ABSTRACT
Objectives: The effect of methotrexate (MTX)-related adverse reaction on hematologic neoplasms patients is controversial. We performed this meta-analysis to assess the association between methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C polymorphism and the adverse reaction after MTX using.
Methods: We searched for qualified studies according to PubMed, the Cochrane Library, and the Web of Science. The meta-analysis was performed by Review Manager 5.3. The analysis was conducted to compare risk ratios (RRs) with the corresponding 95% confidence interval (95% CI) to evaluate the relationship between different toxicity reactions and the genotype of MTHFR.
Results: We included 17 studies which satisfied with the criteria in this meta-analysis. The results of our statistical analysis showed that no significant correlation between MTHFR C677T/A1298C genetic polymorphism and patients’ toxicity or the relapse and survival associated with MTX chemotherapy (P > .05). But we observed that a tendency toward increased risk of hepatotoxicity was also present for acute lymphoblastic leukemia in the mutation model (CT/TT vs. CC: RR: 1.92, 95% CI: 1.01–3.67; P = .05).
Conclusion: The polymorphism of MTHFR C677T/A1298C may not be an important indicator for the accurate detection of side effects of chemotherapy after using MTX. More relative research is needed.
Introduction
Hematological malignancies are a group of neoplastic diseases that occur in the hematopoietic system, hematopoietic tissues, and organs, including leukemia, lymphoma, and bone marrow proliferative tumor. Acute lymphoblastic leukemia (ALL) is a common malignant tumor; it has an extremely high incidence over the world, especially in children [Citation1].
Methotrexate (MTX) is widely used in the treatment of rheumatoid arthritis, psoriasis, and hematological malignancies. Because of the highly competitive combination with dihydrofolate reductase, MTX can interfere with the synthesis of DNA, RNA, and protein in cells [Citation2]. Since the 1870s, different doses of MTX for treating ALL were widely applied in clinics. Data showed that 5-year event-free survival (EFS) and overall survival (OS) of patients after chemotherapy were significantly improved [Citation3]. However, a large number of patients had been reported after chemotherapy with MTX had different kinds of adverse reactions. Like many anti-tumor medications, MTX is associated with severe toxicities, including toxicities of the central nervous system (CNS), liver, bone marrow, and gastrointestinal system, particularly oral mucosa. And people had different reactions after MTX administration; this suggests that pharmacogenomics may be one of the important factors affecting the adverse effects of MTX on hematological malignancy patients [Citation4].
Methylenetetrahydrofolate reductase (MTHFR) is an important enzyme involved in the folic acid cycle. It can catalyze the reduction of 5-methyl-10-methyl-THF to 5-methyl-THF. The association of MTHFR genetic polymorphism and toxicities after giving MTX has also become a hot issue. In recent years, studies mainly focus on two single-nucleotide polymorphisms: 677C>T (rs1801133) and 1298A>C (rs1801131). The mutation of 677 C/T in MTHFR gene resulted in the substitution of alanine by valine, which can increase the intracellular homocysteine concentration and change the folic acid distribution. Glutamic acid will be replaced by alanine when base A mutates into base C in the MTHFR 1298 site, and it may cause the reduction of MTHFR activity [Citation5]. Related reports showed that patients with MTHFR 677TT are associated with MTX clearance and are associated with the increased risk of mucositis, anemia, and so on [Citation6–8]. There were also partial reports that suggested that the MTHFR genetic polymorphism may not be an accurate predictor for MTX-related toxicity [Citation9,Citation10].
Toxicity may cause chemotherapy to be interrupted, thereby increasing the risk for relapse, which can result in patients’ poor quality of life. According to this meta-analysis, we collected relevant literature data and expanded the sample size to analyze the correlation between MTHFR C677T/A1298C polymorphism and MTX-induced toxicities, and the relationship between MTHFR polymorphism and relapse/survival of patients after receiving MTX.
