (C). In 14 patients with CR after induction therapy, four patients received allogeneic HSCT. Three of these four patients remained in remission at 7, 23, and 28 months, respectively, from the diagnosis of BP.
Figure 3. OS from the diagnosis of BP in all patients (n = 35) (A). OS from BP in patients who transformed to BP with a previous diagnosis of MPN or MDS (n = 7) and in those with a diagnosis of primary BP (n = 28) (B). OS from BP grouped according to the response with a landmark of 2 months after BP (C).
Patients receiving allogeneic HSCT
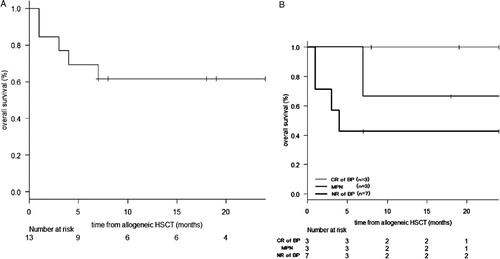
Allogeneic HSCT was performed in 14 patients: 3 with MPN, three with transformed BP, and eight with primary BP. Survival data from diagnosis, BP, and allogeneic HSCT were available for 10, 8 and 13 patients, respectively. The 1-year OS from diagnosis, BP, and allogenic HSCT was 68.6% (95% CI, 30.5–88.7%), 56.2% (95% CI, 14.7–84.2%), and 61.5% (95% CI, 30.8–81.8%), respectively (A). The hazard ratio of mortality for allogeneic HSCT as a time-dependent covariate was 0.34 (95% CI, 0.08–1.51, P = 0.15). With regard to the effect of disease status at HSCT, the 1-year OS from allogenic HSCT of those with MPN (n = 3), those in CR of BP (n = 3), and those in NR of BP (n = 7) was 66.7% (95% CI, 5.4–94.5%), 100% (95% CI, 100–100%), and 42.9% (95% CI, 9.8–73.4%), respectively (B).
Discussion
The aim of the present study was to evaluate the clinical outcomes and therapeutic strategies for hematological malignancies associated with FGFR1 rearrangement. In previous reports, EMS was reported to have a poor prognosis [Citation3]. However, no previous report has provided detailed survival data from patients with the FGFR1 rearrangement. We conducted a retrospective review of a case series from the published literature. This study showed that the prognosis in patients with FGFR1 rearrangement was poor, but allogeneic HSCT would provide a clinical benefit in selected patients.
Compared to a previous report of 65 cases with EMS [Citation3], 16 of whom were also included in the current analysis, this study showed high CR rates (46.9% vs. 27%) and a prolonged survival from allogeneic HSCT (median OS; not reached vs. 15months) in patients with FGFR1 rearrangement. A possible explanation for this difference is that the previous study included cases reported between 1970s and 2000s, and improvements in induction therapies and supportive care might have improved the treatment outcome. On the other hand, the 1-year OS was not improved in this study (median OS; 11 months vs. 15 months). This was possibly due to the lower proportion of cases diagnosed as MPN in the current study (31% vs. 41%).
There is no established induction therapy for myeloid/lymphoid neoplasms with FGFR1 rearrangement, and therefore various treatments, including ALL and AML regimens, were used in this cohort. The present study showed that the CR rate of patients with ALL/LBL associated with FGFR1 rearrangement was equivalent to that of patients with de novo ALL/LBL, but the CR rate for AML and AL of ambiguous lineage was extremely low. These results suggested that FGFR-1 rearrangement may be associated with chemoresistance in myeloid neoplasms. In a mouse model, BCR-FGFR1-driven AML blasts remarkably increased the phosphoactivation of FLT3, MAPK3/1, and STAT/3/5, as well as the expression of anti-apoptotic BCL2 [Citation44]. For myeloid neoplasms with FGFR1 rearrangement, it is worth trying to target these molecules. In fact, one patient with AL of ambiguous lineage showed chemotherapy resistance, but achieved CR with the use of ponatinib [Citation4]. Sorafenib, a multikinase inhibitor, has also been used for the treatment of myeloproliferative disorders with FGFR1 abnormalities, but the hematological response was short term [Citation45]. TKIs alone may be insufficient to produce a sufficient response, and therefore TKIs could possibly serve as a bridge to allogeneic HSCT.
