ABSTRACT
Objectives: This meta-analysis evaluated the impact of granulocyte colony-stimulating factor (G-CSF) added to chemotherapy on treatment outcomes including survival and disease recurrence in patients with acute myeloid leukemia (AML).
Methods: Medline, Cochrane, EMBASE, and Google Scholar databases were searched until 19 September 2016 using search terms. Studies that investigated patients with AML who underwent stem-cell transplantation were included.
Results: The overall analysis revealed a significant improvement in overall survival (OS) (P = .019) and disease-free survival (DFS) (P = .002) for patients receiving G-CSF with chemotherapy. Among patients without prior AML treatment, there was a significant improvement in DFS (P = .014) and reduction in incidence of relapse (P = .015) for those who received G-CSF. However, subgroup analyses found no significant difference between G-CSF (+) and G-CSF (−) treatments in rates of OS (P = .104) and complete remission (CR) (P = .572) for patients without prior AML treatment. Among patients with relapsed/refractory AML, there was no significant difference found between G-CSF (+) and G-CSF (−) groups for OS (P = .225), DFS (P = .209), and CR (P = .208).
Discussion: Treatment with chemotherapy plus G-CSF appears to provide better survival and treatment responses compared with chemotherapy alone, particularly for patients with previously untreated AML.
Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; CR, complete remission; DFS, disease-free survival; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; HR, hazard ratio; MDS, myelodysplastic syndrome; OR, odds ratio; OS, overall survival; RCTs, randomized control trials; RR, relative risk
Introduction
The majority of younger adult patients with acute myeloid leukemia (AML) are treated with curative intent. However, relapse is a significant treatment issue due to residual leukemic cells escaping the cytotoxic effects of the chemotherapy [Citation1]; consequently, AML treatment is still associated with considerable morbidity and mortality [Citation2].
Several studies have evaluated the use of the myeloid growth factors, granulocyte colony-stimulating factor (G-CSF), or granulocyte macrophage colony-stimulating factor (GM-CSF), used in conjunction with chemotherapy with the idea that these molecules may sensitize the leukemic cells to the cytotoxic effects of the chemotherapy, and hence reduce the rate of relapse [Citation3–5]. In support of this, studies in AML tissue culture cells have shown that priming with G-CSF or GM-CSF may alter the cell cycle of AML cells, making them more susceptible to the cytotoxicity of chemotherapy [Citation6,Citation7].
In addition, colony-stimulating factors (CSFs) can reduce complications resulting from chemotherapy suppression of bone marrow. Previous meta-analyses have found that the addition of CSFs to treatment in adults and children cancer patients reduced the risk of chemotherapy-associated complications and thus the need to reduce or delay dosing of chemotherapy [Citation8–11]. CSFs are commonly used to accelerate neutrophil recovery following stem-cell transplantation to reduce the risk of infectious complications and the subsequent need of supportive care [Citation12].
However, in AML, disease recurrence can result from leukemic clones surviving high-dose chemo- and/or radiotherapy or contaminating autologous stem cells used for transplantation [Citation13,Citation14]. Hence, the use of G-CSF in AML is controversial as surviving leukemic blasts may express G-CSF receptors and the interaction of the receptor with the cytokine may increase the risk of relapse and reduce disease-free survival (DSF) [Citation15–17]. However, findings from prior studies evaluating the use of G-CSF as part of the treatment regimen for patients with AML receiving stem-cell transplantation have been inconsistent, and hence the effect of G-CSF on disease-related outcomes and survival in AML is not clear. This meta-analysis evaluated the impact of G-CSF on outcomes including survival and disease recurrence in patients with AML undergoing stem-cell transplantation.
Methods
Search strategy
This study was performed in accordance with PRISMA. Medline, Cochrane, EMBASE, and Google Scholar databases were searched until 19 September 2016 using the following search terms: acute myeloid leukemia or AML, relapse, granulocyte colony-stimulating factors or G-CSF. Randomized control trials (RCTs), two-arm, and prospective studies were included. All studies had patients with AML who planned to undergo stem-cell transplantation, and had at least two treatment arms: one in which G-CSF was administered as part of the treatment regimen (either as priming or given prior to or at transplantation) and another in which G-CSF was not given. Studies not published in English, letters, comments, editorials, case reports, proceedings, and personal communications were excluded. All potential studies were searched by two independent reviewers, with a third reviewer acting as consultant to resolve any uncertainty regarding eligibility.