Materials and methods
Search strategy
Relevant studies were published since the establishment of the library in January 2018 on the Electric database, including PubMed, Web of Science, and Cochrane. Subjects were searched using the following keywords: ‘methotrexate’ or ‘MTX’, ‘methylenetetrahydrofolate reductase’ or ‘MTHFR’ and ‘polymorphism’. The language was restricted to English. We also searched the reference lists from the previous meta-analysis to evaluate more potential articles. The meta-analysis protocol for this study was registered on Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD42018091337).
Selection criteria
The retrieved data sources were screened independently by two researchers (P.Y. and X.H.), and the obviously irrelated studies would be removed after reading topics and abstracts. Then, two evaluators read the full text for further evaluation. Papers would be brought into our meta-analysis once it meets the selection criteria. The third researcher (R.Z.) would be asked for evaluation when any disagreement aroused between the two reviewers.
Inclusion: (1) All published clinical studies focused on the relationship between MTHFR C677T/A1298C genetic polymorphism and the occurrence of an adverse reaction after offering MTX; (2) The diseases were limited to hematologic neoplasms; (3) Detailed data about the genotyping and adverse reactions would be required.
Exclusion: (1) Abstracts, summaries, unpublished reports, documents that cannot be extracted from exhaustive data, and repeated research; (2) Other non-hematological malignancies.
Data extraction and quality assessment
These following information characteristics were included: the first author, year and country of publication, sample size (male/female), patients (children/adults), age of the sample, MTX dosage, diseases, types of toxicity criteria, genotype, MTX-related toxicities, relapse data, and survival data.
The Newcastle–Ottawa Quality Assessment Scale [Citation11] (NOS) was applied to assess the selected studies, which can be used to evaluate case–control and cohort studies. The whole evaluation composed of multiple questions assessing the potential risk for selection bias, comparability bias, and outcome bias. Reviewers then used these questions to judge each study. The ⋆ represents scores: a full score of 9 points and the comparability is a maximum of two ⋆. The specific issues are as follows: point 1: representativeness of the exposed cohort; point 2: selection of the non-exposed cohort; point 3: ascertainment of exposure; point 4: demonstration that outcome of interest was not present at start of study; points 5 and 6: comparability of cohorts on the basis of the design or analysis; point 7: assessment of outcome; point 8: was follow-up long enough for outcomes to occur; point 9: adequacy of follow-up of cohorts. Researchers appraised them according to principles; studies that scored more than six points had a good research value and would be engaged in the meta-analysis owing to the poorer impact of other factors such as selection bias, comparability bias, and outcome bias [Citation12].
Statistical analysis
Statistical analysis was performed with Review Manager (Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark). We examined only those groups that contained more than three articles according to the data in the study. Adverse reactions mainly refer to the incidence of total adverse reactions, neutropenia, hepatotoxicity, gastrointestinal reactions, and mucositis.
The results were expressed as risk ratio (RR) with corresponding 95% confidence interval (CI) to assess the association between toxicity, relapse, survival, and MTHFR genetic polymorphism. The chi-square test (Q statistic) and I2 statistic were performed to assess heterogeneity. If P ≥ .10 and/or I2 ≤ 50%, the heterogeneity was recognized to be too low and we would select the fixed-effect model. If not, we would choose the random-effects model. For evaluating association, a Z-test was performed to determine the statistical significance of RRs and a P-value < .05 was considered to be statistically significant. When I2 ≤ 50%, subgroups (children, HD-MTX dosage, ALL) would be analyzed again. Considering the large number of groups in this meta-analysis, forest map would only be present when the groups contain more than five articles.
