ABSTRACT
Objectives: In adults with sickle cell disease (SCD), pain often necessitates opioid use. Few studies have examined the relationship between opioid use and health-related quality of life (HRQOL) in adults with SCD. We tested the hypothesis that higher doses of opioids are associated with worse HRQOL.
Methods: A cross-sectional cohort study was performed in adults with SCD who completed standardized and validated HRQOL questionnaires: Patient Health Questionnaire-15 (PHQ-15), Patient Health Questionnaire-9 (PHQ-9), Medical Outcome Study 36 Item Short Form (SF-36), and Generalized Anxiety Disorder questionnaire (GAD-7). Daily outpatient opioid dose was converted into morphine milligram equivalents (MME) and categorized as < 90 mg/day or ≥ 90 mg/day. Subject's questionnaire scores were compared by opioid dose.
Results: Ninety-nine adults completed questionnaires. The majority had HbSS and median age was 30 years. The median MME was 80 mg/day. When the association between HRQOL and opioid dose was compared, those prescribed ≥ 90 MME had significantly lower SF-36 subscale scores in 7 of 8 domains, and significantly higher severity scores in the PHQ-15, GAD-7, and the PHQ-9 in comparison those prescribed < 90 MME. Using a multivariable regression tree analysis, in addition to the presence of chronic pain, mental health, physical health, and somatic burden were key predictors of ≥ 90 MME opioid use.
Conclusion: Higher daily opioid dose is associated with chronic pain. Among those with chronic pain, opioid dose ≥ 90 MME is associated with worse HRQOL.
Introduction
Sickle cell disease (SCD) is a hematologic disorder characterized by sickle-shaped red blood cells [Citation1,Citation2]. Individuals affected with HbSS, the most common type of SCD, are homozygous for a single amino acid substitution in the beta globin gene [Citation3]. Under conditions of hypoxia, normally oval-shaped erythrocytes become sickle-shaped, non-deformable, and poorly able to traverse the microvasculature [Citation3]. The result is vascular occlusion, with consequent ischemia and organ damage [Citation3,Citation4]. When ischemia occurs within the bone marrow, the result is painful vaso-occlusive crises (VOC) [Citation4]. These self-limited episodes of bony pain are the most common morbidity in patients with SCD [Citation5]. While these acute episodes typify the pain seen in children with SCD, adults have a more complex pain syndrome. In addition to episodes of VOC, 50% of adults suffer from a chronic pain syndrome [Citation5,Citation6]. For many adults with chronic pain, long-term opioids are the mainstay of treatment [Citation4].
Few studies have examined how opioids affect the life of an adult with SCD in areas besides pain. The only large, prospective study to do so was the Pain in Sickle Cell Epidemiology Study (PiSCES), in which 230 adults with SCD were administered measures of physical function, mental health and health-related quality of life (HRQOL) [Citation7,Citation8]. Not surprisingly, adults with SCD demonstrated a higher somatic symptom burden compared to primary care patients, even when common pain sites were excluded in the SCD subjects [Citation7]. High somatic symptom burden in adults with SCD was also associated with increased rates of depression, anxiety, and lower HRQOL [Citation7,Citation8]. A major reason for the low HRQOL scores was pain [Citation7]. When the association between opioid use and HRQOL was examined, those adults with SCD who used opioids scored higher on measures of somatic symptom burden, stress, physical and mental HRQOL than non-opioid users [Citation9]. Although this study showed that opioid use was associated with worse HRQOL, a limitation was that opioid dose was not examined. In the general population, high-dose opioids are known to be associated with more side effects, an increased risk of overdose and death, and the development of opioid-induced hyperalgesia. Although these risks have not been demonstrated in SCD patients, the impact of opioid dose on HRQOL in these patients is still a knowledge gap that needs to be addressed [Citation10–13].
