ABSTRACT
Objectives: A multicenter, noninterventional, observational study was conducted in the Latin American countries including Argentina, Brazil, Colombia, Mexico, and Venezuela to assess the prevalence of liver and cardiac iron overload using magnetic resonance imaging (MRI) in patients with chronic anemias except thalassemia.
Methods: Patients aged >10 years with transfusion-dependent anemias, except thalassemia, either with <20 units of red blood cell (RBC) transfusions with serum ferritin (SF) levels >2000 ng/mL or with ≥20 units of RBC transfusions regardless of SF level in their lifetime, were enrolled. Iron overload was assessed using MRI.
Results: Among 175 patients included, the majority had sickle cell disease (SCD; 52%), followed by aplastic anemia (AA; 17.7%), myelodysplastic syndrome (MDS; 8.6%), Diamond-Blackfan anemia (DBA; 4%), pure red cell aplasia (1.1%), and others (16.6%). Liver iron overload was observed in 76.4% of patients, while cardiac iron overload was seen in 19.2% when assessed by MRI. The prevalence of iron overload was 80.2% in patients with SCD, 73.3% in MDS, 77.4% in AA, 100% in pure red cell aplasia, 71.4% in DBA, and 68.9% in other transfusion-related disorders. A moderate correlation between liver iron concentration (LIC) and SF was observed in patients with SCD and MDS (r = 0.47 and r = 0.61, respectively). All adverse events reported were consistent with the published data for deferasirox or underlying disease.
Conclusion: A high prevalence of iron overload in this patient population in Latin American countries indicates that a better diagnosis and management of iron overload is required in these countries.
Introduction
Iron overload, caused by regular transfusions in patients with hemoglobinopathies, is managed by iron chelation therapy using, deferoxamine, deferiprone, or deferasirox. However, accurate assessment of iron overload is required for the diagnosis and management with iron chelation therapy. Traditionally, iron overload has been evaluated by serum ferritin (SF) levels, which is an indirect method of measurement of iron deposition in the liver, and is reasonably well correlated with the direct measurement of liver iron concentration (LIC) for patients with thalassemia [Citation1,Citation2]. However, for diseases like sickle cell disease (SCD), factors like chronic inflammation may cause elevated SF resulting in inaccurate assessment of iron overload [Citation3]. Hence, in recent years, assessment of liver and cardiac iron using magnetic resonance imaging (MRI) has enabled more direct, accurate, and noninvasive monitoring of iron overload of the liver and heart [Citation4].
The prevalence and extent of iron overload vary significantly across different geographical areas, with a comparatively higher transfusion burden observed in countries in the Asia-Pacific region, Europe, and Africa and patients are dependent on the management of iron overload [Citation5]. Earlier, the retrospective RELATH study in Latin America, using SF as a marker of iron overload, demonstrated that iron overload and related complications are significant clinical problems in this region as well; hepatic complications were found in 65.3% of the patients, followed by cardiac (27.5%) and endocrine complications (18.2%) [Citation6]. The diagnosis [Citation7] and monitoring of iron overload may improve in the region if SF is complemented with the MRI of the liver and heart [Citation8]. Therefore, to assess the prevalence of iron overload accurately using liver and cardiac MRI, ASIMILA (assessment of iron overload in transfusion-dependent patients by MRI in Latin America) study was conducted in transfusion-dependent patients with chronic anemias with the exception of thalassemia.
Methods
Patient population
Patients aged >10 years and with a confirmed diagnosis of homozygous sickle cell anemia (SCA), low- and intermediate–1- International Prognostic Scoring System (IPSS) risk myelodysplastic syndrome (MDS), aplastic anemia (AA), Diamond-Blackfan anemia (DBA), congenital sideroblastic anemias, and other rare anemias were included in the study. Patients were required to have received (in their lifetime history) <20 units (or ∼100 mL/kg) of red blood cell (RBC) transfusions with SF levels >2000 ng/mL or ≥20 units (or ∼100 mL/kg) of RBC transfusions regardless of SF level. Patients with thalassemia and any disease with hemosiderosis not related to blood transfusion (ie, hereditary hemochromatosis, idiopathic pulmonary hemosiderosis, etc), or with a life expectancy of <12 months, or pregnant women were excluded from the study. Patients with SCA facing clinical infections or pain crisis, at the time of enrollment or during sample collection and those for which MRI evaluation was contraindicated were also excluded.