Results
Study characteristics
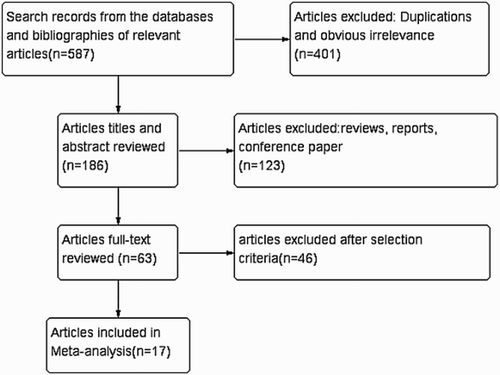
A total of 587 related documents were retrieved through our initial search. After reading articles and screening in accordance with inclusion and exclusion criteria, 17 studies were included in our meta-analysis. The flow chart of the screening process is described in .
Quality assessment was conducted for all the 17 included studies. The full mark is 9 points, and scores greater than 6 were included in our study judged by the NOS. Specific assessment results are shown in . Only three articles had the highest points; others were not appropriate in terms of comparability and the follow-up time of outcome.
Table 1. Quality of studies included in the meta-analysis.
The 17 articles were all cohort studies published since 2007–2016, with a total of 2133 patients included. These studies had a broad research background: three of them were published in Italy, two in Japan, another two in Egypt, and other countries like China, Brazil, and Korea also been covered. Four of them investigated adults or children and adults; others were only restricted to pediatrics. Studies mainly focused on the treatment of ALL with a high dose of MTX, but there were still eight articles in which the dosage of MTX was less than 1 g m−2 or the dosage was not clear, and four articles were concerned with other diseases. We would discuss in subgroups for the differences among subjects, dosage, and disease in the later analysis. World Health Organization (WHO) evaluation standard was used to appraise toxicity while most researchers chose the National Cancer Institute–Common Terminology Criteria for Adverse Events (NCI-CTCAE). Details are shown in .
Table 2. Characteristics of studies included in the meta-analysis study.
Adverse reaction
Correlation between MTHFR C677T and MTX-related toxicity (CC vs. CT + TT)
All the 17 studies measured the association between MTHFR C677T polymorphism and MTX-induced toxicities. Detailed data of mutation group and wild group models regarding different toxicities are summarized in . Four articles [Citation13,Citation20,Citation21,Citation29] described the occurrence of overall adverse reactions, and six articles [Citation17,Citation18,Citation23,Citation25–27] had detailed statistics on neutropenia. There were nine articles [Citation14–18,Citation23,Citation24,Citation26,Citation28] with statistics on hepatotoxicity, of which three were studies involving adults, four involved unknown dose or low dose group, and four involved other disease groups. Subgroup analysis would be performed again after getting rid of adult subjects, MTX dosage was too low or not clear, and other diseases. However, we did not observe any correlation between the genetic polymorphism of MTHFR C677T and the occurrence of adverse reactions after MTX treatment (P > .05). Without the interference of other diseases, a tendency toward increased risk of hepatotoxicity was also present for ALL disease in the mutation model (CT/TT vs. CC: RR: 1.92, 95% CI: 1.01–3.67; P = .05). For hepatotoxicity grades 1–4, no pattern could be detected in the limited available data from the included studies, and further stratified analysis based on ethnicity was not possible.
Table 3. The association between MTHFR C677T and MTX-induced toxicities in patients with malignancies.
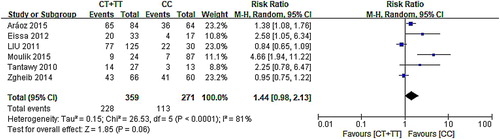
The forest map of the correlation between C677T and neutropenia in six articles showed that 95% CI of three groups of data intersected with invalid lines, indicating that C677T had nothing to do with neutropenia, and 95% CI of the other three studies were distributed on the right side of the invalid line. These results suggest that the mutation of C677T may cause the occurrence of neutropenia. The results of the meta-analysis showed that six had heterogeneity (χ2 = 26.53, P < .0001) adopting the random-effect model (CT/TT vs. CC: RR: 1.44, 95% CI: 0.98–2.13; P = .06). According to the analysis results (), MTHFR C677T can be considered as having no correlation with neutropenia.
