ABSTRACT
Background: Increasing evidence has suggested that miR-29b plays an antitumor effect in multiple malignancies via the regulation of cell proliferation, apoptosis, invasion and migration. In the present study, we aimed to explore the underlying function and mechanism of miR-29b in multiple myeloma (MM).
Methods: The expression of miR-29b in MM cell lines and tissues was detected by quantitative real-time PCR (qRT–PCR). CCK-8 and flow cytometry analyses were performed to assess cell proliferation, cycles and apoptosis. Bioinformatics, Dual-Luciferase reporter and qRT–PCR assays were employed to explore the possible correlation between miR-29b and FOXP1. Xenograft model was established to confirm the role of miR-29b on tumor growth in vivo.
Results: miR-29b expression was markedly decreased in MM cell lines and tissues and downregulated miR-29b was closely related to International Staging System (ISS) stages. Exogenous overexpression of miR-29b inhibited the proliferation but induced MM cell cycle arrest and apoptosis. FOXP1 was identified as a direct target gene for miR-29b, and restoration of FOXP1 weakened miR-29b-induced anti-proliferation and pro-apoptosis in MM cell lines. Finally, the inhibitory effects of miR-29b on the growth of MM tumors were validated in mice.
Conclusions: miR-29b recedes the progression of MM via downregulating FOXP1, which may provide a potential biological target for MM treatment.
KEYWORDS:
1. Introduction
Multiple myeloma (MM) is a common hematological malignant disease, characterized by the abnormal proliferation of malignant plasma cells in bone marrow (BM) along with the overproduction of monoclonal immunoglobulin (M protein) [Citation1,Citation2]. It has been reported that the accumulated plasma cells usually involved in multiple bone lesions were similar to cancer metastasis [Citation3]. Although the survivability and prognosis for MM patients have improved greatly in the past 15 years with the treatment of monoclonal antibodies, cell cycle-specific drugs, immunomodulatory drugs, proteasome inhibitors, etc. [Citation4-6], most of them eventually passed away because of this disease. Thus, more studies should be carried out for the determination of the molecular mechanisms underlying MM progression, in order to provide more effective strategies to ameliorate the survivability of MM patients.
Forkhead box P1 transcription factor (FOXP1), one of the four members of the forkhead box P subfamily, involved in transcriptional regulation and DNA repair in various physiological and pathological processes [Citation7]. In cancers, FOXP1 was considered as a potential oncogene or tumor suppressor depending on cellular context. For instance, decreased FOXP1 was reported in epithelial ovarian cancer and ectopic expression of FOXP1 was tightly correlated with aggressive pathological characteristics, poor prognosis and enhanced chemotherapy resistant [Citation8,Citation9]. Inversely, FOXP1 level was prominently elevated in glioma [Citation10] and breast cancer [Citation11], which commonly predicted the adverse clinical outcome. However, the precise role of FOXP1 in MM has not been clearly stated.
MicroRNAs (miRNAs) are a type of endogenous and conserved non-coding RNAs with approximately 21–23 nucleotides in length [Citation12]. Accumulated evidence has suggested that miRNAs negatively regulated gene expression in the post-transcriptional levels through imperfect pairing with the 3'-untranslated region (UTR) of the target mRNAs [Citation13]. These small molecules play a critical role in the modulation of various biological processes involved in the development and progression of cancers [Citation14,Citation15]. MiR-29b, a member of miR-29 family (miR-29a, miR-29b, miR-29c), exerts gene regulatory function via interacting with other members of miR-29 family [Citation16]. Ectopic expression of miR-29b observed in various cancers is thought to be a potential tumor suppressor through repressing the translation and stability of targeted oncogenes [Citation17]. However, the underlying molecular of miR-29b in the development of MM remains to be fully expounded.
In the present study, we observed a notable downregulation of miR-29b in both MM tissues and cell lines and ectopic expression of miR-29b was closely associated with International Staging System (ISS) stages. In particular, FOXP1 was identified as a direct target gene of miR-29b. Restoration of miR-29b depressed the growth of MM cells through negatively modulating FOXP1 expression. Moreover, the inhibitory effects of miR-29b on MM tumor growth were further validated in vivo. Our study elaborated a novel miR-29b/FOXP1 pathway in the development of MM, providing a potential biomarker for MM therapy in clinical settings.