In this study, approximately half of the patients who were diagnosed with MPN transformed to BP within one year. However, two of three patients who received allogeneic HSCT before BP achieved long-term remission. These results suggest that allogeneic HSCT may improve the prognosis of patients with MPN associated with FGFR1 abnormalities, when performed before BP transformation.
This study has several limitations. For example, this study included a small number of patients with heterogeneous characteristics. The retrospective nature of this study also limits the reliability of statistical analyses. In addition, a potential publication bias can not be excluded, since patients who successfully underwent allogenic HSCT might have been more likely to be reported. However, hematological malignancies associated with FGFR1 are very rare, and our analysis provides the current best evidence that may help physicians.
In conclusion, we demonstrated that patients with FGFR1 rearrangement were associated with a poor prognosis. The available literature suggested that allogeneic HSCT could provide a potential clinical benefit in selected patients. The further accumulation of clinical data is needed to determine whether allogeneic HSCT could be an optimal therapeutic approach and improve clinical outcomes.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Macdonald D, Aguiar RC, Mason PJ, et al. A new myeloproliferative disorder associated with chromosomal translocations involving 8p11: a review. Leukemia. 1995;9(10):1628–1630. PubMed PMID: 7564500; eng.
- Vega F, Medeiros LJ, Bueso-Ramos CE, et al. Hematolymphoid neoplasms associated with rearrangements of PDGFRA, PDGFRB, and FGFR1. Am J Clin Pathol. 2015;144(3):377–392. doi:10.1309/ajcpmorr5z2ikcem. PubMed PMID: 26276769; eng.
- Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Human Pathol. 2010;41(4):461–476. doi:10.1016/j.humpath.2009.11.003. PubMed PMID: 20226962; eng.
- Khodadoust MS, Luo B, Medeiros BC, et al. Clinical activity of ponatinib in a patient with FGFR1-rearranged mixed-phenotype acute leukemia. Leukemia. 2016;30(4):947–950. doi:10.1038/leu.2015.136. PubMed PMID: 26055304; eng.
- Duckworth CB, Zhang L, Li S. Systemic mastocytosis with associated myeloproliferative neoplasm with t(8;19)(p12;q13.1) and abnormality of FGFR1: report of a unique case. Int J Clin Exp Pathol. 2014;7(2):801–807. PubMed PMID: 24551307; PubMed Central PMCID: PMCPmc3925931. eng.
- Morishige S, Oku E, Takata Y, et al. A case of 8p11 myeloproliferative syndrome with BCR-FGFR1 gene fusion presenting with trilineage acute leukemia/lymphoma, successfully treated by cord blood transplantation. Acta Haematol. 2013;129(2):83–89. doi:10.1159/000341289. PubMed PMID: 23171834; eng.
- Haslam K, Langabeer SE, Kelly J, et al. Allogeneic hematopoietic stem cell transplantation for a BCR-FGFR1 myeloproliferative neoplasm presenting as acute lymphoblastic leukemia. Case Rep Hematol. 2012;2012:620967. doi:10.1155/2012/620967. PubMed PMID: 23082258; PubMed Central PMCID: PMCPmc3467796. eng.
- Shaaban H, Dabu J, Al-Rabi K, et al. A rare case report of 8p11 myeloid and lymphoid neoplasm with FGFR1 abnormality in a young adult. Ann Hematol. 2013;92(2):285–286. doi:10.1007/s00277-012-1562-7. PubMed PMID: 22941307; eng.
- Li F, Zhai YP, Tang YM, et al. Identification of a novel partner gene, TPR, fused to FGFR1 in 8p11 myeloproliferative syndrome. Genes Chromosom Cancer. 2012;51(9):890–897. doi:10.1002/gcc.21973. PubMed PMID: 22619110; eng.
