ABSTRACT
Objectives: The primary objective was to determine the prevalence of calreticulin (CALR) mutation in patients with non-JAK2V617F mutated essential thrombocythemia (ET). The secondary objectives were to evaluate the accuracy of CALR mutation analysis by high-resolution melting (HRM) analysis and real-time polymerase chain reaction (PCR) compared with DNA sequencing and to compare clinical characteristics of CALR mutated and JAK2V617F mutated ET.
Methods: This was a prospective cohort study involving ET patients registered at Chiang Mai University in the period September 2015–September 2017 who were aged more than 2 years, and did not harbor JAK2V617F mutation. The presence of CALR mutation was established by DNA sequencing, HRM, and real-time PCR for type 1 and type 2 mutation. Clinical data were compared with that from ET patients with mutated JAK2V617F.
Results: Twenty-eight patients were enrolled onto the study. CALR mutations were found in 10 patients (35.7%). Three patients had type 1 mutation, 5 patients had type 2 mutation, 1 patient had type 18 mutation, and 1 patients had novel mutations (c.1093 C–G, c.1098_1131 del, c.1135 G–A). HRM could differentiate between the types of mutation in complete agreement with DNA sequencing. Patients with a CALR mutation showed a significantly greater male predominance and had a higher platelet count when compared with 42 JAK2V617F patients.
Discussion and Conclusions: The prevalence of CALR mutation in JAK2V617F-negative ET in this study is 35.7%. HRM is an effective method of detecting CALR mutation and is a more advantageous method of screening for CALR mutation.
Introduction
Myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell disorders characterized by the proliferation of one or more myeloid lineages [Citation1–3]. Essential thrombocythemia (ET) is MPNs that has thrombocytosis as the important feature. The major complication associated with ET is vascular thrombosis. A large patient ET cohort shows that 12% of patients suffer from thrombosis [Citation4,Citation5]. Other complications are bleeding, transformation into myelofibrosis, and acute leukemia [Citation2,Citation3].
Analysis of mutation of Janus Kinase 2 (JAK2) gene, commonly JAK2V617F, is an important diagnostic tool for accurate diagnosis of MPNs. However, only approximately 50–70% of patients with ET harbor this mutation [Citation2,Citation3,Citation6].
In 2013, two groups of investigators found the novel calreticulin (CALR) mutation in 67% and 84% of ET patients who did not have JAK2V617F mutation [Citation7,Citation8]. This is the second most common mutation after JAK2V617F mutation and has been included in recent diagnostic criteria of ET [Citation1]. ET patients who harbor CALR mutation have different clinical features from those with JAK2V617F mutation including younger age, higher levels of thrombocytosis, lower hemoglobin and WBC counts, and lower risk of thrombosis [Citation7].
Many variations of CALR mutations are reported, the majority being deletion and insertion mutations in exon 9 which lead to frameshift mutations. The most common mutations are type 1 or 52-base pair (bp) deletion (c.1092_1143del), and type 2 or 5 bp insertion (c.1154_1155insTTGTC) [Citation7,Citation8]. The detection of CALR mutations can be made by direct DNA sequencing [Citation7,Citation8]. The other methods of identifying CALR mutations include fragment analysis [Citation9], high-resolution melting (HRM) analysis [Citation10], real-time polymerase chain reaction (PCR) assay [Citation10], and immunostaining [Citation11].
The primary objective of this study was to determine the prevalence of CALR mutation in ET patients with non-mutated JAK2V617F at Chiang Mai University. The secondary objectives were to study the accuracy of HRM analysis and real-time PCR for detection of the CALR mutation compared to direct DNA sequencing as well as to compare the clinical characteristics of ET patients with mutations of JAK2V617F and CALR.
Material and methods
Study overview
This prospective cohort study was performed at Chiang Mai University between September 2015 and September 2017. This study was approved by the Institutional Review Board of the Faculty of Medicine, Chiang Mai University, Thailand. ET patients aged more than 2 years who did not have JAK2V617F mutation were enrolled onto this study. The patients or their parents (in the case of patients aged less than 18 years) gave their informed consent before being enrolled onto the study. The diagnosis of ET was based on the 2016 World Health Organization (WHO) classification of myeloid neoplasms [Citation1].