2. Materials and methods
2.1. Patients
A cohort of 27 patients with characterized newly diagnosed MM were enrolled in this study, with 27 healthy donors as a control group. MM patients were divided into three subgroups depending on the standard of ISS stages: 7 cases at ISSI, 9 cases at ISSII and 11 cases at ISSIII. Plasma cells isolated from bone marrow specimens of healthy donors and MM patients were collected for the detection of miR-29b expression. This study was approved by the Research Ethic Committee of Luoyang Orthopedics Hospital of Henan Province (Orthopedics Hospital of Henan Province) with the informed consents of all patients and healthy donors.
2.2. Cell culture and transfection
HEK293T cells, normal plasma cells (nPCs) and human MM cell lines OPM-2, U266, MM.1S, H929 were purchased from American type culture collection (ATCC, Rockefeller, MD, USA). All cells were maintained in the RPMI-1640 medium (Gibco, Grand Island, YN, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (HyClone, Logan, UT, USA) in a humidified air with 5% CO2 at 37°C.
miR-29b mimic (miR-29b), miR-29b antisense inhibitor (anti-miR-29b) or controls were obtained from GenePharma (Shanghai, China). FOXP1 overexpression plasmid (pcDNA-FOXP1) was constructed via inserting the full-length sequences of FOXP1 into pcDNA3.1 vector. H929 and U266 cells in logarithmic phase were plated in 24-well plates for 24 h, followed by the transfection of indicated oligonucleotides or plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Afterward, further studies were performed at 48 h post-transfection.
2.3. Quantitative real-time PCR
Total RNAs in bone marrow-derived plasma cells and standard cell lines were isolated using Trizol reagent (Invitrogen) according to the manufacturer’s procedures. miRNA first-stand cDNA synthesis kit (GeneCopoeia, Guangzhou, China) or reverse transcription system kit (Takara, Dalian, China) was exclusively employed for the reverse transcription of miRNA or mRNA. Afterward, quantitative real-time PCR (qRT–PCR) reactions were carried out on Applied Biosystems 7500 Real-time PCR Systems (Thermo Fisher Scientific, Waltham, MA, USA) at 95°C for 2 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. The expression of miRNA and mRNA was quantified using the 2−ΔΔCt method with U6 snRNA or GAPDH as an internal reference. The primers for qRT–PCR were shown as below: miR-29b: 5′-CTCGCTTAGCTAGCCTATACT-3′ (forward) and 5′-ACGTTCGACTTCGACCG TGTC-3′ (reverse); U6: 5′-CGCTTCGGCAGCACATATACTA-3′(forward) and 5′-CGCTTCACGAATTTGCGTGTCA-3′(reverse); FOXP1: 5′-GCAGCAGCTCTGG AAAGAAG-3′ (forward) and 5′-GCAGACTTGGAGAGGGTGAC-3′ (reverse); GAPDH: 5′-TATGATGATATCAAGAGGGTAGT-3′(forward) and 5′-TGTATCC AAACTCATTGTCATAC-3′ (reverse).
2.4. Cell proliferation assay
The cell proliferation rate was measured using Cell Counting Kit-8 (CCK-8 kit) (Dojindo, Tokyo, Japan) according to the manufacturer’s instructions. Suspended H929 and U266 cells were seeded into 96-well plates at a density of 5 × 103/well, and then cells were transfected with indicated oligonucleotides or plasmids followed by the incubation for appropriate periods (0, 24, 48 and 72 h). Then, 10 μl CCK-8 reagent was added for another 2 h incubation and the absorbance at 450 nm was detected using a microplate reader (Thermo Fisher Scientific) to assess cell proliferation.
2.5. Flow cytometry assay
Transfected cells were trypsinized followed by the washing and resuspension with PBS buffer. Afterward, cells were fixed in pre-chilled 70% ethanol at −20°C overnight. For the detection of DNA content, cells were stained with propidium iodide (PI) and incubated at 37°C in the dark for 30 min. Finally, DNA content was measured by flow cytometry (FCM; BD Biosciences, Franklin Lakes, NJ, USA).
Cell apoptosis was performed using Annexin V-FITC/PI Apoptosis Detection Kit (BestBio, Shanghai). Briefly, cells were digested with trypsin and resuspended with binding buffer followed by the introduction of Annexin V-FITC and PI. After incubation at 37°C in the dark for 5 min, apoptotic cells were observed by FCM.