- Dolan M, Cioc A, Cross NC, et al. Favorable outcome of allogeneic hematopoietic cell transplantation for 8p11 myeloproliferative syndrome associated with BCR-FGFR1 gene fusion. Pediatr Blood Cancer. 2012;59(1):194–196. doi:10.1002/pbc.23404. PubMed PMID: 22106025; eng.
- Suzan F, Guasch G, Terre C, et al. Long-term complete haematological and molecular remission after allogeneic bone marrow transplantation in a patient with a stem cell myeloproliferative disorder associated with t(8;13)(p12;q12). Br J Haematol. 2003;121(2):312–314. PubMed PMID: 12694254; eng. doi: 10.1046/j.1365-2141.2003.04269.x
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi:10.1038/bmt.2012.244. PubMed PMID: 23208313; PubMed Central PMCID: PMCPmc3590441. eng.
- Sarthy JF, Reddivalla N, Radhi M, et al. Pediatric 8p11 eosinophilic myeloproliferative syndrome (EMS): a case report and review of the literature. Pediatr Blood Cancer. 2016. doi:10.1002/pbc.26310. PubMed PMID: 27808462; eng.
- Qin YW, Yang YN, Bai P, et al. Chronic myelogenous leukemia-like hematological malignancy with t(8;22) in a 26-year-old pregnant woman: a case report. Oncol Lett. 2016;11(6):4131–4133. doi:10.3892/ol.2016.4505. PubMed PMID: 27313753; PubMed Central PMCID: PMCPmc4888210. eng.
- Malli T, Buxhofer-Ausch V, Rammer M, et al. Functional characterization, localization, and inhibitor sensitivity of the TPR-FGFR1 fusion in 8p11 myeloproliferative syndrome. Genes Chromosom Cancer. 2016;55(1):60–68. doi:10.1002/gcc.22311. PubMed PMID: 26391436; eng.
- Kumar KR, Chen W, Koduru PR, et al. Myeloid and lymphoid neoplasm with abnormalities of FGFR1 presenting with trilineage blasts and RUNX1 rearrangement: a case report and review of literature. Am J Clin Pathol. 2015;143(5):738–748. doi:10.1309/ajcpud6w1jlqqmna. PubMed PMID: 25873510; eng.
- Chao H, Zhou M, Zhang R. A case with ZNF198-FGFR1 gene rearrangement presenting as acute eosinophil myeloid leukemia. Chin Med J. 2015;128(1):131–132. doi:10.4103/0366-6999.147863. PubMed PMID: 25563327; PubMed Central PMCID: PMCPmc4837809. eng.
- Nakamura Y, Ito Y, Wakimoto N, et al. A novel fusion of SQSTM1 and FGFR1 in a patient with acute myelomonocytic leukemia with t(5;8)(q35;p11) translocation. Blood Cancer J. 2014;4:e265. doi:10.1038/bcj.2014.86. PubMed PMID: 25501022; PubMed Central PMCID: PMCPmc4315892. eng.
- Patel RA, Sheehan AM, Finch CJ, et al. Fine-needle aspiration cytology of T-lymphoblastic lymphoma associated FGFR1 rearrangement myeloproliferative neoplasm. Diagn Cytopathol. 2014;42(1):45–48. doi:10.1002/dc.23030. PubMed PMID: 23904402; PubMed Central PMCID: PMCPmc4100463. eng.
- Buijs A, van Wijnen M, van den Blink D, et al. A ZMYM2-FGFR1 8p11 myeloproliferative neoplasm with a novel nonsense RUNX1 mutation and tumor lysis upon imatinib treatment. Cancer Genet. 2013;206(4):140–144. doi:10.1016/j.cancergen.2013.04.001. PubMed PMID: 23751892; eng.
- Lee H, Kim M, Lim J, et al. Acute myeloid leukemia associated with FGFR1 abnormalities. Int J Hemat. 2013;97(6):808–812. doi:10.1007/s12185-013-1337-5. PubMed PMID: 23609419; eng.
- Trimaldi J, Carballido EM, Bowers JW, et al. B-lymphoblastic leukemia/lymphoma associated with t(8;13)(p11;q12)/ ZMYM2 (ZNF198)-FGFR1: rare case and review of the literature. Acta Haematol. 2013;130(3):127–134. doi:10.1159/000347030. PubMed PMID: 23594707; eng.