To expand on the prior work that demonstrated the impact of opioid use on HRQOL in adults with SCD, we prospectively administered Patient Health Questionnaire-15 (PHQ-15), Patient Health Questionnaire-9 (PHQ-9), Medical Outcome Study 36 Item Short Form (SF-36), and the Generalized Anxiety Disorder Questionnaire-7 (GAD-7) to a cohort of adults with SCD. Our primary objective was to determine the association between opioid dose and HRQOL. We hypothesized that, among adults with SCD who managed pain with opioids, those treated with opioids ≥ 90 MME/day would have lower HRQOL scores than those treated with < 90 MME/day.
Methods
The institutional review board at the Medical College of Wisconsin and Froedtert Hospital approved this study. Adults with SCD were recruited from the Adult Sickle Cell Clinic at Froedtert Hospital between January 2014 and July 2017. Eligibility criteria included a diagnosis of SCD and age ≥ 18 years, ability to read and write English, and appropriate cognitive ability to accurately comprehend and complete the written surveys (determined by clinic staff and the study PI). After informed consent (provided verbally and in writing), study personnel administered the following questionnaires: PHQ-15, PHQ-9, SF-36, and GAD-7. Demographic characteristics (age, gender, highest education level, employment, marital status), prior psychiatric history (mental health diagnosis, currently seeing a mental health provider), and social support was obtained from study subjects as a part of a separate written questionnaire. The survey was performed in a private area within the adult SCD clinic, and the survey took, on average, 15 min to complete. The medical chart was abstracted with a standardized data extraction form for number of admissions to the Adult Sickle Cell Clinic, emergency department (ED), and hospital in the year prior to consent. Outpatient opioid regimen was obtained from the chart extraction and also confirmed with the study subjects.
Measures
PHQ: Depression and somatic burden were measured using the Patient Health Questionnaire (PHQ). The PHQ is a widely utilized screening instrument based on subjects’ self-reported symptoms that were originally designed to facilitate the recognition and diagnosis of the most common mental disorders [Citation14]. The PHQ has been found to have excellent reliability and validity across a variety of medical populations [Citation15–17]. Because the questionnaire relies on patient self-report, it does not generate definitive diagnoses, but is highly sensitive [Citation15–17]. There is good agreement between PHQ diagnoses and those of independent mental health professionals [Citation14]. For this study, we specifically utilized the PHQ-15, a 15 item questionnaire that measures the somatic symptom burden regarding the 15 most common somatic symptoms (≤ 4 = normal, 5–9 = low, 10–14 = moderate, > 14 = high) [Citation17]. We also used the PHQ-9, a 9-item survey that measures the frequency by which a subject experiences symptoms of depression (≤ 4 = low symptom burden, 5–9 = mild depression, 10–14 = moderate depression, > 15 = severe depression) (see supplemental figure) [Citation18].
SF-36: The SF-36 measures eight major domains of HRQOL including physical function, physical role functions, emotional role functioning, pain, vitality, general health, mental health, and social function. Scores are measured from 0 to 100, with 0 being the worst and 100 being the best [Citation19]. The SF-36 has been validated in populations with chronic diseases, those with chronic pain, and has been previously used in patients with SCD [Citation20–23]. Based on prior studies, the scores from the 8 sections were also combined into 2 summary scores: physical HRQOL summary (PCS) and mental HRQoL summary (MCS). The first 4 sections (physical functioning, role physical, pain, and general health) represent physical HRQOL, while vitality, social functioning, role emotional, and mental health reflect mental HRQOL (see supplemental figure) [Citation19].
GAD-7: The GAD-7 is a validated 7-item anxiety questionnaire that was developed to identify probable cases of generalized anxiety disorder as defined by the DSM-IVR [Citation24]. The scale is strongly associated with domains of both recent (over the last 2 weeks) functional impairment and disability due to anxiety (≤ 4 = low anxiety, 5–9 = mild anxiety, 10–14 = moderate anxiety, > 14 = severe anxiety) (see supplemental figure) [Citation24].