The forest map of the correlation between C677T and hepatotoxicity in nine articles showed that 95% CI of six groups of data intersected with invalid lines, indicating that C677T had nothing to do with hepatotoxicity, and 95% CI of the other three studies were distributed on the right side of the invalid line suggesting that C677T mutation may cause hepatotoxicity. The results of the meta-analysis showed that nine data had heterogeneity (χ2 = 32.28, P < .01). The random-effect model was used (CT/TT vs. CC: RR: 1.44, 95% CI: 0.88–2.83; P = .15). According to the results () of this analysis, it can be concluded that MTHFR C677T had no correlation between polymorphism and hepatotoxicity.
Figure 3. The forest map of association between C677T polymorphism and hepatotoxicity and the subgroups.
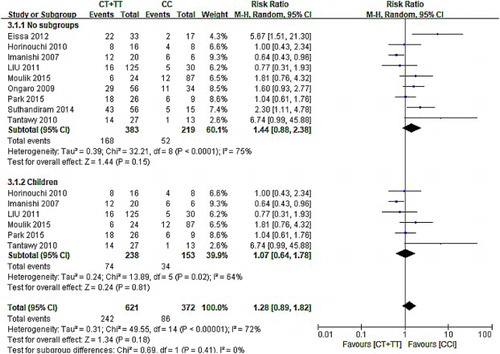
In addition, we analyzed it again after excluding the data from adult patients. The results showed that there is no correlation between C677T polymorphism and hepatotoxicity in children with hematologic tumor (CT/TT vs. CC: RR: 1.28, 95% CI: 0.89–1.82; P = .18).
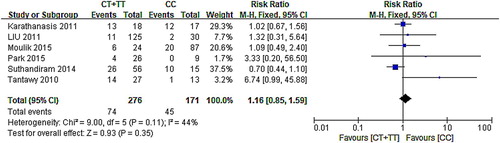
A forest map of six articles [Citation17–19,Citation24,Citation26,Citation28] showed that the 95% CI of the five sets of data intersect the null line, and the other studies’ 95% CI were distributed on the right side of the null line, indicating that the mutation of C677T can be considered to be a result of the mutation. The results of the meta-analysis showed that six of the research had homogeneity (χ2 = 9.00, P = .11), performed using the fixed-effect model (CT/TT vs. CC: RR: 1.16, 95% CI: 0.85–1.59; P = .35). Results () indicated that the polymorphism of MTHFR C677T is not associated with mucositis.
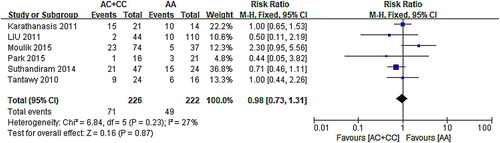
Correlation between MTHFR A1298C and MTX-related toxicity (AA vs. AC + CC).
Thirteen articles [Citation13,Citation15,Citation17–20,Citation22–26,Citation28] had an adequate analysis of A1298C polymorphism and adverse reactions after MTX treatment, five of these articles [Citation17,Citation18,Citation23,Citation25,Citation26] had a detailed statistics on neutropenia, seven [Citation15,Citation17,Citation18,Citation23,Citation24,Citation26,Citation28] for hepatotoxicity, and six [Citation17–19,Citation24,Citation26,Citation28] for mucositis. Similarly, no significant association was found between the MTHFR A1298C polymorphism and MTX-induced hematological toxicities (P > .05). No further stratified analysis based on ethnicity was performed because few studies were available in each comparison ().
Table 4. The association between MTHFR A1298C and MTX-induced toxicities in patients with malignancies.