Study design
The ASIMILA study was an open-label, multicenter, observational study to evaluate the prevalence of iron overload in liver and heart in transfusion-dependent patients with chronic anemias in Latin American countries including Argentina, Brazil, Colombia, Mexico, and Venezuela. Patients were recruited at tertiary-care hematology centers with ≥200 monthly consultations and/or located in cities with ≥1 million people. The study period consisted of day 0 (screening) and day 15, during which 1 MRI test per patient was performed. The study did not test for any hypothesis, and patient evaluation and treatment were performed at the discretion of the investigators. No blinding was performed for the study and no recommendations were made for the treatment of iron overload, and the patients were allowed to have current or previous use of chelation therapy. All potential candidates for each center were considered for the study. The study was conducted in accordance with international and local standards and was approved by the IRB of participating sites and local health authorities. Patients enrolled in the study needed to provide written informed consent.
Assessments
The primary end point was the prevalence and severity of liver and cardiac iron overload as measured by MRI, in patients with transfusional iron burden with special focus on evaluating patients with SCD. Iron overload was defined as the percentage of patients with liver R2 MRI >2 mg Fe/g dry weight (dw) and/or cardiac T2* MRI <20 ms and was assessed by performing MRI (Ferriscan®) within 15 days of evaluation of SF. Cardiac MRI was performed based on the recommendation by the physician and available across all diseases, except SCD, for which it was not required. Echocardiography was performed within 1 week from MRI in all patients who were submitted to T2* MRI. Secondary analyses included correlation between SF, LIC, and cardiac T2*. Safety assessments were made in relation to the treatment of iron overload and included change in dose or interruption and reason for death if any.
Statistical methods
Descriptive statistics (SAS software, version 9.3) was used to summarize the demographics, other baseline characteristics, iron overload assessments including LIC, T2*, and follow-up assessments of subjects in the study, overall and by country. A logistic regression model was used to evaluate the relationship between liver iron overload incidence and SF level. Odds ratios (ORs) are presented with 95% confidence interval (CI).
A supportive analysis to evaluate the relationship between R2 MRI value and SF was done using the Pearson’s correlation coefficient.
Results
Patient disposition
Overall 212 patients were screened, of which, 6 did not meet the inclusion or exclusion criteria, 25 failed to perform key MRI procedures, 4 had protocol deviations not accepted by the ethics committee and 2 withdrew consent; 175 patients were included in the present analysis.
Baseline characteristics
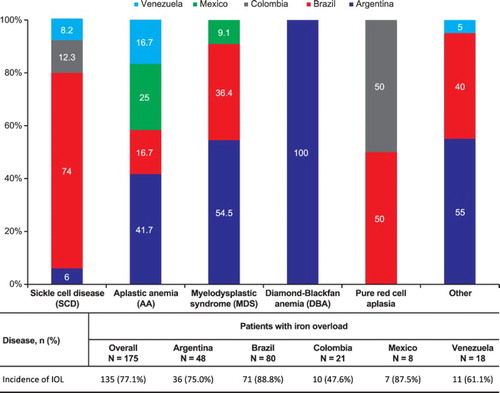
Baseline characteristics at the time of enrollment are summarized in . Prior to study entry, the most common method for assessment of iron overload was SF (89.1%), followed by transferrin saturation (66.9%), echocardiogram (29.7%), liver MRI (24.0%), liver ultrasonography (11.4%), cardiac MRI (8.6%), liver biopsy (2.9%), and cardiac ultrasound (0.6%). All the patients in Mexico and 61.9% in Colombia had undergone liver MRI, compared to only 10% and 27% in Brazil and Argentina, respectively,. Overall, only 8.6% of the patients had undergone evaluation of cardiac iron overload by cardiac MRI prior to the study.