The blood sample from the patients was obtained for analysis of the potential CALR mutation by direct DNA sequencing using an automated DNA Sequencer, HRM, and real-time PCR. Data including baseline characteristics, symptoms, signs (including splenomegaly), complete blood count (CBC) at the diagnosis, the International Prognostic Score for ET-thrombosis (IPSET-thrombosis) [Citation4], IPSET-survival [Citation12], medication, response to treatment according to international criteria [Citation13], and complications were collected from patients and medical records. The same clinical data were also collected from medical records of ET patients who had a JAK2V617F mutation to give a comparison with patients who harbored the CALR mutation.
Direct DNA sequencing
For the CALR gene amplification [Homo sapiens calreticulin; RefSeqGene (LRG_828) on chromosome 19; Accession No. NG_029662.1], PCR was set up with 5 μl of 30 ng/μl genomic DNA, 0.3 μΜ of each primer [forward primer: 5′-TAACAAAGGTGAGGCCTGGT-3′ (10023-10042), reverse primer: 5′-GCCTCTCTACAGCTCGTCCTT-3′ (10300-10320)], 1× HotStarTaq Master Mix Kit (QIAGEN GmbH, Hilden, Germany) in a final volume of 50 µl.
Amplification was carried out using 30 cycles of denaturation at 95oC for 1 minute, annealing at 60oC for 1 minute, and extension at 72oC for 1 minute on a PCR thermal cycler (Veriti; Applied Biosystems, Foster City, California, U.S.A.).
The PCR products were further purified using the QIA quick PCR purification kit (QIAGEN GmbH). Sequencing was done in an automated 3130 Genetic Analyzer system (Applied Biosystem; Life Technologies, Carlsbad, California, U.S.A.). Reference sequences (NG_029662.1) were analyzed using Chromas Lite version 2.01 (Technelysium Pty Ltd, South Brisbane, Australia).
HRM analysis
The HRM analysis was performed as described previously [Citation10]. The method was performed in duplicate on a Biorad CFX 96 instrument, using the Bio-Rad Precision Melt Analysis Software. (Bio-Rad Laboratories, Irvine, California, U.S.A.). The amplification 20 μl mixture included 5 μl of 30 ng/μl genomic DNA, with 0.3 μΜ final concentration for each primer (forward primer: 5′-TAACAAAGGTGAGGCCTGGT-3′, reverse primer 5′-GCCTCTCTACAGCTCGTCCTT-3′), 1.5 mM MgCl2, 0.2 µM of dNTPs and 2 µM of SYTO-9, 1.25 unit of Platinum® Taq DNA polymerase (Invitrogen, Carlsbad, California, U.S.A.), and 1× PCR buffer. Primers were designed to flank the wild-type region in exon 9 of the CALR gene. The reaction mixture was subjected initially to denaturing conditions of 95°C for 2 minutes, followed by 45 cycles of amplification consisting of denaturation at 95°C for 10 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 20 seconds. After amplification, the HRM melting program was begun at 95°C for 10 seconds, then increased from 75 to 95°C with a transitional rate of 0.2°C per 10 seconds.
Real-time PCR assay
The method was performed as described previously [Citation10]. For the detection of CALR mutations, 5 μl of 30 ng/μl genomic DNA was amplified in 25 μl reaction volume with 0.3 μΜ each of forward and reverse primers and 0.5 μΜ of probe. The forward primers (CALR-F) used were 5′-TAACAAAGGTGAGGCCTGGT-3′ for wild-type CALR, 5′-AAACAGGACGAGGAGCA GAGGA-3′ for the type 1 mutation and 5′-GAGGAGGCAGAGGACAATTG-3′ for the type 2 mutation. A common reverse primer (CALR-R) 5′-GCCTCTCTACAGCTCGTCCTT-3′ and a common probe FAM-TGAGGATGAGGAGGATGAGG-TAMRA were used for all three assays. The common probe is designed to anneal to a sequence close to the area of the mutation. The reaction was carried out in 1× TaqMan Universal PCR Master Mix (Applied Biosystem) at 50°C for 2 minutes, 95°C for 10 minutes followed by 45 cycles at 95°C for 15 seconds, annealing, and extension at 68°C (gradient with 0.3°C decrement per cycle) for 1 minute. Detection takes place in the extension step of real-time PCR. All samples were tested with wild-type primers as an internal control for the assay.