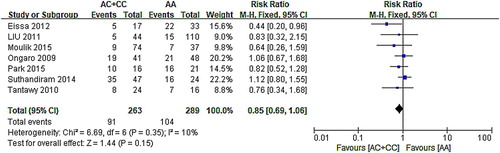
The relationship between A1298C and hepatotoxicity was studied in seven articles, six of which intersected with invalid lines. The other one’s 95% CI was distributed to the left of the invalid line, suggesting that the mutation of C677T may cause hepatotoxicity. The results of the meta-analysis showed that the seven data exist homogeneity (χ2 = 6.69, P = .35). Results () showed in the fixed-effect model (AC/CC vs. AA: RR: 0.85, 95% CI: 0.69–1.06; P = .15) that the polymorphism of MTHFR A1298C is not associated with hepatotoxicity.
The forest map of the correlation between A1298C and mucositis showed that 95% CI of six groups of data intersected with invalid lines. Meta-analysis results () indicated that A1298C had no relationship with mucositis (AC/CC vs. AA: RR: 0.98, 95% CI: 0.73–1.31; P = .87).
Relapse and survival
Besides, overall relapse and OS also be analyzed in our study. For C677T, five papers [Citation13,Citation17,Citation20,Citation21,Citation23] described the relapse and two of them were adults, and six papers [Citation13,Citation17,Citation22,Citation23,Citation26,Citation29] had an accurate description for OS. For A1298C, four articles [Citation13,Citation17,Citation20,Citation23] described the relapse and OS rate was counted in six articles [Citation13,Citation17,Citation22,Citation23,Citation26,Citation29]. As shown in , we did not find any correlation between polymorphism and the relapse and survival rate of the patients (P > .05).
Table 5. The association between MTHFR genetic polymorphism and relapse and survival.
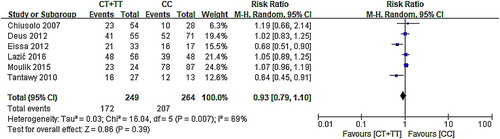
According to the forest map, 95% CI of three groups of data intersected with invalid lines indicated that C677T had no relationship with survival, while another two studies that were distributed on the left side of the invalid line suggested that CT/TT may be a hazard factor for patients with MTX. Owing to the high heterogeneity (χ2 = 16.04, P = .007), the RR of random effect model was used (CT/TT vs. CC: RR: 0.93, 95% CI: 0.79–1.10; P = .39). The results () of this analysis indicated that MTHFR C677T had no correlation between polymorphism and survival.
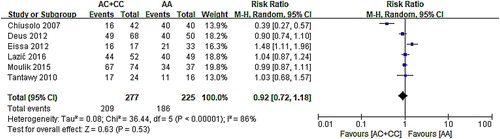
According to the forest map, 95% CI of five types of research intersected with invalid lines, indicating that A1298C had no relationship with survival, while another two studies were distributed apart on the left and right sides of the invalid line. Owing to the high heterogeneity (χ2 = 36.44, P < .0001), the RR of random effect model was used (AC/CC vs. AA: RR: 0.92, 95% CI: 0.72–1.18; P = .53). The results () of this analysis indicated that MTHFR A1298C had no correlation between polymorphism and survival.
Discussion
MTX has been widely used in most chemotherapy protocols for hematological malignancies. As a major component in current treatment protocols for childhood ALL, MTX, especially high-dose MTX (HD-MTX), is significantly associated with clinical success. At the same time, HD-MTX may cause toxicity, leading to a dose reduction or treatment interruption, which could compromise the survival [Citation18,Citation30]. Due to the higher dosage (>1.0 g m–2), clinicians paid more attention to HD-MTX toxicities, and there is extensive inter-patient variability in toxicity.
The detection of C677T genotypes has been used to judge the metabolic status of MTX in order to avoid serious adverse reactions for leukemia patients. In this meta-analysis, we cannot observe whether these two genetic polymorphisms may be associated with the occurrence of adverse reactions and the occurrence of relapse and survival after MTX using. But we cannot certainly conclude that there is no relationship between C677T and MTX-related hepatotoxicity (P = .05). This suggested that different results may be obtained when expanding the sample size.