Table 1. Baseline characteristics.
Prevalence of iron overload by disease and by country
Liver MRI was performed on 174 patients. summarizes iron overload in patients by disease and by country. The overall prevalence of iron overload, including liver and/or cardiac iron overload, was 77.1% (n = 135). Among the countries, the prevalence of iron overload was 88.8% in Brazil, 87.5% in Mexico, 75.0% in Argentina, 47.6% in Colombia, and 61.1% in Venezuela. Upon evaluation of iron overload by disease, high prevalence of iron overload was seen in the patients with pure red cell aplasia and SCD. Liver iron overload (>2 mg Fe/g dw) was prevalent in 76.4% (n = 133) of 174 enrolled patients. Of the 73 patients who underwent cardiac MRI, cardiac iron overload (T2* <20 ms) was seen in 19.2% (n = 14).
Iron overload assessments
High rates of iron overload was observed in patients across various hemoglobinopathies and other anemias, as assessed by SF and LIC ( and Supplementary Tables 1 and 2). Overall, median SF was high with 1401 ng/mL with highest levels observed in the patients in Brazil followed by Venezuela and Colombia, while the patients in Argentina and Mexico had comparatively lower median SF values. High median SF levels >1800 ng/mL were observed specifically in the patients with SCD and AA in Brazil and in the patients with SCD and MDS in Argentina.
Table 2. Serum ferritin, LIC, and cardiac T2* by disease and by country.
Iron overload assessments based on LIC were different than those with SF ( and Supplementary Table 2). Overall, mean LIC was 14.0 ± 14.0 mg Fe/g dw; highest mean LIC was seen in the patients in Mexico followed by Brazil and Argentina; the patients in Colombia and Venezuela had a comparatively lower mean LIC. Patients with MDS had highest mean LIC (mg Fe/g dw) (19.0 ± 14.4) followed by AA (16.5 ± 15.8), and SCD (14.3 ± 13.8). Among the patients analyzed for cardiac iron overload, mean cardiac T2* of 27.3 ± 10.7 ms was within normal range (>20 ms). ( and Supplementary Table 3). Patients with SCD in Brazil and Columbia had low mean cardiac T2*, however, due to low patient numbers any meaningful inference cannot be derived.
Of the 165 patients who had performed echocardiography, 71.5% (n = 118) and 28.5% (n = 47) of the patients had normal and abnormal echocardiography results, respectively.
Iron chelation treatment
In the study, 97 patients (55.4%) were treated with iron chelation therapy and 4.6% of the patients received hydroxyurea as a treatment for the underlying disease. The most commonly used chelator was deferasirox (n = 88; 50.3%), followed by deferoxamine (n = 15; 8.6%), and deferiprone (n = 7; 4.0%), and a consistent trend was observed across countries. Duration of iron chelation also showed considerable variation across the diseases and countries. Overall, the mean duration of iron chelation was higher in DBA (n = 6; 50 ± 32.2 months) and other transfusion-dependent disorders (n = 14; 50.6 ± 74.3 months) followed by MDS (n = 8; 30.6 ± 29.1 months), SCD (n = 48; 26.3 ± 33.1 months), and AA (n = 14; 17.4 ± 19.2 months). In Brazil and Colombia, the mean duration of chelation for the patients with SCD was reasonably high (n = 40; 29.4 ± 35.1 months and n = 2; 21.6 ± 25 months, respectively); no patients in Mexico were treated with chelation therapy. None of the patients with MDS were chelated in Colombia, Mexico, and Venezuela, but the mean duration of chelation in Brazil was high (n = 4; 53.9 ± 22.2 months) and low in Argentina (n = 4; 7.4 ± 6.6 months). The SF and LIC seemed to be lower in patients without chelation for almost all the diseases except pure red cell aplasia and for SF in patients with AA (Supplementary Table 4).