SCD Disease Severity: Disease severity was assessed using a modified version of a previously validated chronic disease severity score, where 1 point is given for each of the following: pulmonary dysfunction as defined by the presence of pulmonary hypertension or oxygen saturation < 92%; avascular necrosis of the hip or shoulder; central nervous system abnormality, as defined by a history of stroke, seizure, or transient ischemic attack; and kidney dysfunction, as defined by creatinine clearance < 60 ml/min. The total score was interpreted on a scale of 0 (mildest disease) to 4 (most severe disease) [Citation25].
Key Definitions of Disease severity:
Chronic pain: Chronic pain was defined as the presence of disease-related pain on 3 or more days per week for 6 months or the use of daily opioids for the management of pain [Citation26].
Pulmonary hypertension: Pulmonary hypertension was defined by the presence of any one of the following: symptomatic pulmonary hypertension, a tricuspid jet velocity of ≥ 3.0 m/s or greater, or a mean pulmonary arterial pressure of 25 mmHg on right heart catheterization.
Chronic renal insufficiency: Chronic renal insufficiency was defined as a creatinine clearance < 60 ml/min.
Statistical methods
Subject demographics were summarized as medians with interquartile ranges (IQR) for continuous variables, and frequencies with percent, as applicable, for categorical variables. Outpatient doses of opioids were converted into morphine milligram equivalent doses (MME) in order to calculate an oral morphine equivalent dose per day. Because the distribution of MME was skewed, with a long right tail, subjects were dichotomized into opioid dose groups (< 90 MME/day and ≥ 90 MME/day) for comparison. The decision to use ≥ 90 mg MME as the cutoff was based on recent published guidelines [Citation27]. We recognize that there is literature that defines ≥ 50 MME as a cutoff for high-dose opioids, but, due to sample size, we were limited in the number of groups that we could divide our patient population into. We did, however, explore differences between those subjects receiving 0–49 MME/day and 50–89 MME/day and found no differences in HRQOL (data not shown). To compare subject characteristics between the < 90 MME/day and ≥ 90 mg MME groups, we used Chi-Square or Fisher's exact tests for categorical variables and Mann-Whitney U tests for continuous variables.
Multivariable regression analysis was done by using a classification and regression tree analysis (CART). A regression tree analysis was used to examine important factors associated with MME. The CART analysis was specifically used for this study because: (1) the method allows for the inclusion of all predictor variables, regardless of established or possible interactions; (2) the method allows for inclusion of data that are missing at random; and (3) as we suspected that patients with chronic pain would be markedly different than those with no chronic pain, we selected this method because it is the best regression technique available when interactions between variables are predicted to be asymmetric [Citation28]. The tree was optimized by least absolute deviation with a 10-fold cross validation. The split criteria were 10 for parent node and 5 minimum for the terminal nodes to mitigate over fitting of the model [Citation28]. The variables included in the tree model were demographics (age, genotype), SCD characteristics (presence of chronic pain, number of ED or hospital admissions in the last 12 months, education level, employment level, and substance use), and HRQOL scores (PHQ-15, SF-36, MCS, PCS, PHQ-9, GAD-7). Median and interquartile range (IQR) were used as a summary of continuous or ordinal variables. A two-sided p-value of < 0.05 was considered as significant. Statistical analyses were done by using SAS 9.4, SPSS 23 for Windows and Salford Systems CART.
Results
Study subjects
Ninety-nine adults with SCD were administered questionnaires to assess somatic symptom burden, depression, anxiety and HRQOL (). Most of the cohort was female (65%), the majority had HbSS (76%), and about half were prescribed hydroxyurea. Most subjects in this cohort were at least high school educated (57%), and a minority of subjects reported a prior mental health diagnosis (25%). When subjects were categorized by MME, those in the ≥ 90 MME/day group, compared to < 90 MME/day, had a higher prevalence of unemployment (N = 41, 83%, p = 0.010) as well as chronic pain (N = 47, 96%, p < 0.0001). And, although more in the ≥ 90 MME/day group (N = 27, 55%, p = 0.006) were treated with hydroxyurea or chronic transfusions (N = 15, 31%), the median rate of ED (2 admissions/year, p = 0.015) and hospital admissions (2 admissions/year, p < 0.0001) was greater in the ≥ 90 MME/day group compared to the < 90 MME/day group.
