ABSTRACT
Objective: Mature B-cell non-Hodgkin lymphoma (B-NHL) comprises more than 50% of all non-Hodgkin lymphoma (NHL) in children and adolescents. An official report published by the Mexican National Center for the Control and Prevention of Cancer in the Pediatric and Adolescent Populations, reported a lymphoma OS of 71% (including all Hodgkin and NHL). The Mexican Association of Pediatric Oncology and Hematology conducted a retrospective study to analyze the clinical characteristics and outcomes of children with diagnosis of B-NHL in Mexico, in order to perceive the main areas of improvement in the health care.
Methods: From 1 January 2000 to 31 December 2016, 166 pediatric patients were diagnosed with B-cell NHL at the participant institutions.
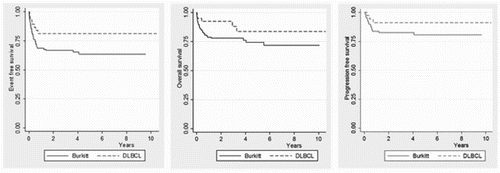
Results: According to histology the outcomes were 5-year EFS 63%, for BL/BLL, and 80% DLBCL, (P = .051), 5-year PFS 81%, for BL/BLL, and 91% for DLBCL, (P = .126), and 5-year OS 71%, for BL/BLL, and 83% for DLBCL, (P = .095).
Discussion: Overall, 18 patients died due to acute treatment toxicity, resulting in a cumulative incidence of toxic death of 10.84% and an early death rate of 7.23%, defined as <30 days after initial treatment. In conclusion, there is an urgent need to establish an academic collaboration to create strategies to improve pediatric cancer care according to our resources, especially in diseases with expected excellent prognosis as B-NHL. These strategies must include comprehensive supportive care, early referral, and the creation of easy communication between pediatric and adults centers as well as late-effects clinics.
Introduction
Lymphomas are the second most common tumors (12%) among children in Mexico [Citation1]. Mature B-cell non-Hodgkin lymphoma (B-NHL) comprises more than 50% of all non-Hodgkin lymphoma (NHL) in children and adolescents [Citation2]. B-NHL consists mainly of two histological subtypes, namely Burkitt lymphoma (BL), which includes Burkitt leukemia (BLL), and diffuse large B-cell lymphoma (DLBCL). Multidisciplinary pediatric group collaborations over the past 25 years have reported a 99% overall survival (OS) rate in limited-risk patients, a 90% OS rate in intermediate-risk patients, and an approximate 70–80% OS rate in children with advanced-risk mature B-cell NHL [Citation3]. However, an official report published by the Mexican National Center for the Control and Prevention of Cancer in the Pediatric and Adolescent Populations (CENSIA), reported a lymphoma OS of 71% (including all Hodgkin and NHL) [Citation1]. B-NHL are usually characterised by a high degree of malignancy and aggressive behavior and treatment, is associated with considerable toxicity, which calls for a comprehensive supportive care [Citation4]. In light of these data, the Mexican Association of Pediatric Oncology and Hematology (AMOHP) conducted this retrospective study. The aim of the study was to analyze the clinical characteristics and outcomes of children with a diagnosis of B-NHL in Mexico, in order to pinpoint the main areas of improvement in health care.
Materials and methods
An invitation to participate in this study was sent by AMOHP to all pediatric cancer centers in Mexico. Thirteen centers accepted. Medical records of patients younger than 18 years of age with newly diagnosed B-NHL were reviewed at the participant centers from January 2001 to December 2016 although follow-up was updated until December 2017. B-NHL subtypes were classified according to the 2016 WHO Classification of Haematological Malignancies [Citation5]. Disease staging was performed according to the St. Jude staging system including a physical examination, bone marrow smears, cerebrospinal fluid (CSF) analyses, and adequate imaging techniques [Citation6]. Central nervous system (CNS) was considered positive at diagnosis if there were ≥5 lymphoma cells/µl in the CSF and/or cranial nerve palsy and/or cerebral lesions on neuroimaging. Lactate dehydrogenase (LDH) level was considered elevated if it was ≥ twice the upper normal limit. B symptoms were defined as fever, drenching night sweats during the last 6 months, and weight loss at > 10% of baseline weight. Toxicity was graded as per CTCAE v4.