- Matikas A, Tzannou I, Oikonomopoulou D, et al. A case of acute myelogenous leukaemia characterised by the BCR-FGFR1 translocation. BMJ Case Rep. 2013;2013. doi:10.1136/bcr-2013-008834. PubMed PMID: 23519513; PubMed Central PMCID: PMCPmc3618816. eng.
- Yamamoto S, Ebihara Y, Mochizuki S, et al. Quantitative polymerase chain reaction detection of CEP110-FGFR1 fusion gene in a patient with 8p11 myeloproliferative syndrome. Leuk Lymphoma. 2013;54(9):2068–2069. doi:10.3109/10428194.2013.767455. PubMed PMID: 23327291; eng.
- Gervais C, Dano L, Perrusson N, et al. A translocation t(2;8)(q12;p11) fuses FGFR1 to a novel partner gene, RANBP2/NUP358, in a myeloproliferative/myelodysplastic neoplasm. Leukemia. 2013;27(5):1186–1188. doi:10.1038/leu.2012.286. PubMed PMID: 23041776; eng.
- Mayeur-Rousse C, Sorel N, Voldoire M, et al. Unique association of systemic mastocytosis and myeloid/lymphoid neoplasm in blast crisis with abnormality of FGFR1 gene. Leuk Res. 2012;36(3):377–381. doi:10.1016/j.leukres.2011.10.009. PubMed PMID: 22030335; eng.
- Kim SY, Oh B, She CJ, et al. 8p11 Myeloproliferative syndrome with BCR-FGFR1 rearrangement presenting with T-lymphoblastic lymphoma and bone marrow stromal cell proliferation: a case report and review of the literature. Leuk Res. 2011;35(5):e30–e34. doi:10.1016/j.leukres.2010.12.014. PubMed PMID: 21239058; eng.
- Zhou L, Fu W, Yuan Z, et al. Complete molecular remission after interferon alpha treatment in a case of 8p11 myeloproliferative syndrome. Leuk Res. 2010;34(11):e306–e307. doi:10.1016/j.leukres.2010.06.027. PubMed PMID: 20650528; eng.
- Baldazzi C, Iacobucci I, Luatti S, et al. B-cell acute lymphoblastic leukemia as evolution of a 8p11 myeloproliferative syndrome with t(8;22)(p11;q11) and BCR-FGFR1 fusion gene. Leuk Res. 2010;34(10):e282–e285. doi:10.1016/j.leukres.2010.05.009. PubMed PMID: 20594995; eng.
- Post GR, Holloman D, Christiansen L, et al. Translocation t(3;8;9)(p25;p21;q34) in a patient with features of 8p11 myeloproliferative syndrome: a unique case and review of the literature. Leuk Res. 2010;34(11):1543–1544. doi:10.1016/j.leukres.2010.05.017. PubMed PMID: 20541805; eng.
- Zhang WW, Habeebu S, Sheehan AM, et al. Molecular monitoring of 8p11 myeloproliferative syndrome in an infant. J Pediatr Hematol Oncol. 2009;31(11):879–883. doi:10.1097/MPH.0b013e3181b83fd0. PubMed PMID: 19829149; eng.
- Soler G, Nusbaum S, Varet B, et al. LRRFIP1, a new FGFR1 partner gene associated with 8p11 myeloproliferative syndrome. Leukemia. 2009;23(7):1359–1361. doi:10.1038/leu.2009.79. PubMed PMID: 19369959; eng.
- Park TS, Song J, Kim JS, et al. 8p11 myeloproliferative syndrome preceded by t(8;9)(p11;q33), CEP110/FGFR1 fusion transcript: morphologic, molecular, and cytogenetic characterization of myeloid neoplasms associated with eosinophilia and FGFR1 abnormality. Cancer Genet Cytogenet. 2008;181(2):93–99. doi:10.1016/j.cancergencyto.2007.11.011. PubMed PMID: 18295660; eng.