2.6. Dual-Luciferase reporter assay
Luciferase reporter plasmid wild-type FOXP1 (FOXP1-WT) and mutated FOXP1 (FOXP1-MUT) were generated via inserting the amplified wild-type or mutated FOXP1 sequences into psiCHECK™-2 luciferase plasmid (Promega, Madison, WI, USA). Subsequently, luciferase reporter constructs were transfected into HEK 293 T cells with miR-29b mimics or miR-NC using lipofectamine 2000. Approximately 48 h post-transfection, cells were harvested and the activity of luciferase reporters was determined using Dual-Luciferase Reporter assay system (Promega).
2.7. Western blot assay
H929 and U266 cells were lysed with cell lysis buffer (Huashun, Shanghai, China). Lysated cells containing equal amounts of protein were separated by SDS-PAGE, and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk solution for 2 h at 37°C, followed by the addition of specific antibodies against FOXP1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for overnight at 4°C. After the membranes were incubated with HRP-conjugated anti-rabbit secondary antibody (Cell Signaling Technology, Inc, Danvers, MA, USA) for 1 h, protein bands were visualized by chemiluminescence assay kits (BestBio, Shanghai, China).
2.8. Tumor xenograft formation assay in vivo
Precursor sequences of miR-29b were digested using EcoR I and BamH I and then subcloned into pCDH-CMV-MCS-EF1-copGFP vector to obtain the recombinant plasmid LV-miR-29b, with vector (LV-NC) as a control. Male BALB/c nude mice (six weeks old) were purchased from Shanghai Experimental Animal Center of the Chinese Academy of Sciences. H929 cells transfected with LV-miR-29b or LV-NC were subcutaneously inoculated into the mice with a dose of 1 × 107 cells per mouse. Tumor growth was monitored by the measurement of tumor volumes every other week after injection. All mice were sacrificed at five weeks after transplantation and tumors were collected for weighing and detecting miR-29b and FOXP1 expression. The procedures were performed following the Guidelines for Care and Use of Laboratory Animal and approved by Ethics Committee of Luoyang Orthopedics Hospital of Henan Province (Orthopedics Hospital of Henan Province).
2.9. Statistical analysis
Statistical analysis was performed by the SPSS 20.0 and described as mean ± standard deviation. All data between groups were estimated with Student’s t-test and one-way analysis of variance. P < .05 represented that the difference was statistically significant.
3. Results
3.1. miR-29b was upregulated in MM tissues and cell lines
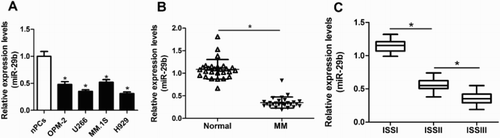
To explore the role of miR-29b in MM, miR-29b expression in MM cell lines and tissues were detected by qRT–PCR. Results showed that the levels of miR-29b in MM cell lines were lower than those of nPCs cells, especially in U266 and H929 cells ((A)). Besides, miR-29b was weakly expressed in MM tissues than in normal controls ((B)). Further analysis revealed that miR-29b showed remarkably lower expression in MM patients at stage III than patients at stage I and stage II ((C)), which indicated the correlation of miR-29b and ISS stages of MM patients.
Figure 1. miR-29b expression was upregulated in MM cell lines and patients. (A) The expression of miR-29b in nPCs and MM cells was detected by qRT–PCR. (B) miR-29b expression in bone marrow (BM)-derived plasma cells was determined by qRT–PCR in MM patients (n = 27) and healthy donors (n = 27). (C) The levels of miR-29b in MM patients of diverse ISS stages (ISSI: n = 7, ISSII: n = 9 and ISSIII: n = 11).
3.2. miR-29b inhibited the proliferation and promoted apoptosis of MM cells
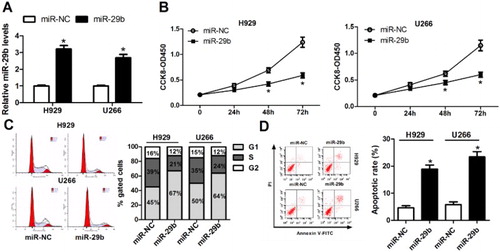
To further investigate the function of miR-29b in MM, H929 and U266 cells were transfected with miR-29b mimics or miR-NC for 48 h. Afterward, cells were harvested for the following experiments. qRT–PCR showed that the expression of miR-29b was induced with the transfection of miR-29b mimics ((A)). CCK-8 and FCM analyses suggested that upregulation of miR-29b notably attenuated cell proliferation ((B)), but induced cell cycle arrest ((C)) and apoptosis ((D)) in H929 and U266 cell lines.