Table 1. Patient demographics by daily opioid dose (MME) overall and for those with chronic pain.
Relationship between PHQ-15, SF-36, PHQ-9, GAD-7 and opioid dose
Overall, the adult cohort demonstrated deficits in SF-36 physical role limitations, pain, and general health scale scores compared to the general US population (mean of 50) (). The cohort overall also had moderate somatic burden (median score, IQR = 12, 8–16), mild anxiety (median score, IQR = 5, 2–9) and depression (median score, IQR = 6, 2–10).
Table 2. Health related quality of life scores by daily opioid use overall and for those with chronic pain.
When subjects were compared by opioid dose, those in the ≥ 90 MME/day group had a significantly diminished physical functioning, physical role, emotional role, mental health, social functioning, pain, and general health. The ≥ 90 MME/day group also reported significantly greater somatic symptom burden, depression, and anxiety ().
Since chronic pain confounds the relationship between opioid dose and HRQOL, we also chose to examine only those subjects who were diagnosed with chronic pain (N = 65). Among subjects with chronic pain, those who were treated with ≥ 90 MME/day reported a lower metal health score (SF-36), and higher median somatic burden (PHQ-15) and depression (PHQ-9) scores in comparison to those using < 90 MME/day. ≥ 90 MME/day users in this cohort also had more hospital utilization for pain (p = 0.01).
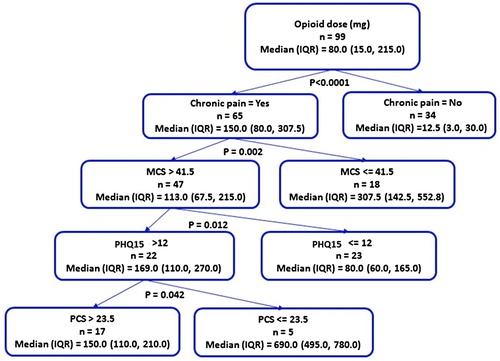
Classification tree analysis to predict opioid use
To examine the important predictors of opioid dose, a multi-variable analysis was completed by using CART. In the best fit model, the presence of chronic pain was the most important predictor of opioid dose (). Among those with chronic pain, a low SF-36 mental health composite score (MCS, p = 0.002) was the next most important predictor, followed by high somatic burden (PHQ15, p = 0.012), and a low SF-36 physical health composite score (PCS, p = 0.042).
Figure 1. Regression tree analysis representing the significant predictors of median daily opioid dose in adult patients with SCD (N = 99). The diagnosis of chronic pain is the most important predictor of opioid dose. Other key predictors include a low SF-36 mental component summary score (MCS, p = 0.002), high somatic burden (PHQ15, p = 0.012), and a low SF-36 physical component summary score (PCS, p = 0.042).
Discussion
In this study, the daily use of opioid doses ≥ 90 MME/day was associated with a significantly higher somatic symptom burden, depression, anxiety, and overall worse HRQOL. Chronic pain confounded these relationships; however, even among those with chronic pain, an opioid dose ≥ 90 MME/day was associated with worse mental health, somatic burden, and depression. Many adults with SCD require long-term opioid treatment for management of acute and chronic pain [Citation29]. Over time, opioid dose may escalate because of perceived tolerance, persistent pain, or repeat admissions on moderate doses [Citation29]. Although no therapeutic ceiling is known to exist for opioids, there is also little evidence to suggest that escalation to high doses in the general population provides long-term benefit [Citation30–35]. Although many adults with SCD suffer from severe, daily pain, our data suggests that these higher daily opioid doses may not meaningfully improve the quality of life for these patients.