Statistical analysis
The analysis of CALR mutation by direct DNA sequencing by an automated DNA Sequencer, HRM, and real-time PCR was presented by descriptive analysis. The differences between baseline characteristics, laboratory findings, and outcomes of ET patients with CALR and JAK2 V6 17F mutation were compared using an independent t-test for continuous variables and a Chi-square test for categorical variables. The statistical significance of differences was determined if the P-value was less than 0.05. All data were analyzed using the SPSS version 16.0.
Results
Clinical characteristics of non-JAK2V6 17F-mutated ET
Twenty-eight patients with non-JAK2 V6 17F-mutated ET were enrolled onto this study. () The median age was 49 years with the range from 8 to 85 years. There was female predominance (60.7%). The majority of patients (35.7%) showed no symptoms or presented with thrombosis (35.7%) which occurred commonly in arterial sites (90%). All patients except one received treatment with antiplatelet (89.3%), anticoagulant (10.7%), or cytoreductive therapy (89.3%). The indications for cytoreductive therapy were mainly a high risk of thrombosis (had a history of thrombosis or were aged more than 60 years [Citation3]; (64%) or extreme thrombocytosis (32%). Hydroxyurea was the most common cytoreductive agent prescribed in this study (92%).
Table 1. Clinical characteristics of ET patients with non-mutated JAK2V617F, mutated CALR, and mutated JAK2V617F.
CALR mutation analysis
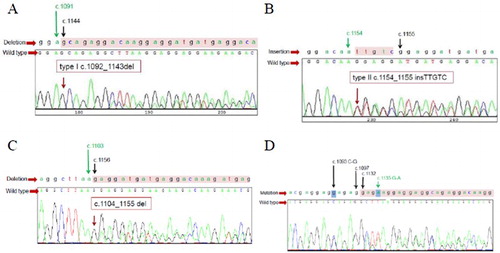
From direct sequencing method, 10 out of 28 (35.7%) patients harbored the CALR mutation including 1 pediatric patient (aged 8 years). Three patients (30%) had a type 1 mutation whereas five patients (50%) had a type 2 mutation. One patient had type 18 mutation [Citation1] which is type 1-like mutation (52 bp deletion; c.1104_1155del). One patient harbored compound mutation (c.1093 C–G, c.1098_1131del, c.1135 G–A) ().
Figure 1. DNA sequencing for CALR mutation shows results of type 1 mutation (52-base pairs deletion; c.1092-1143del) (A), type 2 mutation (5-base pair insertion; c.1154_1155insTTGTC) (B), type 1-like mutation (52-base pair deletion; c.1104_1155del) (C), and compound mutation (c.1093 C–G, c.1098_1131 del, c.1135 G–A) (D) that found in this study.
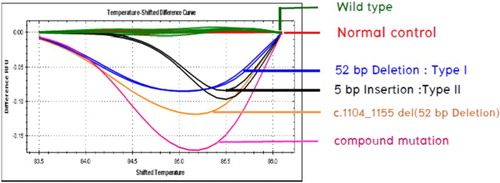
The results of HRM showed a 100% correlation with direct sequencing. The melting curve in patients with a CALR mutation type 1, type 2, and other two types were different from the wild-type ().
Figure 2. HRM analysis showing different melting curves between wild-type CALR, CALR mutation type 1, CALR mutation type 2, and another two mutations found in this study.
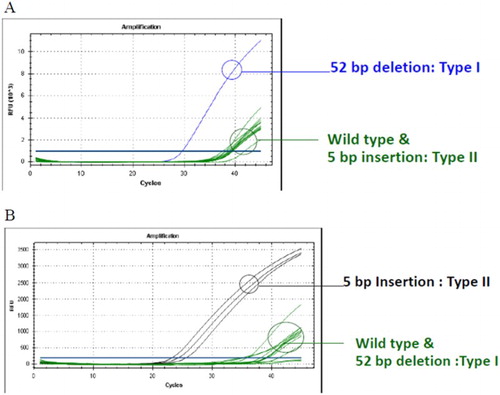
To determine the type of CALR mutation, real-time PCR could identify patients with type 1 and type 2 mutation () in three and five patients, respectively, which correlated with the results of the direct sequencing method and HRM. However, this method could not detect the other types of CALR mutation in two patients (7.1%).