Data extraction
The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, number of participants in each treatment group, patients’ age and gender, type of AML (relapsed or primary/secondary AML with no prior treatment), and outcomes of interest.
Quality assessment
Quality assessment was performed using the Cochrane Risk of Bias Tool (Cochrane Handbook for Systematic Reviews of Interventions) [Citation18].
Outcome measures
The primary outcomes were overall survival (OS) and DFS between AML patients receiving G-CSF plus chemotherapy (G-CSF (+)) compared with patients receiving chemotherapy alone (G-CSF (−)). The secondary outcomes were complete remission and incidence of relapse between two groups.
Statistical analysis
Hazard ratios (HRs) with 95% confidence interval (CI) for survival outcomes were extracted for each individual study. If the available data were presented from the Kaplan–Meier curve, we extracted their survival rates at some specified times in order to reconstruct the HR estimate and its variance, with the assumption that the rate of patients censored was constant during the study follow-up [Citation19]. Odds ratios (ORs) with 95% CIs were calculated for the dichotomous outcomes in the G-CSF (+) group compared with the G-CSF (−) group. For OS and DFS, an HR/OR <1 indicated that the patients treated with G-CSF plus chemotherapy were favored. For CR, an OR <1 indicated that the patients treated with G-CSF alone were favored. A χ2-based test of homogeneity was performed and the inconsistency index (I2) and Q statistics were determined. If the I2 statistics were >50%, a random-effects model was used. Otherwise, fixed-effect models were employed. Pooled effects were calculated and a 2-sided P value <0.05 was considered to indicate statistical significance. A prospectively planned subgroup analysis was performed according to AML status (untreated AML or relapsed/refractory AML). Sensitivity analysis was carried out using the leave-one-out approach. Publication bias analysis was not performed because the number of studies was too few (<10) to detect an asymmetric funnel [Citation20]. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Search results
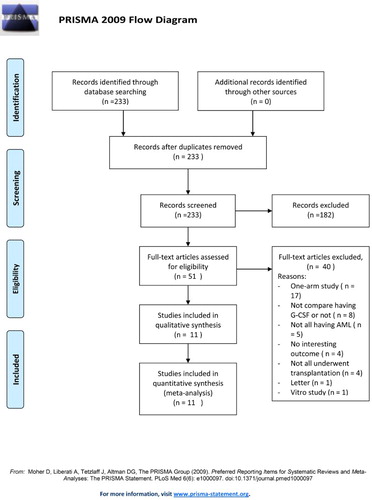
The initial database search identified 233 studies that were screened (). Of these, 182 were removed for not being relevant. Fifty-one studies were fully reviewed and 40 were excluded for being one-arm studies, not investigating patients with AML, not reporting outcomes of interest, not administering G-CSF prior to transplantation, being a letter, being an in vitro study, and not all patients underwent stem-cell transplantation.
Eleven studies were included in the analysis with a total of 5076 patients (range 18–1029 patients): 2466 in the G-CSF (+) group and 2610 in the non-G-CSF (−) group () [Citation21–31]. The age of patients ranged from 10 to 60 years and the percentage of males and females was similar between treatment groups. Of the 11 included studies, 7 focused on patients with untreated AML (primary or secondary) and 4 were on patients with relapsed or refractory AML. The type of chemotherapy used and the dosage of G-CSF varied across studies (Supplemental Table 1).
Table 1. The characteristics of the studies included in this study.
The HR for OS ranged from 0.513 to 1.1 and for DFS from 0.63 to 0.94 in the G-CSF (+) group relative to the G-CSF (−) group across studies (). The incidence of relapse ranged from 29.8% to 59% in the G-CSF (+) group and from 36.6% to 60.7% in the G-CSF (−) group.
Table 2. Summarized reported outcomes of the included studies.