Figure 2. miR-29b depressed the growth of MM cells. (A) miR-29b expression was notably elevated following the treatment of miR-29b mimics in H929 and U266 cells. (B) CCK-8 assay was performed for the measurement of cell proliferation after transfecting with miR-NC or miR-29b mimics into H929 and U266 cell. (C and D) After treatment with miR-NC or miR-29b for 48 h, DNA content of PI-stained MM cells and cell apoptosis rate were determined by the FCM analysis.
3.3. FOXP1 was a target gene of miR-29b
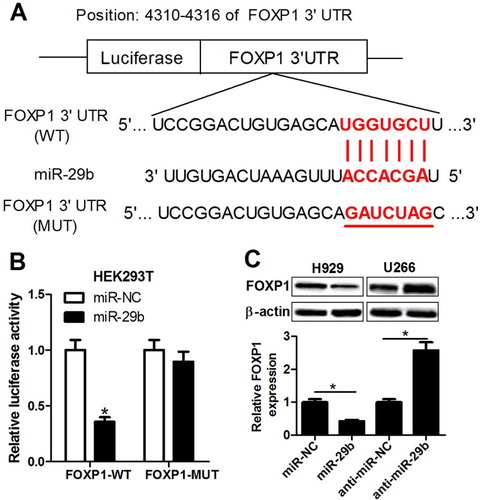
To illuminate the molecular mechanisms underlying the suppression of miR-29b on MM growth, Targetscan bioinformatics was employed to predict the target gene of miR-29b, and FOXP1 was identified to be a potential target for miR-29b ((A)). Next, we further demonstrated the true binding between miR-29b and FOXP1. Dual-Luciferase reporter assay indicated that luciferase activity of reporter plasmid containing wild-type FOXP1 gene was markedly lowered in miR-29b-overexpressed HEK293T cells, while no significant change was observed in the luciferase activity of mutated FOXP1 reporter ((B)). Western blot assay revealed that the protein expression of FOXP1 was significantly inhibited in miR-29b-transfected H929 cell and induced in anti-miR-29b-transfected U266 cell ((C)). Together, miR-29b negatively regulated FOXP1 expression via directly targeting the 3′ UTR of FOXP1 in MM cell lines.
Figure 3. miR-29b negatively regulated FOXP1 expression through direct interaction. (A) The putative binding sites between FOXP1 3’ UTR and miR-29b. (B) Luciferase activity of reporter containing wild-type or mutated FOXP1 gene was assessed in HEK293T cells after transfection of miR-NC or miR-29b. (C) The protein expression of FOXP1 was measured after transfection of miR-29b mimic or inhibitor in H929 and U266 cells.
3.4. FOXP1 reversed the suppression of miR-29b on MM progression
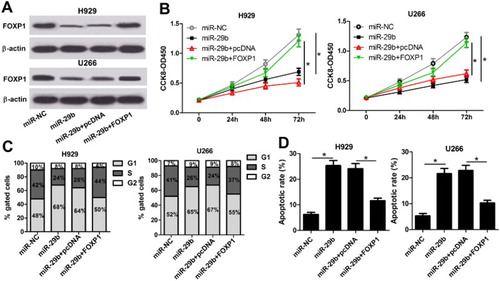
To further investigate whether miR-29b led to the reduction of MM cell growth through acting as an inhibitory element for FOXP1, restoration experiments were performed via upregulating FOXP1 in miR-29b overexpression of MM cell lines. qRT–PCR showed that pcDNA-FOXP1 introduction weakened miR-29b-reduced FOXP1 expression ((A)). Further functional studies manifested that miR-29b-induced anti-proliferation of H929 and U266 cells was inversed by FOXP1 overexpression ((B)). Moreover, cell cycle arrest ((C)) and apoptosis ((D)) derived by miR-29b were also abolished following the treatment of pcDNA-FOXP1 in H929 and U266 cells.