Correlation analysis
The correlation analysis between LIC (R2 MRI) and SF suggests a fair degree of relationship with a Pearson correlation coefficient (r) of 0.48 (95% CI: 0.35, 0.58), which was significant at 0.05 (Supplementary table 5). The logistic regression model suggests that for an increase of 1000 ng/mL in the SF values, the chance of presence of liver iron overload by MRI (R2 MRI >2 mg Fe/g dw) increases by 76%, and this association is significant at 0.05 level (OR, 1.76; 95% CI: 1.25, 2.47). The relationship between the prevalence of liver iron overload and SF categories of ≤1500 ng/mL or > 1500 ng/mL was explored. The results suggest that the odds of liver iron overload is 7.33 times higher if an SF level is >1500 ng/mL (OR, 7.33; 95% CI: 2.88, 18.63). This relationship was significant at 0.05 level.
The Pearson correlation coefficient between T2* MRI and SF suggests no linear relationship between the two parameters (r = −0.17; 95% CI: −0.39, 0.07). The logistic regression model, suggests that by increasing SF by 1000 ng/dL, the chance of evidence of cardiac iron overload by MRI increases by 40%, and this association was significant at 0.05 level (OR, 1.40; 95% CI: 1.04, 1.90). The presence of echocardiography abnormality with clinical significance increases the chance of evidence of iron overload by MRI by 190%. However, the 95% CI indicates that the association between the two parameters is not significant at 0.05 level
Safety
Safety data were limited to the 15-day data collection period and are listed in . Fourteen patients (8.0%) had at least 1 AE. Two patients (1.1%) had grade 3 AEs and 4 patients had AEs requiring concomitant medication (2.3%). Three patients had AEs assessed as related to current treatment: diarrhea (0.6%), hepatitis (0.6%), and thrombosis (0.6%). One patient (0.6%) experienced serious AE (urinary tract infection) requiring prolonged hospitalization without interruption of deferasirox treatment and 1 patient (0.6%) had an AE (hepatobiliary disorder) that resulted in permanent discontinuation of current treatment. No deaths were reported during the study period.
Table 3. AEs during the 15-day study period.
Discussion/Conclusion
The severity of anemia, transfusion requirements, and extent of iron overload may vary across hemoglobinopathies and other anemias, and also across various geographical regions, especially for hemoglobinopathies other than thalassemia [Citation9]. Patients with thalassemia major have typically higher transfusional burden compared to the patients with SCD and MDS [Citation10,Citation11]. Data from the EPIC study showed that across Europe, Middle East/Africa, and Asia Pacific, the mean proportion of lifetime on transfusion was 90% for the patients with thalassemia major compared to 60% in SCD and less than 10% in MDS, though the median SF levels across diseases was >2500 ng/mL [Citation9]. Even though guidelines recommend transfusions as supportive care in the patients with MDS and as prophylaxis in SCD, the extent of transfusions and subsequent chelation therapy in these patients was less. The current observational study across Brazil, Argentina, Mexico, Colombia, and Venezuela showed high prevalence of liver iron overload as seen with abnormal MRI in 76% of the patients, and was most evident in the patients with SCD, MDS, and AA. Similar results were seen in the retrospective, epidemiological registry RELATH study in Latin America, which showed that approximately 90% of the patient population had SF greater than 1000 ng/mL [Citation6]. Half of the patients recruited in RELATH had SCD, followed by thalassemia, which compares to the current study, as among those recruited highest proportion of patients was with SCD. Though patients with thalassemia were not included in the study, high prevalence of SCD in this region is evident.