Meta-analysis
Overall survival
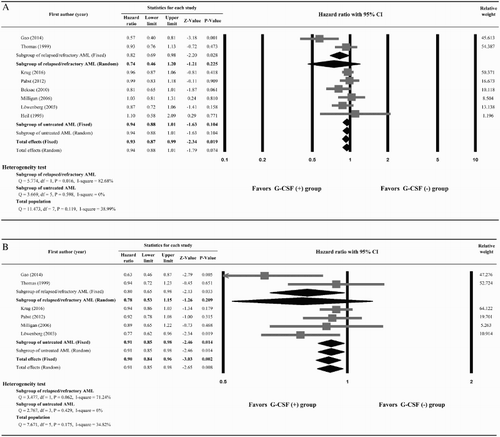
Three studies, Kern et al. [Citation27], Laver et al. [Citation29], and Estey et al. [Citation31], did not provide enough information to calculate HRs for OS and were excluded from the meta-analysis. Heterogeneity of the data was not significant among the seven included studies, and therefore a fixed-effect model was used (Q statistic = 11.473, I2 = 38.99%). The overall analysis revealed a significant survival advantage in the G-CSF (+) group compared with the G-CSF (−) (pooled HR = 0.93; 95%CI, 0.87–0.99; P = .019) ((A)). However, subgroup analysis found no significant difference in OS between patients without prior AML treatment (pooled HR = 0.94; 95% CI, 0.88–1.01; P = .104), or in those with relapsed/refractory AML (pooled HR = 0.74; 95% CI, 0.46–1.20; P = .225).
Figure 2. Meta-analysis of (A) overall survival, (B) disease-free survival, (C) complete remission rate, and (D) incidence of relapse.
Disease-free survival
Six studies quantitatively reported DFS and were included in the meta-analysis. A fixed-effect model was used because no heterogeneity was seen among the studies (Q statistic = 7.671, I2 = 34.82%) [Citation21–23,Citation25,Citation26,Citation28]. The overall analysis revealed there was a benefit of DFS in favor of patients treated with G-CSF plus chemotherapy (pooled HR = 0.90; 95% CI, 0.84–0.96; P = .002). In the untreated AML subgroup, patients treated with G-CSF (+) were associated with significant improvement in DFS compared with those who did not receive G-CSF (−) [pooled HR = 0.91; 95% CI, 0.85–0.98; P = .014]. No significant difference between G-CSF (+) and G-CSF (−) groups was observed in the patients with relapsed or refractory AML (pooled HR = 0.78; 95% CI, 0.53–1.15; P = .209) ((B)).
Complete remission
One study, Gao et al. [Citation22], did not provide data for CR and was omitted from the analysis. No heterogeneity was observed among the nine included studies; therefore, a fixed-effect model was used (Q statistic = 13.566, I2 = 33.66%). The overall analysis revealed there was no difference in CR between G-CSF (+) and G-CSF (−) groups (pooled OR = 0.99; 95%CI, 0.87–1.12; P = .870) ((C)). Subgroup analysis found that the addition of G-CSF to chemotherapy compared to chemotherapy alone did not alter the rate of CR in both untreated AML (pooled OR = 0.96; 95% CI, 0.85–1.10, P = .572) and relapsed/refractory AML subgroups (pooled OR = 1.31; 95% CI, 0.86–1.99, P = .208).
Incidence of relapse
For evaluation of the incidence of relapse, we only assessed the subgroup of previously untreated AML patients due to lack of reported data in the studies. Four studies, Pabst et al. [Citation23], Beksac et al. [Citation24], Milligan et al. [Citation25], and Lowenberg et al. [Citation26], provided the frequency of relapse rates for the two intervention groups’ subjects without prior AML treatment. No heterogeneity was observed among these four studies, so a fixed-effect model was used (Q statistic = 1.512, I2 = 0%). The overall analysis revealed patients receiving G-CSF plus chemotherapy had a significantly lower incidence of relapse than those receiving chemotherapy alone (pooled OR = 0.81; 95%CI, 0.68–0.96; P = .015) ((D)).