Figure 4. miR-29b suppressed the growth of MM cells via targeting FOXP1. (A) pcDNA-FOXP1 introduction abolished the inhibitory effect of miR-29b on FOXP1 expression in H929 and U266 cells. (B) miR-29b-derived anti-proliferation was reversed with FOXP1 overexpression in H929 and U266 cells. (C and D) Cell cycle arrest and apoptosis induced by miR-29b overexpression were repressed with the transfection of pcDNA-FOXP1.
3.5. miR-29b inhibited tumor growth in vivo
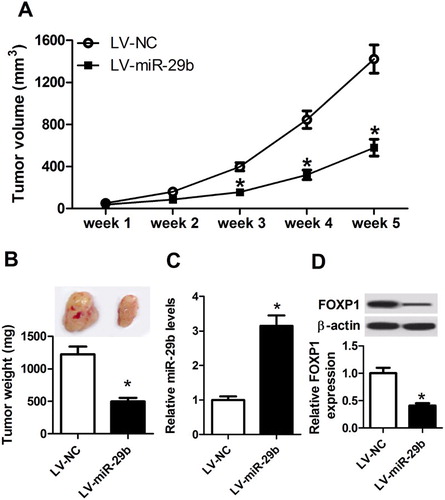
To explore the impact of miR-29b on tumorigenesis in vivo, H929 cells transfected with LV-NC or LV-miR-29b were subcutaneously injected into the BALB/c nude mice to establish xenograft models. Five weeks after inoculation, tumor growth was assessed via the detection of tumor volume and weight, results displayed that LV-miR-29b-transfection significantly decreased the volume ((A)) and weight ((B)) of transplantation tumors compared with those in control groups. Afterward, the expression of miR-29b or FOXP1 in different groups of lumps was measured by qRT–PCR or western blot. As shown in (C,D), miR-29b expression was induced, while FOXP1 expression was reduced in tumor masses derived from LV-miR-29b-transfected H929 cells. These results confirmed the inhibitory effects of miR-29b in MM progression in vivo.
Figure 5. miR-29b suppressed MM tumor growth in vivo. (A) Tumor volume was measured every other week after inoculation. (B) Xenograft tumors in LV-NC and LV-miR-29b groups were taken photos and weighed at five weeks after inoculation. (C and D) miR-29b mRNA and FOXP1 protein expression in tumor tissues were detected by qRT–PCR or western blot.
4. Discussion
With the fast development of medical research, the molecular mechanism underlying MM tumorigenesis has been disclosed gradually over the past years. Growing evidence indicates the central roles of miRNAs in the modulation of human malignancies via acting as inhibitory factors of downstream target genes, including MM [Citation18]. For instance, Morelli et al. [Citation19] found that the tumor-suppressor activity of miR-125b-5p inhibited the formation of MM tumor via negatively modulating IRF4 and its downstream effectors BLIMP-1, c-Myc, caspase-10 and cFlip. Maria et al. [Citation20] suggested the knockdown of miR-221/222 against the growth of MM cells, as well as the tumor formation of xenografted mice. Recent findings highlighted that miRNA was a promising option for the treatment of incurable MM patients [Citation21,Citation22].
Recent studies have confirmed the involvement of miR-29b in various tumors via interacting with cancer-related genes. For instance, miR-29b diminished cell migration and invasion activity and led to a partial blocking of transforming growth factor beta 1 (TGFβ1)-induced epithelial–mesenchymal transition (EMT) in TGFβ1-responsive ovarian cancer cells via directly binding to the 3′ UTR fragment of Id-1 [Citation23]. MiR-29b markedly inhibited EMT in colorectal cancer (CRC), and overexpression of miR-29b resulted in the inhibition of CRC tumor growth and metastasis by downregulation of Tiam1 [Citation24]. Through the reduction of Sp1 expression and blocking PTEN-AKT signaling pathway, miR-29b depressed the proliferation, migration and invasion of tongue squamous cell carcinoma cells [Citation25]. In MM, miR-29b was also considered to be a potential tumor suppressor. As reported by Nicola, miR-29b targeted DNA methyltransferase 3A and 3B (DNMT3A and 3B) and reduced global DNA methylation in MM cells, leading to a significant anti-tumor effect [Citation26]. Amodio et al. [Citation27] indicated that miR-29b upregulation involved in the demethylation of SOCS-1 gene promoter and negatively modulated the growth and migration of MM cells. Xie et al. [Citation28] reported that genistein stimulated miR-29b expression resulting in the blockage of NF-κB pathway and then dented the proliferation of human MM cells. Jagannathan et al. [Citation29] suggested that miR-29b replacement impaired the growth of MM cells and xenotransplants by targeting PSME4 and enhanced the bortezomib sensitivity of MM patients. In the present study, we also showed that miR-29b was notably downregulated in a cohort of MM patients, as well as in multiple MM cell lines. Dysregulated miR-29b was intimately correlated with ISS stages of MM patients. More importantly, the silencing of miR-29b led to anti-tumor effects in MM cell lines, as reflected by the reduced cell proliferation and the induced cell cycle arrest and apoptosis. Experiments in vivo further supplemented the results in vitro, in which miR-29b knockdown significantly depressed the growth of MM transplantation tumor.