- Etienne A, Gelsi-Boyer V, Carbuccia N, et al. Combined translocation with ZNF198-FGFR1 gene fusion and deletion of potential tumor suppressors in a myeloproliferative disorder. Cancer Genet Cytogenet. 2007;173(2):154–158. doi:10.1016/j.cancergencyto.2006.10.004. PubMed PMID: 17321332; eng.
- Yamamoto K, Kawano H, Nishikawa S, et al. A biphenotypic transformation of 8p11 myeloproliferative syndrome with CEP1/FGFR1 fusion gene. Eur J Haematol. 2006;77(4):349–354. doi:10.1111/j.1600-0609.2006.00723.x. PubMed PMID: 16879608; eng.
- Wong WS, Cheng KC, Lau KM, et al. Clonal evolution of 8p11 stem cell syndrome in a 14-year-old Chinese boy: a review of literature of t(8;13) associated myeloproliferative diseases. Leuk Res. 2007;31(2):235–238. doi:10.1016/j.leukres.2006.04.015. PubMed PMID: 16777224; eng.
- Mozziconacci MJ, Carbuccia N, Prebet T, et al. Common features of myeloproliferative disorders with t(8;9)(p12;q33) and CEP110-FGFR1 fusion: report of a new case and review of the literature. Leuk Res. 2008;32(8):1304–1308. doi:10.1016/j.leukres.2007.11.012. PubMed PMID: 18096225; eng.
- Hidalgo-Curtis C, Chase A, Drachenberg M, et al. The t(1;9)(p34;q34) and t(8;12)(p11;q15) fuse pre-mRNA processing proteins SFPQ (PSF) and CPSF6 to ABL and FGFR1. Genes Chromosom Cancer. 2008;47(5):379–385. doi:10.1002/gcc.20541. PubMed PMID: 18205209; eng.
- Belloni E, Trubia M, Gasparini P, et al. 8p11 myeloproliferative syndrome with a novel t(7;8) translocation leading to fusion of the FGFR1 and TIF1 genes. Genes Chromosom Cancer. 2005;42(3):320–325. doi:10.1002/gcc.20144. PubMed PMID: 15609342; eng.
- Vizmanos JL, Hernandez R, Vidal MJ, et al. Clinical variability of patients with the t(6;8)(q27;p12) and FGFR1OP-FGFR1 fusion: two further cases. Hematol J. 2004;5(6):534–537. doi:10.1038/sj.thj.6200561. PubMed PMID: 15570299; eng.
- Mugneret F, Chaffanet M, Maynadie M, et al. The 8p12 myeloproliferative disorder. t(8;19)(p12;q13.3): a novel translocation involving the FGFR1 gene. Br J Haematol. 2000;111(2):647–649. PubMed PMID: 11122115; eng. doi: 10.1046/j.1365-2141.2000.02355.x
- Guasch G, Mack GJ, Popovici C, et al. FGFR1 is fused to the centrosome-associated protein CEP110 in the 8p12 stem cell myeloproliferative disorder with t(8;9)(p12;q33). Blood. 2000;95(5):1788–1796. PubMed PMID: 10688839; eng.
- Popovici C, Zhang B, Gregoire MJ, et al. The t(6;8)(q27;p11) translocation in a stem cell myeloproliferative disorder fuses a novel gene, FOP, to fibroblast growth factor receptor 1. Blood. 1999;93(4):1381–1389. PubMed PMID: 9949182; eng.
- Cowell JK, Qin H, Chang CS, et al. A model of BCR-FGFR1 driven human AML in immunocompromised mice. Br J Haematol. 2016;175(3):542–545. doi:10.1111/bjh.13877. PubMed PMID: 27785808; eng.
- Wakim JJ, Tirado CA, Chen W, et al. t(8;22)/BCR-FGFR1 myeloproliferative disorder presenting as B-acute lymphoblastic leukemia: report of a case treated with sorafenib and review of the literature. Leuk Res. 2011;35(9):e151–e153. doi:10.1016/j.leukres.2011.05.013. PubMed PMID: 21628071; eng.