Sensitivity analysis
Sensitivity analyses were performed using the leave-one-out approach for each outcome (). For OS, when we removed each study in turn, the pooled estimates for OS generally remained in the same direction (all pooled HR <1), except the removal of two studies, Gao et al. [Citation22] and Beksac et al. [Citation24], caused the findings to become non-significant (P = .076 and .062, respectively). For DFS and CR, the direction of the pooled estimates did not vary markedly with the removal of each study individually, indicating the findings are robust. The pooled estimates for relapse rate remained <1 after each study was removed in turn; though removal of Lowenberg et al. [Citation26] caused the difference between treatments to become non-significant. Therefore, sensitivity analysis found that individual studies may have overly influenced the findings for OS, and relapse rate, but not for DFS and CR.
Table 3. Results of sensitivity analysis.
Quality analysis
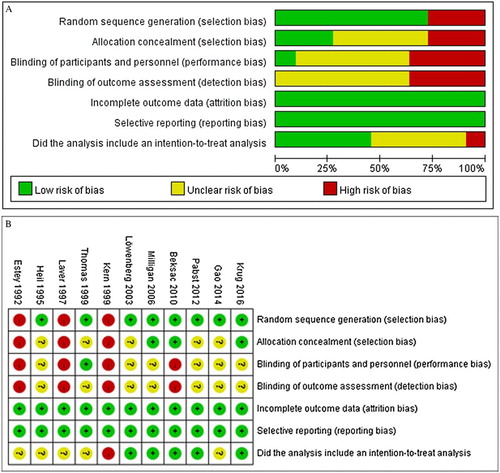
Quality analysis indicated that three of the studies, Kern et al. [Citation27], Laver et al. [Citation29], and Estey et al. [Citation31], had potential selection bias and performance bias and did not describe an intent-to-treat analysis ((A,B)). Across the studies, it was unclear if there was detection bias due to blinding of outcome assessments. There was no reporting bias among the studies.
Discussion
The prevention of relapse remains a significant challenge in the treatment of AML patients because of residual leukemic cells escaping the cytotoxic effect of chemotherapy. To optimize results of standard chemotherapy, the use of G-CSF or GM-CSF concurrently with induction chemotherapy has been studied in several randomized trials. In this meta-analysis, we evaluated the efficacy of treatment of AML with chemotherapy plus G-CSF. We found that for the overall analysis, significant advantages in OS and DFS were observed in patients receiving G-CSF plus chemotherapy compared with chemotherapy alone. Subgroup analysis found no significant difference between treatments in patients without prior AML treatment in OS or CR. However, patients with previously untreated primary/secondary AML who received G-CSF had significantly longer DFS and reduced incidence of relapse than those who did not receive G-CSF therapy. In the subgroup of patients with relapsed/refractory AML, no difference was found between treatment groups for OS, DFS, or CR. Sensitivity analysis suggests that the data for DFS are robust, but the findings for OS, CR, and relapse rate may have been overly impacted by certain studies. The findings of our meta-analysis suggest that treatment with G-CSF plus chemotherapy may provide better survival and treatment outcomes than with chemotherapy alone, particularly in patients with previously untreated AML.
Several prior meta-analyses have assessed the effect of G-CSF containing regimens in the treatment of AML [Citation32–35]. Gurion et al. [Citation34] evaluated the impact of CSFs (e.g. GM-CSF, G-CSF, etc.) added to chemotherapy in the treatment of patients with AML [Citation34]. Their analysis included 19 studies comprising a total of 5256 patients. In contrast to our study, the addition of CSF to chemotherapy gave no additional benefit in all-cause mortality at 30 days and at the end of follow-up (relative risk (RR), 0.97; 95% CI 0.80–1.18 and RR 1.01; 95% CI 0.98–1.05, respectively) or in OS (HR 1.00; 95% CI 0.93–1.08). There was no difference between treatment with or without CSF in complete remission (RR 1.03; 95% CI 0.99–1.07), relapse rates (RR 0.97; 95% CI 0.89–1.05), or disease-free survival (HR 1.00; 95% CI 0.90–1.13). The presence of CSF also did not decrease the risk of bacteremia (RR 0.96; 95% CI 0.82–1.12) or invasive fungal infections (RR 1.40; 95% CI 0.90–2.19), and slightly increased adverse events (RR 1.33; 95% CI 1.00–1.56). Their findings suggest that CSF did not add benefit for treating patients with AML. However, their analysis pooled findings from studies including different CSFs, while our study focused only on G-CSF.