FOXP1, a function gene associated with gene transcription and DNA repair, has been widely reported as oncogene or tumor suppressor gene involved in several cancers. For example, FOXP1 elicited a repressive effect on androgen receptor-induced transcriptional activity and acted as a tumor inhibitor as well as a prognostic factor in prostate cancer through inhibiting cell proliferation and migration [Citation30]. Ectopic expression of FOXP1 was associated with unfavorable prognosis in neuroblastoma, and FOXP1 induction resulted in cell cycle arrest and apoptosis of neuroblastoma cells [Citation31]. Knockdown of FOXP1 in ovarian cancer cells significantly decreased spheroid formation and tumor size in vivo by repressing cancer stem cell-like characteristics [Citation32]. To further explore a novel regulatory mechanism of miR-29b in MM progression, in the following study, FOXP1 was identified to be a potential target gene of miR-29b, and the expression of FOXP1 was negatively modulated by miR-29b. Function and mechanism analyses revealed that restoration of FOXP1 weakened miR-29b-induced anti-proliferative and pro-apoptotic effects in MM cell lines.
In conclusion, our studies revealed that miR-29b suppressed proliferation but induced cell cycle arrest and apoptosis of MM cells via downregulating FOXP1. These findings demonstrated a novel miR-29b/FOXP1 regulatory pathway in the development and progression of MM, indicating that miR-29b might be a potential therapeutic target for incurable MM patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Hongyan Wang http://orcid.org/0000-0002-6109-1036
References
- Röllig C, Knop S, Bornhäuser M. Multiple myeloma. The Lancet. 2015;385(9983):2197–2208. doi: 10.1016/S0140-6736(14)60493-1
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–2972. doi: 10.1182/blood-2007-10-078022
- Umezu T, Tadokoro H, Azuma K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–3757. doi: 10.1182/blood-2014-05-576116
- Leaf RK, Cho HJ, Avigan D. Immunotherapy for multiple myeloma, past, present, and future: monoclonal antibodies, vaccines, and cellular therapies. Curr Hematol Malig Rep. 2015;10(4):395–404. doi: 10.1007/s11899-015-0283-0
- Maes A, Menu E, Veirman K, et al. The therapeutic potential of cell cycle targeting in multiple myeloma. Oncotarget. 2017;8(52):90501–90520. doi: 10.18632/oncotarget.18765
- Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32. doi: 10.1038/leu.2009.236
- Katoh M, Igarashi M, Fukuda H, et al. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328(2):198–206. doi: 10.1016/j.canlet.2012.09.017
- Hu Z, Zhu L, Tan M, et al. The expression and correlation between the transcription factor FOXP1 and estrogen receptors in epithelial ovarian cancer. Biochimie. 2015;109:42–48. doi: 10.1016/j.biochi.2014.12.001
- Hu Z, Zhu L, Gao J, et al. Expression of FOXP1 in epithelial ovarian cancer (EOC) and its correlation with chemotherapy resistance and prognosis. Tumour Biol. 2015;36(9):7269–7275. doi: 10.1007/s13277-015-3383-5
- Cui R, Guan Y, Sun C, et al. A tumor-suppressive microRNA, miR-504, inhibits cell proliferation and promotes apoptosis by targeting FOXP1 in human glioma. Cancer Lett. 2016;374(1):1–11. doi: 10.1016/j.canlet.2016.01.051
- Oskay HS. FOXP1 enhances tumor cell migration by repression of NFAT1 transcriptional activity in MDA-MB-231 cells. Cell Biol Int. 2017;41(1):102–110. doi: 10.1002/cbin.10702
- Lagosquintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921
- Wilczynska A, Bushell M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015;22(1):22–33. doi: 10.1038/cdd.2014.112
- Esquelakerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840
- Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15(1):167–166. doi: 10.1186/s12935-015-0185-1
- Yan B, Guo Q, Fu F, et al. The role of miR-29b in cancer: regulation, function, and signaling. Oncotargets Ther. 2015;8(default):539–548.