Higher opioid dose was associated with worse HRQOL in our study. In the general population, it is well known that high-dose opioids are associated with more side effects, a higher risk of overdose, and opioid-induced hyperalgesia, all of which may impact quality of life [Citation30–35]. Similar to Dampier et al. [Citation36] and the PiSCES study [Citation9], we found that opioid use was associated with significantly diminished HRQOL scale scores. What our study adds is the impact of opioid dose. In patients with SCD, HRQOL is known to be worse than the general population, and that other highly inter-related factors, such as daily pain, depression, advancing age, somatic burden, substance abuse, and now opioid dose, may magnify this effect [Citation7,Citation8,Citation37–39].
The impact of chronic pain and opioid use in adults with SCD is difficult to tease apart because the role of opioids and the perception of pain is complex. Previous studies have found that daily pain and opioid use were associated with similar decrements to SF-36 scores [Citation39]. Pain is also independently associated with decrements in HRQOL in other chronic disorders including rheumatoid arthritis [Citation40], inflammatory bowel disease [Citation41], and AIDS [Citation42]. Although it is well established that pain is the most common symptom in adults with SCD, the mechanism(s) of pain, in particular chronic pain in this population for which there is often no anatomic correlate, is an area of active investigation [Citation43,Citation44]. A significant contributor to the chronic pain seen in adults with SCD is peripheral and central sensitization, which is secondary to ischemia and inflammation of nerves, persistent pain input, as well as the effects of long-term opioids [Citation45–47]. In other chronic pain disorders, high-dose opioids are known to cause opioid-induced hyperalgesia, a condition in which changes occur in the peripheral and central nervous system so they become sensitive to pain [Citation12,Citation13,Citation48]. Distinct from opioid tolerance, which is characterized by the requirement of higher doses of opioids to achieve a similar level of pain control, opioid-induced hyperalgesia is the paradoxical association between high-dose opioids and pain with non-painful or minimally painful stimuli [Citation12,Citation13,Citation49]. Potentially, higher opioid doses in our study further contributed to a worsened pain syndrome, as evidenced by the higher somatic symptom burden and lower SF-36 pain score, as well as the higher number of ED and hospital admissions in the ≥ 90 MME/day group compared to the < 90 MME/day group.
Opioid dose escalation occurs in adults with SCD in response to tolerance and/or continued pain [Citation29]. Tolerance to opioids may develop in adults with SCD because they are managed with opioids long-term, sometimes for decades, unlike patients who are treated with short courses of opioids post-operatively or for terminal cancer. Adults with SCD are already known to use higher doses of opioids than other chronic pain populations [Citation50]. After years of treatment and multiple escalations in dose, the amount of oral morphine equivalents prescribed to an adult with SCD, as seen in our patient population, can be high.
Our study had limitations. A key limitation is that we are unable to determine whether worse HRQOL was due to the use of high-dose opioids, chronic pain, or a combination of both. Potentially, the relationship between high-dose opioids and worse HRQOL simply reflects a higher percentage of adults with chronic pain, or poorly controlled pain symptoms. However, our multivariate model and analysis of only chronic pain patients suggests that opioid dose ≥ 90 MME/day is independently associated with worse HRQOL. Additionally, because we chose to administer well-established measures to address HRQOL, we were limited to the questions in these surveys. Important corollary questions about pain history and modulators of pain were not collected.