Statistical analyses
Descriptive statistics were reported as absolute frequencies and percentages for qualitative data, while means, standard deviations (SD) and medians were used to describe quantitative variables. Continuous variables were stratified into categorical variables using appropriate criteria. Overall survival, event-free survival (EFS) and progression-free survival (PFS) were analyzed by the Kaplan–Meier method. OS was calculated as the time from diagnosis to death from any cause, or to the date of the last follow-up. PFS was calculated from the date of diagnosis to the date of progression, relapse, or to the date of the last follow-up. EFS was calculated from the date of diagnosis to relapse, disease progression, abandonment of treatment, second malignancy, death from any cause or to the date of the last follow-up. A formal cumulative incidence analysis was performed regarding toxic death versus death related to relapse or progression. Comparisons between survival functions were performed by means of the Log-Rank Test, whereas the Cox regression model was used to evaluate the risk factors affecting the EFS and PFS. All P-values are two-sided, with a type I error rate fixed at 0.05. Statistical procedures were performed using STATA® statistical analysis software (version 11.0; StataCorp LP, College Station, TX).
Results
Patient characteristics
From 1 January 2000 to 31 December 2016, 166 pediatric patients were diagnosed with B-cell NHL at the participant institutions. Median age at diagnosis was 8 years (range 0.7–18 years). Male to female ratio was 1.3:1. The most frequent primary site of involvement was abdomen (48%). Among all B-NHL patients, 82% had the advanced-stage disease [86 (52%) patients had stage III and 49 (30%) patients had stage IV], 50% had elevated LDH, 39% had B symptoms, 24% had bone marrow (BM) involvement and 10% had CNS involvement. BL was diagnosed in 126 patients (76%) (Including 26 BLL), and DLBCL in 39 patients (23%). See .
Table 1. Patients baseline characteristics.
Upfront treatment and acute toxicities
Most of the patients received treatment according to the BFM90 (52%) and LMB96 protocols (46%), two patients received CHOP (1%), and two patients received CHOP plus Rituximab (1%). Rates of acute severe (grade 3 and 4) toxicities were as follow: Hematological toxicity was the most common, with 85% of the patients having at least one episode. Regarding non-hematologic toxicity, infection was the single most common, occurring at least once in 61% of patients. Nausea/vomit and stomatitis were also frequent, occurring at least once in 60% and 52% of patients, respectively. The rates of liver, renal, cardiac and neurological toxicities were 16%, 18%, 9% and 9% respectively. Overall, 17 patients died due to acute treatment toxicity, resulting in a cumulative incidence of toxic death of 10.84% and an early death rate of 7.23%, defined as <30 days after initial treatment was. Infection was the main cause of death in 14 patients; other causes included tumor lysis syndrome in 2 patients and bleeding in 1 patient. In addition, two more patients died upon arrival at the hospital (within 24hrs) without receiving any type of treatment other than supportive care.