In the RELATH study, SF was the most common method of iron overload assessment in this region, followed by transferrin saturation and echocardiogram [Citation6]. In ASIMILA, though SF and transferrin saturation remained the most common methods, even with the limited availability of MRI, about one-quarter of the patients had undergone liver MRI to assess their iron burden. A small fraction of the patients also underwent liver biopsy and liver ultrasonography. Disparities in using MRI to assess iron overload within the countries were also observed, with some countries like Mexico and Columbia focused more on using MRI compared to others in the study, however, due to comparatively low patient numbers in these countries, any conclusion cannot be drawn. Inconsistencies were seen in median SF measurements and mean LIC across diseases and countries, further highlighting the need to complement SF by MRI measurements for the accurate measurement of iron overload.
While SF correlates reasonably with LIC for patients with transfusion-dependent thalassemia, for other hemoglobinopathies SF may not provide an accurate assessment [Citation2,Citation12]. Even though LIC by MRI measures stored iron, higher values are associated with increasing morbidity in patients with hemoglobinopathies and is considered a gold standard for the measurement of iron overload [Citation13]. In the present study, moderate and significant correlation of SF with LIC was seen only for the patients with SCD and MDS. High SF levels were observed in the patients with SCD across all countries with the median of 1897 ng/mL, but moderate overall LIC of 9 mg Fe/g dw. This could be due to higher SF levels observed in these patients secondary to inflammation [Citation12], which further highlights the importance of MRI in assessing iron overload. In Brazil, which had the highest number of patients with SCD enrolled, liver MRI was performed in only 10% of the patients prior to the study.
Normal cardiac T2* was observed in most patients due to the fact that high cardiac iron overload is mostly seen in patients with thalassemia compared to SCD. High SF in these patients could be due to increased inflammation, less duration of exposure of iron to the target organs, lower nontransferrin bound iron (NTBI), and comparatively less ineffective erythropoiesis can lead to lesser iron deposition in heart and endocrine organs [Citation7,Citation14–16]. Although the study was not designed to correlate iron overload severity and treatment patterns, it was evident that the high prevalence of iron overload was due to lack of appropriate chelation or lack of compliance. Of 76% of patients with liver and cardiac iron overload, only 58.9% received chelation therapy. In addition, the duration of chelation treatment was low compared to the duration of iron overload, and perhaps for some patients, it may have been too early to observe a change in SF. The reason for higher liver iron overload in patients with chelation could be due to many factors, such as lack of compliance, inappropriate monitoring, and lack of proper management. One limitation of the study was that the that the duration of chelation treatment was collected from medical charts retrospectively, and therefore, it was difficult to determine the exact overall treatment duration. Another limitation was that the patient numbers in some groups were very small to make meaningful comparisons. Overall, the assessment of iron overload by MRI of the liver and heart continues to demonstrate value in complementing SF measurements, both in the patients with or without iron chelation therapy. This study demonstrates the need to monitor and assess the patients on chelation treatment closely to optimize treatment. With the increasing availability of MRI in Latin America, hematologists should use this tool for appropriate diagnosis. Better monitoring, compliance, and appropriate dosing of iron chelation therapy are required as indicated by increased iron burden despite chelation therapy.
Supplemental Material
Download MS Word (118.5 KB)Acknowledgments
This study was sponsored by Novartis Pharma AG. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Amrita Dutta, PhD and Haritha Nekkanti, PharmD of Novartis Healthcare Pvt. Ltd. for their medical editorial assistance with this manuscript.
Disclosure statement
Guillermo Drelichman reports to Speakers' Bureau for Novartis Pharma. Nora Watman reports personal fees from Sanofi, Shire, Alexion, and Novartis. Juan Pablo Zarate is a full time employee of Novartis Pharmaceuticals Corporation. The other authors declare no conflict of interest.
ORCID
Miriam Park http://orcid.org/0000-0001-5558-6166
Luis Marfil http://orcid.org/0000-0002-1807-7810
Additional information
Funding
Notes
* All the authors designed study, performed research, collected and interpreted data, performed statistical analysis, wrote and reviewed manuscript. All authors except Juan Pablo Zarate were institutional principal investigators and contributed equally to the manuscript. Juan Pablo Zarate contributed in protocol writing and trial management.