Conclusion
In conclusion, opioid use of ≥ 90 MME/day was associated with worsened HRQOL in adults with SCD. Although we cannot determine with absolute certainty whether lower HRQOL scores were due to opioid dose versus chronic pain, being mindful of opioid dose is good clinical practice. Fifty percent of adults with SCD have a severe chronic pain syndrome. In the era of the ‘opioid epidemic,’ however, some providers may be reluctant to prescribe opioids for patients with SCD, despite the fact that, in many cases, it may be appropriate. The opioid epidemic was not caused by patients with SCD, and opioids remain a cornerstone in the management of SCD pain. However, the benefits of long-term opioid therapy, especially high-dose opioid therapy, have not been established in patients with SCD, or any chronic pain population. For this reason, any benefit in terms of pain relief from an escalation in opioid dose should be weighed against the potential risks.
Supplemental Material
Download TIFF Image (135.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Arun Singavi http://orcid.org/0000-0002-5634-5951
Additional information
Funding
References
- Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000;151:839–845. doi: 10.1093/oxfordjournals.aje.a010288
- Hassell K. Sickle cell disease population estimation: application of available contemporary data to traditional methods. In: 35th Anniversary Convention of the National Sickle Cell Disease Program. Washington, DC; 2007.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–769. doi: 10.1056/NEJM199709113371107
- Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. doi: 10.1002/ajh.20336
- Sogutlu A, Levenson JL, McClish DK, et al. Somatic symptom burden in adults with sickle cell disease predicts pain, depression, anxiety, health care utilization, and quality of life: the PiSCES project. Psychosomatics. 2011;52:272–279. doi: 10.1016/j.psym.2011.01.010
- Levenson JL, McClish DK, Dahman BA, et al. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosom Med. 2008;70:192–196. doi: 10.1097/PSY.0b013e31815ff5c5
- Smith WR, McClish DK, Dahman BA, et al. Daily home opioid use in adults with sickle cell disease: the PiSCES project. J Opioid Manag. 2015;11(3):243–253. doi: 10.5055/jom.2015.0273
- Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain [Evidence Report/Technology Assessment No. 218]. Agency for Healthcare Research and Quality. Available from: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/chronic-pain-opioid-treatment_research.pdf.
- Multiple cause of death data. CDCWONDER. Centers for Disease Control and Prevention. Available from: http://wonder.cdc.gov/mcd.html.
- Hutchinson MR, Bland ST, Johnson KW, et al. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci World J. 2007;7:98–111. doi: 10.1100/tsw.2007.230
- Hutchinson MR, Shavit Y, Grace PM, et al. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. doi: 10.1124/pr.110.004135
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737
- Lowe B, Grafe K, Zipfel S, et al. Detecting panic disorder in medical and psychosomatic outpatients: comparative validation of the hospital anxiety and depression scale, the patient health questionnaire, a screening question, and physicians’ diagnosis. J Psychosom Res. 2003;55:515–519. doi: 10.1016/S0022-3999(03)00072-2
- Lowe B, Spitzer RL, Grafe K, et al. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians? diagnoses. J Affect Disord. 2004;78:131–140. doi: 10.1016/S0165-0327(02)00237-9
- Kroenke K, Spitzer RL, Williams JBW, et al. The patient health questionnaire somatic, anxiety, and depressive symptom scales (PHQ-SADS): a systematic review. Gen Hosp Psychiatry. 2010;32:345–359. doi: 10.1016/j.genhosppsych.2010.03.006
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Int Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002
- Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the international quality of life assessment (IQOLA) project. Qual Life Res. 2004;13(2):283–298. doi: 10.1023/B:QURE.0000018472.46236.05
- Rijken M, van Kerkhof M, Dekker J, et al. Comorbidity of chronic diseases: effects of disease pairs on physical and mental functioning. Qual Life Res. 2005;14(1):45–55. doi: 10.1007/s11136-004-0616-2