Treatment outcomes
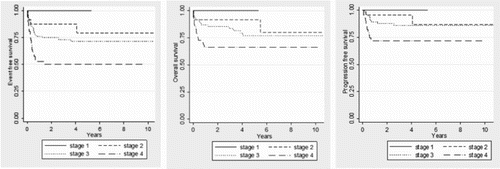
Five-year EFS for all patients was 66.6% (SE: 3.9%), 5-year PFS was 82.7% (SE: 3.5%), and 5-year OS 76.3% (SE: 3.7%). According to histology the outcomes were as follows: 5-year EFS 63% (SE: 4.7%) for BL/BLL, and 80% DLBCL (SE: 6.5%, P = .051), 5-year PFS 81% (SE: 4.1%), for BL/BLL, and 91% for DLBCL (SE: 4.7%, P = .126), and 5-year OS 71% (SE: 4.9%), for BL/BLL, and 83% for DLBCL (SE: 7.0%, P = .095). See . The 5-year EFS, PFS and OS according to clinical stage were 100% for stage I, 80% (SE: 9.5%), 91.4% (SE: 5.7%), and 87.2% (SE: 8.8%) for stage II, 70.5% (SE: 5.2%), 76.2% (SE: 5.5%), and 85.9% (SE: 4.1%) for stage III, 50% (SE: 7.4%), 66% (SE: 6.9%), and 73.1% (SE: 6.9%) for stage IV, (P = .001), (P = .023) and (P = .024) respectively. See . We evaluated the impact on outcomes in the following variables: Histology, stage, age (≥ 15 years old), gender, LDH (> 2× ULN), B-symptoms, treatment protocol, BM involvement, and CNS involvement. The stage was the only significant factor in the multivariate analysis for EFS, OS and PFS. Overall, twenty-four patients progressed/relapsed at a median time of 4 months, 9 in primary site only (38%), 2 in CNS alone (8.3%), 2 in bone marrow alone (8.3%), 1 each in abdomen only, neck only and mediastinum only (4% each site), and 8 (33.3%) in multiple sites. Nine (37%) of those patients are alive in remission with a median follow-up time of 22 months (range 13–77 months).
Follow-up and late-effects
The incidence of long-term toxicity, other than second malignancies, was 3%. To date, only one second malignancy has occurred, an acute myeloblastic leukemia (AML) at 4 years after the first diagnosis; that patient died at 2.5 months from diagnosis of AML. There was no statistical significance in any outcome when a comparison we compared children and adolescents; however, the median follow-up was, for children 36 months and for adolescents 22 months.
Discussion
Overall, the demographic and clinical baseline characteristics of our patients do not have a significant deviation from the literature [Citation4,Citation7-10]. However, the high percentage of patients with stage III and IV disease is of note, which is concordant with what has been reported in other lower and middle-income countries [Citation11,Citation12]. Although the more important prognostic determinants are the biology of the tumor and its related clinical behavior, and in many instances, there is no clear relationship between lag times and survival [Citation13]. It is important to mention that given the high percentage of advance disease, the good results for limited disease, and the fact that two patients were in such a bad condition that died few time later they arrived to the hospital, make us highlight the importance of early diagnosis and prompt referral to specialized institutions.
The incidence of grade 3 and 4 acute toxicities was also similar to that reported in the literature and expected due to the aggressiveness of the disease and the type of treatment required [Citation4,Citation7]. Outcomes for stage III and IV were well below those reported in developed countries [Citation10,Citation14] but were similar to those reported for low and middle-income countries [Citation8,Citation9,Citation12,Citation15,Citation16], which might be explained by our economic disparity. Despite Mexico belongs to an upper middle-income category, our country has striking levels of income disparity. Among its 123 million inhabitants, 53 million live below the poverty line and 10 million live in extreme poverty [Citation17-19]. The high early mortality rate and the fact that more patients died of TRM than from disease progression reveals the huge need to improve supportive care. This area of improvement was described before in a recent AMOHP study regarding pediatric cancer care in Mexico, where it was found that the availability of specialized services varies substantially among centers, and that additional capacity in supportive and palliative care was needed [Citation20].
However, these results are also a reflection of the poor baseline health and economic condition of our patients. According to data from the Mexican National Institute of Statistics and Geography 34% of children, 0 to 17 years old present some degree of malnutrition [Citation21], putting our patients in disadvantage to endure a treatment designed in a developed world for a population with better conditions of living. Other point to highlight is the lack of long-term follow-up in most of the patients, especially in the adolescents. In the aforementioned study about pediatric cancer care in Mexico, it was also found that a substantial proportion of adolescents are lost to follow-up in the transition from pediatric to adult programs and that late effect clinics were only available at 13% of the centers [Citation20].