While screening the literature, we found that four studies had a discussion on this issue. Yang et al. [Citation31] and Lopez-Lopez et al. [Citation9] performed two meta-analyses earlier. Yang et al. [Citation31] expounded that MTHFR C677T mutation was significantly correlated with liver toxicity, and A1298C was only associated with dermatotoxicity (mucositis) according to the research. Lopez-Lopez et al. [Citation9] limited the study to children with a higher prevalence rate, and the results showed that the mutation of C677T and A1298C was less correlated with the use of HD-MTX in children. Recently, Zhao et al. [Citation32] and Zhu et al. [Citation33] carried out two analyses in adults and children separately, and their results suggested that an obvious relativity existed between C677T and the occurrence of adverse reactions such as hepatotoxicity and gastrointestinal reactions but had no unified conclusion in A1298C. There is a bit similarity with our results that a tendency toward increased risk of hepatotoxicity was also present for ALL disease in the mutation model (CT/TT vs. CC: RR: 1.92, 95% CI: 1.01–3.67; P = .05). Compared with previous studies, our analysis has the following advantages. First, more documents are included and the sample size is increased. Second, adults’ data were included and group analysis of patients with large heterogeneity was carried out. The most important is that we performed analyzed the relapse and OS. Previous studies did not carry out a meta-analysis on this issue.
In the same way, we are wondering the reasons for various results produced in this meta-analysis. The major limitations of our study are as follows:
Host factors may also be one of the main reasons for negative results: the host’s own disease status, the specific medication plan, and medication compliance of chemotherapy may reduce the applicability and reliability of the research results.
The reference and evaluation criteria of adverse reactions in different studies may lead to differences in results.
The fact that the sample size of some studies is too small while some of the samples are too large means that clinical diversity may cause severe heterogeneity in our study.
Owing to the divergence that existed in the follow-up time and follow-up plan, there may be differences between the results.
In the whole process of MTX entering the body, besides MTHFR, there are many important enzymes and transportations that affect its metabolic and excretory pathway, such as FPGSG, GGH SLCO1B1, and so on. Whether the polymorphism of these genes will affect the effect of MTHFR in vivo and whether they were the interfering factors cannot be ruled out.
As mentioned above, the CNS or neurologic toxicity is a significant MTX-related toxicity, but what is interesting, there were only two papers according to the included articles [Citation28,Citation29]. Therefore, based on our meta-analysis principle, only the adverse reactions mentioned in more than the three articles were analyzed. Therefore, a meta-analysis of MTX-related neurologic toxicity was not performed in this article. Certainly, the publication time of these two articles is relatively new, suggesting that CNS toxicity may have attracted clinicians’ and patients’ attention in recent years. In China, according to clinical experience, HD-MTX-related neurologic toxicity is less observed, may suggest that the neurotoxic response induced by HD-MTX may be the direction we need to focus on in the future, and genetic polymorphisms may be an important factor in different performances among people.
Although most of our results are negative, this does not mean that our meta-analysis is meaningless. First of all, there may be a big problem with the sample selection. Perhaps, when we use larger cohorts, the results will be different, which suggests that future research needs to be carried out with a greater sample size and strict control of other influencing factors. In addition, because our analysis process is very strict, we believe that the results are quite credible in the case of ensuring the quality of the literature included. So, whether or not the polymorphism of MTHFR will affect the toxicity of MTHFR is worthy to be studied further.