- Dampier C, Lieff S, LeBeau P, et al. Health-related quality of life in children with sickle cell disease: a report from the comprehensive sickle cell centers clinical trial consortium. Pediatr Blood Cancer. 2010;55(3):485–494. doi: 10.1002/pbc.22497
- Asnani MR, Lipps GE, Reid ME. Validation of the SF-36 in Jamaicans with sickle-cell disease. Psychol Health Med. 2009;14(5):606–618. doi: 10.1080/13548500903016567
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092
- Afenyi-Annan A, Kail M, Combs MR, et al. Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease. Transfusion. 2008;48(5):917–924. doi: 10.1111/j.1537-2995.2007.01622.x
- Dampier C, Palermo TM, Darbari DS, et al. Aapt diagnostic criteria for chronic sickle cell disease pain. J Pain. 2017;18(5):490–498. doi: 10.1016/j.jpain.2016.12.016
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464
- Cook EF, Goldman L. Empiric comparison of multivariate analytic techniques: advantages and disadvantages of recursive partitioning analysis. J Chronic Dis. 1984;37:721–731. doi: 10.1016/0021-9681(84)90041-9
- Field JJ. Five lessons learned about long-term pain management in adults with sickle cell disease. Hematology Am Soc Hematol Educ Program. 2017;2017(1):406–411.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–1953. doi: 10.1056/NEJMra025411
- Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100(3):213–217. doi: 10.1016/S0304-3959(02)00422-0
- Gwira Baumblatt JA, Wiedeman C, Dunn JR, et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796–801. doi: 10.1001/jamainternmed.2013.12711
- Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370
- Dart RC, Severtson SG, Bucher-Bartelson B. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(16):1573–1574.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med. 2015;162(4):276–286. doi: 10.7326/M14-2559
- Dampier C, LeBeau P, Rhee S, et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011;86(2):203–205. doi: 10.1002/ajh.21905
- Adam SS, Flahiff CM, Kamble S, et al. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Adv. 2017;1(23):1983–1992. doi: 10.1182/bloodadvances.2017006940
- Levenson JL, McClish DK, Dahman BA, et al. Alcohol abuse in sickle cell disease: the pisces project. Am J Addict. 2007;16(5):383–388. doi: 10.1080/10550490701525434
- Barakat LP, Patterson CA, Daniel LC, et al. Quality of life among adolescents with sickle cell disease: mediation of pain by internalizing symptoms and parenting stress. Health Qual Life Outcomes. 2008;6:60. doi: 10.1186/1477-7525-6-60
- Rupp I, Boshuizen HC, Jacobi CE, et al. Comorbidity in patients with rheumatoid arthritis: effect on health-related quality of life. J Rheumatol. 2004;31:58–65.
- Palm O, Bernklev T, Moum B, et al. Non-inflammatory joint pain in patients with inflammatory bowel disease is prevalent and has a significant impact on health related quality of life. J Rheumatol. 2005;32:1755–1759.
- Rosenfeld B, Breitbart W, McDonald MV, et al. Pain in ambulatory AIDS patients. II: impact of pain on psychological functioning and quality of life. Pain. 1996;68:323–328. doi: 10.1016/S0304-3959(96)03220-4
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer. 2015;62(9):1501–1511. doi: 10.1002/pbc.25574
- Tran H, Gupta M, Gupta K. Targeting novel mechanisms of pain in sickle cell disease. Blood. 2017;130(22):2377–2385. doi: 10.1182/blood-2017-05-782003
- Treede RD, Meyer RA, Raja SN, et al. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-C
- Brandow AM, Farley R, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer. 2014;61:512–517. doi: 10.1002/pbc.24838
- Ezenwa MO, Molokie RE, Wang ZJ, et al. Safety and utility of quantitative sensory testing among adults with sickle cell disease: indicators of neuropathic pain? Pain Pract. 2016;16(3):282–293. doi: 10.1111/papr.12279
- Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 2001;93(1):204–209. doi: 10.1097/00000539-200107000-00040
- Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679–684.
- Carroll CP, Lanzkron S, Haywood C, et al. Chronic opioid therapy and central sensitization in sickle cell disease. Am J Prev Med. 2016;51:S69–S77. doi: 10.1016/j.amepre.2016.02.012