Clinical characteristics of ET patients with mutated CALR and JAK2V617F
To compare the clinical characteristics of CALR mutated and JAK2V617F mutated ET, clinical data from 42 ET patients with a JAK2V617F mutation were collected. As shown in , the median age of ET patients with a CALR mutation was 57 years which was lower than patients with a JAK2V617F mutation (61.5 years) but was not statistically significant. The group with CALR mutation had a significantly higher proportion of male patients when compared to the group with JAK2V617F mutation (60.0 vs. 21.4%, p = 0.024). As regards the clinical presentation, the majority of both patients with CALR mutation and JAK2V617F mutation were asymptomatic (40.0 vs. 42.9%, respectively). Thrombotic events at presentation occurred slightly less frequently in patients with CALR mutation (10 vs. 19%) whereas bleeding complications were more common in patients with CALR mutation than patients with JAK2V617F mutation (20 vs. 4.8%) but the difference did not reach statistical significance.
According to CBC at presentation, the group with CALR mutation had a significantly higher platelet count (1449.0 × 109/l vs. 1037.5 × 109/l, p = 0.03) but tended to have lower hemoglobin levels (12.4 vs. 13.2 g/dl) and a lower WBC count (10.1×109/l vs. 12.9×109/l) when compared with the group with JAK2V617F mutation but the result was not statistically different.
Almost all patients received antiplatelet therapy (100% in CALR mutated vs. 92.9% in JAK2V617F mutated) with around 10% in both groups receiving an anticoagulant. Cytoreductive therapies were initiated in all patients with a CALR mutation and in 88.1% of cases with a JAK2 V6 17F mutation. However, the indication for cytoreductive treatment due to extreme thrombocytosis was more common in patients with CALR mutation (40 vs. 24.3%) whereas high risk of thrombosis was the main indication in patients with JAK2V617F mutation (50 vs. 73%). Hydroxyurea was commonly used as a cytoreductive therapy in both groups (80 vs. 94.6%) and hydroxyurea intolerance occurred in just over one-third of patients (37.5 vs. 37.1% in cases of CALR mutation and JAK2V617F mutation, respectively). The responses to hydroxyurea were not statistically different with a complete response rate of 70.0% in patients with CALR mutation and 88.1% in patients with JAK2V617F mutation.
The median follow-up time for the group with a CALR mutation and a JAK2V617F mutation were 31.5 months and 51.5 months, respectively. One patient (10%) in CALR mutation group and three patients (7.1%) in JAK2V617F mutation group suffered from thrombotic complications during treatment whereas a patient in the JAK2V617F mutation group (2.4%) had bleeding complications. One patient in the JAK2V617 mutation group (2.4%) died from acute myeloid leukemia.
Discussion
The prevalence of CALR mutation in cases of non-mutated JAK2V617F ET in this study was 35.7%. This proportion is lower than that found in studies from Western countries (47.6–67.5%) [Citation7,Citation14] as well as those reported in China (54.5%) [Citation15]. However, the prevalence from the current study is comparable to other reports from Thailand (26.4 and 35.7%) [Citation16,Citation17]. The difference in the prevalence in these studies may be partially explained by the accuracy of diagnosis of ET based on WHO criteria [Citation1]. Since the criteria required exclusion of reactive thrombocytosis if there is no JAK2, CALR, or MPL mutation [Citation1], it is possible to misdiagnose some patients as ET until the causes of reactive thrombocytosis are present. Moreover, prefibrotic PMF is sometimes confused with ET unless the bone marrow biopsy is carefully interpreted by an experienced hematopathologist [Citation1]. Another reason is possibly the difference between genetic predisposition and environmental factors in each population.
There was one 8-year-old child with ET who has shows mutated CALR in this study. The prevalence of CALR-mutated ET (1.6–23.5%) together with JAK2V617F mutated ET (15.7–44%) in children is reported to be lower than that in adults [Citation18–20]. The median age of CALR-mutated ET in children is about 12 years [Citation18,Citation19] with the reported minimal age being 7.9 years [Citation18]. It is possible that underdiagnosed familial thrombocytosis or non-MPN disorders are included in the pediatric ET population [Citation18].