References
- Thuret I. Post-transfusional iron overload in the haemoglobinopathies. C R Biol. 2013 Mar;336(3):164–172. doi:10.1016/j.crvi.2012.09.010. PubMed PMID: 23643400.
- Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007 May;92(5):583–588. PubMed PMID: 17488680. doi: https://doi.org/10.3324/haematol.10842
- Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2013;2013:447–456. doi:10.1182/asheducation-2013.1.447. PubMed PMID: 24319218.
- Wood JC. Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program. 2011;2011:443–450. doi:10.1182/asheducation-2011.1.443. PubMed PMID: 22160072.
- Cappellini MD, Porter J, El-Beshlawy A, et al. Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010 Apr;95(4):557–566. doi:10.3324/haematol.2009.014696. PubMed PMID: 19951979; PubMed Central PMCID: PMCPMC2857545.
- Lobo C, Angulo IL, Aparicio LR, et al. Retrospective epidemiological study of Latin American patients with transfusional hemosiderosis: the first Latin American epidemiological study in iron overload--the RELATH study. Hematology. 2011 Sep;16(5):265–273. doi:10.1179/102453311X13085644680302. PubMed PMID: 21902889.
- Beard JL, Dawson H, Pinero DJ. Iron metabolism: a comprehensive review. Nutr Rev. 1996 Oct;54(10):295–317. PubMed PMID: 9063021. doi: https://doi.org/10.1111/j.1753-4887.1996.tb03794.x
- Araujo A, Drelichman G, Cancado RD, et al. Management of transfusional iron overload in Latin america: current outlook and expert panel recommendations. Hematology. 2009 Feb;14(1):22–32. doi:10.1179/102453309X385179. PubMed PMID: 19154661.
- Viprakasit V, Gattermann N, Lee JW, et al. Geographical variations in current clinical practice on transfusions and iron chelation therapy across various transfusion-dependent anaemias. Blood Transfus. 2013 Jan;11(1):108–122. doi:10.2450/2012.0012-12. PubMed PMID: 22871821; PubMed Central PMCID: PMC3557481.
- Marsella M, Borgna-Pignatti C. Transfusional iron overload and iron chelation therapy in thalassemia major and sickle cell disease. Hematol Oncol Clin North Am. 2014 Aug;28(4):703–727, vi. doi:10.1016/j.hoc.2014.04.004. PubMed PMID: 25064709.
- Steensma DP, Gattermann N. When is iron overload deleterious, and when and how should iron chelation therapy be administered in myelodysplastic syndromes? Best Pract Res Clin Haematol. 2013 Dec;26(4):431–444. doi:10.1016/j.beha.2013.09.009. PubMed PMID: 24507819.
- Porter JB. Pathophysiology of transfusional iron overload: contrasting patterns in thalassemia major and sickle cell disease. Hemoglobin. 2009;33(Suppl 1):S37–S45. doi:10.3109/03630260903346627. PubMed PMID: 20001631.
- Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009 Oct;97(3):185–197. doi:10.1111/j.1423-0410.2009.01207.x. PubMed PMID: 19663936.
- Walters MC. Sickle cell anemia and hematopoietic cell transplantation: when is a pound of cure worth more than an ounce of prevention? Pediatr Transplant. 2004 Jun;8(Suppl 5):33–38. doi:10.1111/j.1398-2265.2004.00188.x. PubMed PMID: 15125704.
- Fillet G, Beguin Y, Baldelli L. Model of reticuloendothelial iron metabolism in humans: abnormal behavior in idiopathic hemochromatosis and in inflammation. Blood. 1989 Aug 1;74(2):844–851. PubMed PMID: 2502204.
- Bourantas KL, Dalekos GN, Makis A, et al. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol. 1998 Jul;61(1):49–54. PubMed PMID: 9688292. doi: https://doi.org/10.1111/j.1600-0609.1998.tb01060.x