Wei et al. [Citation33] performed a meta-analysis to assess the overall treatment efficacy and the adverse events of a treatment regimen that contained the topoisomerase inhibitors cytarabine and aclarubicin with G-CSF (CAG) in the treatment of patients with AML or myelodysplastic syndrome (MDS) [Citation33]. The CAG treatment is widely used in China and Japan for these two diseases [Citation33]. Their analysis included 814 AML and 215 MDS patients. They found that the CR rate was greater in AML patients (57.9%) than MDS patients (45.7%) (P < .01). They saw no difference in CR between previously untreated (new) (56.7%) and relapsed/refractory (60.1%) AML with CAG treatment (P > .05). Interestingly, CAG-containing regimens were associated with a significantly higher CR rate than non-CAG regimes (OR, 2.43). Four of our studies included cytarabine and another the topoisomerase inhibitor idarubicin [Citation24,Citation26,Citation28,Citation30]. However, a sufficient number of studies were not included to perform subgroup analysis to evaluate the efficacy of different chemotherapies with or without G-CSF in treating AML.
Lyman et al. performed a systematic review and meta-analysis that included 25 RCTs in which patients with AML or MDS received chemotherapy with (n = 6058) or without (n = 6746) G-CSF [Citation35]. After a mean follow-up across studies of 60 months, AML/MDS was reported in 22 control patients and 43 G-CSF-treated patients, with an estimated relative risk (RR) of 1.92 (P = .009). Deaths were reported in 1845 patients treated with G-CSF and 2099 controls (RR 0.897 and absolute risk of 3.40% (P values < .001). The findings of the study indicate that the RR for AML/MDS relapse is increased but all-cause mortality is decreased in patients receiving chemotherapy with G-CSF support. Importantly the authors note that it is not possible to distinguish any leukemia risk associated with G-CSF from the known dose-dependent leukemia risk associated with myelosuppressive chemotherapeutic agents. In contrast to the findings of Lyman et al., we found that in the subgroup of patients with previously untreated AML, the priming of G-CSF reduced the relapse rate. The difference between studies may be due to the significantly different patient populations evaluated, and suggests that different types of patients might have responded to G-CSF therapy differently. Future studies are necessary to address this question.
A chemotherapy regimen that includes fludarabine, cytarabine, and G-CSF (FLAG) has been shown to be effective in treating relapsed and relapsed/refractory acute lymphoblastic leukemia (ALL) and AML [Citation36–38]. Other commonly used related chemotherapies include FLAG plus mitoxantrone (FLANG), FLAG plus idarubicin (FLAG-IDA), and liposomal daunorubicin, fludarabine, cytarabine, G-CSF (L-DNR/FLAG) [Citation36–38]. These combinations maximize favorable cytotoxic interactions between cytarabine and G-CSF, and between cytarabine and fludarabine [Citation36]. FLAG-based regimens take advantage of the fact that fludarabine with cytarabine increases the intracellular retention of cytarabine’s active metabolite, cystosine arabinoside 5′ triphosphate, resulting in a synergistic antitumor effect. In addition, increasing cell cycling with G-CSF treatment response is thought to improve treatment response by causing dormant leukemic cells to be more sensitive to cytotoxic drugs [Citation36]. Also, G-CSF increases the effect of cytarabine by increasing its incorporation into DNA [Citation36].
There are several limitations to this study that should be considered when evaluating the findings. The number of studies was small and the type of chemotherapy, the different dosages of chemotherapy and G-CSF, and treatment cycles differed across studies. Moreover, patients across the studies differed with regard to AML-specific risk factors. These differences in treatment regimens and patient populations may have affected the response to treatments, which may have confounded our results. In addition, sensitivity analysis suggested that OS, CR, and relapse rate may have been overly impacted by certain studies, making these findings less robust.
In conclusion, this study examined the responses to G-CSF priming in patients with previously untreated or recurrent/relapsed AML who planned to undergo stem-cell transplantation. We found that receiving G-CSF together with chemotherapy might have provided better survival and treatment responses than with chemotherapy alone, particularly in patients with previously untreated AML. This and prior meta-analysis suggest that addition of G-CSF may improve survival.