Conclusion
The polymorphism of MTHFR C677T/A1298C may not be an important indicator for the accurate detection of side effects of chemotherapy after using MTX. However, we cannot directly conclude that the MTHFR C677T/A1298C polymorphism is not related to the side effects of MTX, survival rate, and recurrence rate. Therefore, high-quality articles with larger sample size and detailed statistical indicators are still needed.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Pingli Yao http://orcid.org/0000-0002-5786-7081
Additional information
Funding
References
- Pui CH. Genomic and pharmacogenetic studies of childhood acute lymphoblastic leukemia. Front Med. 2015;9:1–9. doi: 10.1007/s11684-015-0381-3
- Fotoohi AK, Albertioni F. Mechanisms of antifolate resistance and methotrexate efficacy in leukemia cells. Leukemia Lymphoma. 2008;49(3):410–426. doi: 10.1080/10428190701824569
- Pui CH, Campana D, Pei D, et al. Treatment of childhood acute lymphoblastic leukemia without prophylactic cranial irradiation. New Engl J Med. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386
- Relling M V, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol 1994;12(8):1667. doi: 10.1200/JCO.1994.12.8.1667
- El-Khodary NM, El-Haggar SM, Eid MA, et al. Study of the pharmacokinetic and pharmacogenetic contribution to the toxicity of high-dose methotrexate in children with acute lymphoblastic leukemia. Med Oncol. 2012;29(3):2053–2062. doi: 10.1007/s12032-011-9997-6
- Wang S M, Sun L L, Zeng W X, et al. Influence of genetic polymorphisms of FPGS, GGH, and MTHFR on serum methotrexate levels in Chinese children with acute lymphoblastic leukemia. Cancer Chem Pharmacology. 2014;74(2):283–289. doi: 10.1007/s00280-014-2507-8
- Ayad MW, El Naggar AA, El NM. MTHFR c677T polymorphism: association with lymphoid neoplasm and effect on methotrexate therapy. Eur J Haematol. 2014;93(1):63–69. doi: 10.1111/ejh.12302
- Haase R, Elsner K, Merkel N, et al. High dose methotrexate treatment in childhood ALL: pilot study on the impact of the MTHFR 677C>T and 1298A>C polymorphisms on MTX-related toxicity. Klin Paditr. 2012;224(03):156–159. doi: 10.1055/s-0032-1304623
- Lopez-Lopez E, Martinguerrero I, Ballesteros J, et al. A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Pharmacogenomics J. 2013;13(6):498–506. doi: 10.1038/tpj.2012.44
- Shimasaki N, Mori T, Torii C, et al. Influence of MTHFR and RFC1 polymorphisms on toxicities during maintenance chemotherapy for childhood acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol. 2008;30(5):347–352. doi: 10.1097/MPH.0b013e318165b25d
- Wells GA, Shea B, O'connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. 2014 [cited 2018 March 24]. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z
- Chiusolo P, Reddiconto G, Farina G, et al. MTHFR polymorphisms’ influence on outcome and toxicity in acute lymphoblastic leukemia patients. Leukemia Res. 2007;31(12):1669–1674. doi: 10.1016/j.leukres.2007.03.028
- Imanishi H, Okamura N, Yagi M, et al. Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet. 2007;52(2):166–171. doi: 10.1007/s10038-006-0096-z
- Ongaro A, De MM, Della Porta MG, et al. Gene polymorphisms in folate metabolizing enzymes in adult acute lymphoblastic leukemia: effects on methotrexate-related toxicity and survival. Haematologica. 2009;94(10):1391–1398. doi: 10.3324/haematol.2009.008326
- Horinouchi M, Yagi M, Imanishi H, et al. Association of genetic polymorphisms with hepatotoxicity in patients with childhood acute lymphoblastic leukemia or lymphoma. Pediatric Hemat Oncol. 2010;27(5):344. doi: 10.3109/08880011003739422