- Nguyen T, Kuo C, Nicholl MB, et al. Downregulation of microRNA-29c is associated with hypermethylation of tumor-related genes and disease outcome in cutaneous melanoma. Epigenetics. 2011;6(3):388–394. doi: 10.4161/epi.6.3.14056
- Leva GD, Garofalo M, Croce CM. MicroRNAs in cancer. Annual Rev Pathol. 2014;9(1):287–314. doi: 10.1146/annurev-pathol-012513-104715
- Morelli E, Leone E, Cantafio ME, et al. Selective targeting of IRF4 by synthetic microRNA-125b-5p mimics induces anti-multiple myeloma activity in vitro and in vivo. Leukemia. 2015;29(11):2173–2183. doi: 10.1038/leu.2015.124
- Di MMT, Annamaria G, Gallo CME, et al. In vitro and in vivo anti-tumor activity of miR-221/222 inhibitors in multiple myeloma. Oncotarget. 2013;4(2):242–255.
- Rossi M, Amodio N, Di MM, et al. From target therapy to miRNA therapeutics of human multiple myeloma: theoretical and technological issues in the evolving scenario. Curr Drug Targets. 2013;14(10):1144–1149. doi: 10.2174/13894501113149990186
- Tagliaferri P, Rossi M, Di MM, et al. Promises and challenges of microRNA-based treatment of multiple myeloma. Curr Cancer Drug Targets. 2012;12(7):838–846. doi: 10.2174/156800912802429355
- Teng Y, Zhao L, Zhang Y, et al. Id-1, a protein repressed by miR-29b, facilitates the TGFβ1-induced epithelial-mesenchymal transition in human ovarian cancer cells. Cellular Physiol Biochem. 2014;33(3):717–730. doi: 10.1159/000358647
- Wang B, Li W, Liu H, et al. miR-29b suppresses tumor growth and metastasis in colorectal cancer via downregulating Tiam1 expression and inhibiting epithelial-mesenchymal transition. Cell Death Dis. 2014;5(7):e1335–e1335. doi: 10.1038/cddis.2014.304
- Jia LF, Huang YP, Zheng YF, et al. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN–AKT signaling pathway by targeting Sp1. Oral Oncol. 2014;50(11):1062–1071. doi: 10.1016/j.oraloncology.2014.07.010
- Nicola A, Marzia L, Dina B, et al. DNA-demethylating and anti-tumor activity of synthetic miR-29b mimics in multiple myeloma. Oncotarget. 2012;3(10):1246–1258.
- Amodio N, Bellizzi D, Leotta M, et al. miR-29b induces SOCS-1 expression by promoter demethylation and negatively regulates migration of multiple myeloma and endothelial cells. Cell Cycle. 2013;12(23):3650–3662. doi: 10.4161/cc.26585
- Xie J, Wang J, Zhu B. Genistein inhibits the proliferation of human multiple myeloma cells through suppression of nuclear factor-κB and upregulation of microRNA-29b. Mol Med Rep. 2016;13(2):1627–1632. doi: 10.3892/mmr.2015.4740
- Jagannathan S, Vad N, Vallabhapurapu S, et al. MiR-29b replacement inhibits proteasomes and disrupts aggresome + autophagosome formation to enhance the antimyeloma benefit of bortezomib. Leukemia. 2015;29(3):727–738. doi: 10.1038/leu.2014.279
- Takayama K, Suzuki T, Tsutsumi S, et al. Integrative analysis of FOXP1 function reveals a tumor-suppressive effect in prostate cancer. Mol Endocrinol. 2014;28(12):2012–2024. doi: 10.1210/me.2014-1171
- Ackermann S, Kocak H, Hero B, et al. FOXP1 inhibits cell growth and attenuates tumorigenicity of neuroblastoma. BMC Cancer. 2014;14(1):472–416. doi: 10.1186/1471-2407-14-840
- Choi EJ, Seo EJ, Kim DK, et al. FOXP1 functions as an oncogene in promoting cancer stem cell-like characteristics in ovarian cancer cells. Oncotarget. 2016;7(3):3506.