Supplementary_material.docx
Download MS Word (15.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: https://doi.org/10.1200/JCO.2010.30.1820
- Lowenberg B. Strategies in the treatment of acute myeloid leukemia. Haematologica. 2004;89:1029–2032.
- Terpstra W, Lowenberg B. Application of myeloid growth factors in the treatment of acute myeloid leukemia. Leukemia. 1997;11:315–327. doi: https://doi.org/10.1038/sj.leu.2400561
- Ohno R. Granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor in the treatment of acute myeloid leukemia and acute lymphoblastic leukemia. Leuk Res. 1998;22:1143–1154. doi: https://doi.org/10.1016/S0145-2126(98)00117-9
- Geller RB. Use of cytokines in the treatment of acute myelocytic leukemia: a critical review. J Clin Oncol. 1996;14:1371–1382. doi: https://doi.org/10.1200/JCO.1996.14.4.1371
- Bhalla K, Birkhofer M, Arlin Z, et al. Effect of recombinant GM-CSF on the metabolism of cytosine arabinoside in normal and leukemic human bone marrow cells. Leukemia. 1988;2:810–813.
- Miyauchi J, Kelleher CA, Wang C, et al. Growth factors influence the sensitivity of leukemic stem cells to cytosine arabinoside in culture. Blood. 1989;73:1272–1278.
- Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–3167. doi: https://doi.org/10.1200/JCO.2006.08.8823
- Wittman B, Horan J, Lyman GH. Prophylactic colony-stimulating factors in children receiving myelosuppressive chemotherapy: a meta-analysis of randomized controlled trials. Cancer Treat Rev. 2006;32:289–303. doi: https://doi.org/10.1016/j.ctrv.2006.03.002
- Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. doi: https://doi.org/10.1016/j.ejca.2006.05.002
- Clark OA, Lyman GH, Castro AA, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol. 2005;23:4198–4214. doi: https://doi.org/10.1200/JCO.2005.05.645
- Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: https://doi.org/10.1200/JCO.2006.06.4451
- Brenner MK, Rill DR, Moen RC, et al. Gene-marking to trace origin of relapse after autologous bone-marrow transplantation. Lancet. 1993;341:85–86. doi: https://doi.org/10.1016/0140-6736(93)92560-G
- Feller N, Schuurhuis GJ, van der Pol MA, et al. High percentage of CD34-positive cells in autologous AML peripheral blood stem cell products reflects inadequate in vivo purging and low chemotherapeutic toxicity in a subgroup of patients with poor clinical outcome. Leukemia. 2003;17:68–75. doi: https://doi.org/10.1038/sj.leu.2402781
- Vellenga E, Young DC, Wagner K, et al. The effects of GM-CSF and G-CSF in promoting growth of clonogenic cells in acute myeloblastic leukemia. Blood. 1987;69:1771–1776.
- Lowenberg B, Touw IP. Hematopoietic growth factors and their receptors in acute leukemia. Blood. 1993;81:281–292.