- Tantawy AA, El-Bostany EA, Adly AA, et al. Methylene tetrahydrofolate reductase gene polymorphism in Egyptian children with acute lymphoblastic leukemia. Blood Coagul Fibrin. 2010;21(1):28–34. doi: 10.1097/MBC.0b013e32833135e9
- Liu SG, Li ZG, Cui L, et al. Effects of methylenetetrahydrofolate reductase gene polymorphisms on toxicities during consolidation therapy in pediatric acute lymphoblastic leukemia in a Chinese population. Leukemia Lymphoma. 2011;52(6):1030–1040. doi: 10.3109/10428194.2011.563883
- Karathanasis NV, Stiakaki E, Goulielmos GN, et al. The role of the methylenetetrahydrofolate reductase 677 and 1298 polymorphisms in Cretan children with acute lymphoblastic leukemia. Genetic Test Mol Bioma. 2011;15(1–2):5. doi: 10.1089/gtmb.2010.0083
- D'Angelo V, Ramaglia M, Iannotta A, et al. Methotrexate toxicity and efficacy during the consolidation phase in paediatric acute lymphoblastic leukaemia and MTHFR polymorphisms as pharmacogenetic determinants. Cancer Chem Pharmacol. 2011;68(5):1339–1346. doi: 10.1007/s00280-011-1665-1
- Sepe DM, Mcwilliams T, Chen J, et al. Germline genetic variation and treatment response on CCG-1891. Pediatric Blood Cancer. 2012;58(5):695–700. doi: 10.1002/pbc.23192
- Deus DD, Lima ED, Silva RS, et al. Influence of methylenetetrahydrofolate reductase C677T, A1298C, and G80A polymorphisms on the survival of pediatric patients with acute lymphoblastic leukemia. Leuk Res Treat. 2012;2012:1–6.
- Eissa DS, Ahmed TM. C677T and A1298C polymorphisms of the methylenetetrahydrofolate reductase gene: effect on methotrexate-related toxicity in adult acute lymphoblastic leukaemia. Blood Coagul Fibrinolysis. 2013;24(2):181–188.
- Suthandiram S, Gan GG, Zain SM, et al. Effect of polymorphisms within methotrexate pathway genes on methotrexate toxicity and plasma levels in adults with hematological malignancies. Pharmacogenomics. 2014;15(11):1479–1494. doi: 10.2217/pgs.14.97
- Zgheib NK, Akraismail M, Aridi C, et al. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharmacogenetics Genom. 2014;24(8):387.
- Roy MN, Kumar A, Agrawal S, et al. Role of folate status and methylenetetrahydrofolate reductase genotype on the toxicity and outcome of induction chemotherapy in children with acute lymphoblastic leukemia. Leukemia Lymphoma. 2015;56(5):1379–1384. doi: 10.3109/10428194.2014.947608
- Aráoz HV, D'Aloi K, Foncuberta ME, et al. Pharmacogenetic studies in children with acute lymphoblastic leukemia in Argentina. Leukemia Lymphoma. 2015;56(5):1370–1378. doi: 10.3109/10428194.2014.951844
- Park JA, Shin HY. Influence of genetic polymorphisms in the folate pathway on toxicity after high-dose methotrexate treatment in pediatric osteosarcoma. Blood Res. 2016;51(1):50–57. doi: 10.5045/br.2016.51.1.50
- Lazic J, Kotur N, Krstovski N, et al. Importance of pharmacogenetic markers in the methylenetetrahydrofolate reductase gene during methotrexate treatment in pediatric patients with acute lymphoblastic leukemia. Arch Biol Sci. 2017;69(2):239–246. doi: 10.2298/ABS160325091L
- Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405
- Yang L, Hu X, Xu L. Impact of methylenetetrahydrofolate reductase (MTHFR) polymorphisms on methotrexate-induced toxicities in acute lymphoblastic leukemia: a meta-analysis. Tumour Biol. 2012;33:1445–1454. doi: 10.1007/s13277-012-0395-2
- Zhao M, Liang L, Ji L, et al. MTHFR gene polymorphisms and methotrexate toxicity in adult patients with hematological malignancies: a meta-analysis. Pharmacogenomics. 2016;17(9):1005–1017. doi: 10.2217/pgs-2016-0004
- Zhu C, Liu Y W, Wang S Z, et al. Associations between the C677T and A1298C polymorphisms of MTHFR and the toxicity of methotrexate in childhood malignancies: a meta-analysis. Pharmacogenomics J. 2017;18(3):450–459. doi: 10.1038/tpj.2017.34