The proportions of type 1 and type 2 mutations in this study are also different from previous reports. A large cohort study reveals that type 1 mutation is the most common type accounting for 53%, with a type 2 mutation occurring in 31.7% of cases [Citation7]. In contrast, type 2 CALR mutations are found more than type 1 mutation (50 vs. 30%, respectively) in this study. A larger cohort or nationwide registration study of ET patients in Thailand is warranted to confirm this finding. The type 1-like mutation that found in this study (c.1104_1155del) was previously described as type 18 mutation [Citation1] with frequency of 0.21% in MPNs [Citation7]. The novel compound mutation (c.1093 C–G, c.1098_1131del, c.1135 G–A) that found in this study was resemble to previous reported type 12 mutation [34 bp deletion (c.1098_1131 del)] found in PMF patients [Citation7,Citation8] but it had additional 2 point mutations. The 34 bp deletion CALR mutation has been shown to have the highest variations compare with other insertion–deletion mutations [Citation10]. This novel mutation was different from mutations in updated data [Citation10,Citation21], supports the rationale that direct DNA sequencing is still an important diagnostic method for clarifying types of CALR mutation.
The accuracy of HRM for the detection of CALR mutation is 100% in this study. Although real-time PCR could not detect other types of CALR mutation that occurred in 7.1% of patients with non-JAK2V617F mutations or 20% in patients with CALR mutations in this study, the accuracy for analysis of type 1 and type 2 mutation is 100%. Both are interesting methods to use in the institute that already has a real-time PCR machine because they are simple to perform and the cost of testing is less than direct DNA sequencing [Citation14]. HRM is reported to capture many types of CALR mutation that have different melting curve patterns with a sensitivity and specificity of 96.4 and 96.3%, respectively [Citation14]. As a result, HRM is the preferred method for screening for CALR mutations before confirmation using direct DNA sequencing. Real-time PCR is however limited to the detection of only type 1 and type 2 CALR mutations or other pre-designed mutant primers. The benefit in terms of quantitative testing or CALR allele burdens based on real-time quantitative PCR results for clinical use is in area for further investigation [Citation22].
The difference in clinical phenotypes of CALR-mutated ET and JAK2 V6 17F-mutated ET is also demonstrated in this study. Patients with a CALR mutation are significantly male predominant and have higher platelet counts. They tend to be younger and have lower white blood cell counts and hemoglobin levels but the results did not reach statistical difference. These findings are comparable to the results from previous studies [Citation7,Citation23–26]. The higher platelet count in cases with type 2 mutations of CALR compared to patients with type 1 mutations, as previously described [Citation23,Citation26], is also observed in the current study. However, the lower risk of thrombosis in patients with a CALR mutation which has been shown in previous studies [Citation7,Citation24,Citation25] is not demonstrated in this study, together with the impact of CALR mutation in treatment response and other complications since there is a limitation of number of patients and hence outcome events. Other limitation is the fact that MPL mutations and novel JAK2 mutations are not evaluated and compared with CALR mutation in this study [Citation27].
In conclusion, the prevalence of CALR mutation in JAK2 V6 17F-negative ET in this study is 35.7%. HRM is an effective method of detecting CALR mutation and is a more advantageous method of screening for CALR mutations.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Lalita Norasetthada http://orcid.org/0000-0002-2223-5711
Additional information
Funding
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: https://doi.org/10.1182/blood-2016-03-643544
- Swerdlow SH, Campo E, Harris NL, et al, editors. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008.
- Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770. doi: https://doi.org/10.1200/JCO.2010.31.8436
- Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120(26):5128–5133. doi: https://doi.org/10.1182/blood-2012-07-444067
- Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857–5859. doi: https://doi.org/10.1182/blood-2011-02-339002
- Duangnapasatit B, Rattarittamrong E, Rattanathammethee T, et al. Clinical manifestations and risk factors of complications of Philadelphia chromosome-negative myeloproliferative neoplasms. Asian Pac J Cancer Prev. 2015;16(12):5013–5018. doi: https://doi.org/10.7314/APJCP.2015.16.12.5013
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. doi: https://doi.org/10.1056/NEJMoa1311347
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. doi: https://doi.org/10.1056/NEJMoa1312542
- Chi J, Nicolaou KA, Nicolaidou V, et al. Calreticulin gene exon 9 frameshift mutations in patients with thrombocytosis. Leukemia. 2014;28(5):1152–1154. doi: https://doi.org/10.1038/leu.2013.382
- Chi J, Manoloukos M, Pierides C, et al. Calreticulin mutations in myeloproliferative neoplasms and new methodology for their detection and monitoring. Ann Hematol. 2015;94(3):399–408. doi: https://doi.org/10.1007/s00277-014-2232-8
- Vannucchi AM, Rotunno G, Bartalucci N, et al. Calreticulin mutation-specific immunostaining in myeloproliferative neoplasms: pathogenetic insight and diagnostic value. Leukemia. 2014;28(9):1811–1818. doi: https://doi.org/10.1038/leu.2014.100
- Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867World Health Organization–defined essential thrombocythemia at diagnosis: a study by the International Working Group on myelofibrosis research and treatment. Blood. 2012;120(6):1197–1201. doi: https://doi.org/10.1182/blood-2012-01-403279
- Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121:4778–4781. doi: https://doi.org/10.1182/blood-2013-01-478891
- Park JH, Sevin M, Ramla S, et al. Calreticulin mutations in myeloproliferative neoplasms: comparison of three diagnostic methods. PLoS One. 2015;10(10):e0141010. doi: https://doi.org/10.1371/journal.pone.0141010
- Lin Y, Liu E, Sun Q, et al. The prevalence of JAK2, MPL, and CALR mutations in Chinese patients with BCR-ABL1–negative myeloproliferative neoplasms. Am J Clin Pathol. 2015;144(1):165–171. doi: https://doi.org/10.1309/AJCPALP51XDIXDDV
- Jit-Ueakul D, Mitrpant C, Tassaneetrithep B, et al. Clinical and laboratory characteristics of essential thrombocythemia and primary myelofibrosis patients stratified by presence of JAK2V617F mutation or calreticulin mutation. Poster session presented at: the Thai Society of Hematology Meeting; 2016 March 16; Bangkok, Thailand.
- Singdong R, Siriboonpiputtana T, Chareonsirisuthigul T, et al. Characterization and prognosis significance of JAK2 (V617F), MPL, and CALR mutations in Philadelphia-negative myeloproliferative neoplasms. Asian Pac J Cancer Prev. 2016;17(10):4647–4653.
- Randi ML, Geranio G, Bertozzi I, et al. Are all cases of paediatric essential thrombocythaemia really myeloproliferative neoplasms? Analysis of a large cohort. Br J Haematol. 2015;169(4):584–589. doi: https://doi.org/10.1111/bjh.13329
- Fu R, Liu D, Cao Z, et al. Distinct molecular abnormalities underlie unique clinical features of essential thrombocythemia in children. Leukemia. 2016;30(3):746–749. doi: https://doi.org/10.1038/leu.2015.167
- Giona F, Teofili L, Capodimonti S, et al. CALR mutations in patients with essential thrombocythemia diagnosed in childhood and adolescence. Blood. 2014;123(23):3677–3679. doi: https://doi.org/10.1182/blood-2014-04-572040
- Varricchio L, Falchi M, Dall’Ora M, et al. Calreticulin: challenges posed by the intrinsically disordered nature of calreticulin to the study of its function. Front Cell Dev Biol. 2017;5(96):1–19.
- Verger E, Cassinat B, Chauveau A, et al. Clinical and molecular response to interferon-α therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126(24):2585–2591. doi: https://doi.org/10.1182/blood-2015-07-659060
- Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia. 2016;30(2):431–438. doi: https://doi.org/10.1038/leu.2015.277
- Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552–1555. doi: https://doi.org/10.1182/blood-2013-11-538983
- Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–1551. doi: https://doi.org/10.1182/blood-2013-11-539098
- Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89(8):E121–E124. doi: https://doi.org/10.1002/ajh.23743
- Feenstra JDM, Nivarthi H, Gisslinger H, et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood. 2016;127(3):325–332. doi: https://doi.org/10.1182/blood-2015-07-661835