- Czerw T, Labopin M, Gorin NC, et al. Use of G-CSF to hasten neutrophil recovery after auto-SCT for AML is not associated with increased relapse incidence: a report from the acute leukemia working party of the EBMT. Bone Marrow Transplant. 2014;49:950–954. doi: https://doi.org/10.1038/bmt.2014.64
- Higgins JPT. Cochrane collaboration handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration; 2011 [updated 2011 Mar]. Available from: www.cochrane-handbook.org.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: https://doi.org/10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
- Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. 2011;343:d4002. doi: https://doi.org/10.1136/bmj.d4002
- Krug U, Berdel WE, Gale RP, et al. Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia. 2016;30:1230–1236. doi: https://doi.org/10.1038/leu.2016.25
- Gao L, Wen Q, Chen X, et al. Effects of priming with recombinant human granulocyte colony-stimulating factor on conditioning regimen for high-risk acute myeloid leukemia patients undergoing human leukocyte antigen-haploidentical hematopoietic stem cell transplantation: a multicenter randomized controlled study in southwest China. Biol Blood Marrow Transplant. 2014;20:1932–1939. doi: https://doi.org/10.1016/j.bbmt.2014.08.001
- Pabst T, Vellenga E, van Putten W, et al. Favorable effect of priming with granulocyte colony-stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood. 2012;119:5367–5673. doi: https://doi.org/10.1182/blood-2011-11-389841
- Beksac M, Ali R, Ozcelik T, et al. Short and long term effects of granulocyte colony-stimulating factor during induction therapy in acute myeloid leukemia patients younger than 65: results of a randomized multicenter phase III trial. Leuk Res. 2011;35:340–345. doi: https://doi.org/10.1016/j.leukres.2010.07.005
- Milligan DW, Wheatley K, Littlewood T, et al. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood. 2006;107:4614–4622. doi: https://doi.org/10.1182/blood-2005-10-4202
- Lowenberg B, van Putten W, Theobald M, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349:743–752. doi: https://doi.org/10.1056/NEJMoa025406
- Kern W, Aul C, Maschmeyer G, et al. Granulocyte colony-stimulating factor shortens duration of critical neutropenia and prolongs disease-free survival after sequential high-dose cytosine arabinoside and mitoxantrone (S-HAM) salvage therapy for refractory and relapsed acute myeloid leukemia. German AML cooperative group. Ann Hematol. 1998;77:115–122. doi: https://doi.org/10.1007/s002770050425
- Thomas X, Fenaux P, Dombret H, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) to increase efficacy of intensive sequential chemotherapy with etoposide, mitoxantrone and cytarabine (EMA) in previously treated acute myeloid leukemia: a multicenter randomized placebo-controlled trial (EMA91 trial). Leukemia. 1999;13:1214–1220. doi: https://doi.org/10.1038/sj.leu.2401474
- Laver J, Shearer P, Krance R, et al. A pilot study of continuous infusion Ara-C in combination with rhG-CSF in relapsed childhood acute myeloid leukemia. Leuk Lymphoma. 1997;26:589–593. doi: https://doi.org/10.3109/10428199709050894
- Heil G, Chadid L, Hoelzer D, et al. GM-CSF in a double-blind randomized, placebo controlled trial in therapy of adult patients with de novo acute myeloid leukemia (AML). Leukemia. 1995;9:3–9.
- Estey E, Thall PF, Kantarjian H, et al. Treatment of newly diagnosed acute myelogenous leukemia with granulocyte-macrophage colony-stimulating factor (GM-CSF) before and during continuous-infusion high-dose ara-C + daunorubicin: comparison to patients treated without GM-CSF. Blood. 1992;79:2246–2255.
- Kantarjian H, O'Brien S, Jabbour E, et al. Effectiveness of homoharringtonine (omacetaxine mepesuccinate) for treatment of acute myeloid leukemia: a meta-analysis of Chinese studies. Clin Lymphoma Myeloma Leuk. 2015;15:13–21. doi: https://doi.org/10.1016/j.clml.2014.09.011
- Wei G, Ni W, Chiao JW, et al. A meta-analysis of CAG (cytarabine, aclarubicin, G-CSF) regimen for the treatment of 1029 patients with acute myeloid leukemia and myelodysplastic syndrome. J Hematol Oncol. 2011;4:46. doi: https://doi.org/10.1186/1756-8722-4-46
- Gurion R, Belnik-Plitman Y, Gafter-Gvili A, et al. Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia. Cochrane Database Syst Rev. 2012;6:CD008238.
- Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol. 2010;28:2914–2924. doi: https://doi.org/10.1200/JCO.2009.25.8723
- Jackson GH. Use of fludarabine in the treatment of acute myeloid leukemia. Hematol J. 2004;5(Suppl 1):S62–S67. doi: https://doi.org/10.1038/sj.thj.6200392
- Creutzig U, Semmler J, Kaspers GL, et al. Re-induction with L-DNR/FLAG improves response after AML relapse, but not long-term survival. Klin Padiatr. 2014;226(6-7):323–331.
- Yavuz S, Paydas S, Disel U, et al. IDA-FLAG regimen for the therapy of primary refractory and relapse acute leukemia: a single-center experience. Am J Ther. 2006;13:389–393. doi: https://doi.org/10.1097/01.mjt.0000181690.21601.09