This study has several limitations, being the major one its retrospective nature. Second, there is no information regarding the nutritional status of the patients or biological characteristics of the disease, so we were unable to evaluate their influence on outcomes. Notwithstanding these limitations, this is a multicentre study, which involves Institutions of the three major health care systems in Mexico [the Instituto Mexicano del Seguro Social (IMSS), Popular Medical Insurance (PMI), and Instituto de Seguridad Social al Servicio de los Trabajadores del Estado (ISSSTE)]. Nearly 95% of the population in Mexico receives health care through the public health care systems. Besides, the findings are informative and highlight areas in need of improvement. In conclusion, there is an urgent need to establish an academic collaboration with the aim of creating strategies, according to our resources, for the improvement of pediatric cancer care; this is especially relevant in diseases with expected excellent prognosis as B-NHL. These strategies must include comprehensive supportive care, early referral, and the creation of easy communication between pediatric and adults centers as well as late-effects clinics.
Acknowledgements
The authors are grateful to each of the pediatric cancer units that participated in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Farina Arreguin-Gonzalez http://orcid.org/0000-0002-5552-8369
Gladys García-Becerra http://orcid.org/0000-0003-0639-6360
Marcela Rodriguez-Campos http://orcid.org/0000-0002-5753-835X
Oscar Gonzalez-Ramella http://orcid.org/0000-0002-9669-2632
References
- Comportamiento Epidemiológico del Cáncer en menores de 18 años. México 2008-2014. www.censia.salud.gob.mx/contenidos/descargas/cancer/20160601 Accessed Noveber 20, 2017.
- Worch J, Rohde M, Burkhardt B. Mature B-cell lymphoma and leukemia in children and adolescents-review of standard chemotherapy regimen and perspectives. Pediatr Hematol Oncol. 2013;30(6):465–483. doi: 10.3109/08880018.2013.783891
- Cairo MS, Sposto R, Gerrard M, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but Not adolescent Age (≥ 15 years), Are associated With an increased risk of treatment failure in children and adolescents With mature B-cell Non-hodgkin's lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30(4):387–393. doi: 10.1200/JCO.2010.33.3369
- Baena-Gomez MA, Mora Matilla M, Lassaletta Atienza A, et al. Linfoma no hodgkin: excelentes resultados a expensas de elevada toxicidad del tratamiento. An Pediatr Barc. 2015;82(6):381–387. doi: 10.1016/j.anpedi.2014.09.005
- Chan JK. The new World Health Organization classification of lymphomas: the past, the present and the future. Hematol Oncol. 2001;19(4):129–150. doi: 10.1002/hon.660
- Murphy SB, Fairclough DL, Hutchison RE, et al. Non-Hodgkin's lymphomas of childhood: an analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol. 1989;7(2):186–193. doi: 10.1200/JCO.1989.7.2.186
- Tsurusawa M, Mori T, Kikuchi A, et al. Improved treatment results of children with B-cell non-Hodgkin lymphoma: a report from the Japanese pediatric leukemia/lymphoma study group B-NHL03 study. Pediatr Blood Cancer. 2014;61(7):1215–1221. doi: 10.1002/pbc.24975
- Cervio C, Barsotti D, Ibanez J, et al. Early mortality in children with advanced mature B-cell malignancies in a middle-income country. J Pediatr Hematol Oncol. 2012;34(7):e266–e270. doi: 10.1097/MPH.0b013e31826226b1
- Cunha KC, Oliveira MC, Gomes AC, et al. Clinical course and prognostic factors of children with Burkitt's lymphoma in a developing country: the experience of a single centre in Brazil. Rev Bras Hematol Hemoter. 2012;34(5):361–366. doi: 10.5581/1516-8484.20120093
- Goldman S, Smith L, Anderson JR, et al. Rituximab and FAB/LMB 96 chemotherapy in children with stage III/IV B-cell non-Hodgkin lymphoma: a children's oncology group report. Leukemia. 2013;27(5):1174–1177. doi: 10.1038/leu.2012.255
- Budiongo AN, Ngiyulu RM, Lebwaze BM, et al. Pediatric non-Hodgkin lymphomas: first report from Central Africa. Pediatr Hematol Oncol. 2015;32(4):239–249. doi: 10.3109/08880018.2015.1013231
- Acquatella G, Insausti CL, Garcia R, et al. Outcome of children with B cell lymphoma in Venezuela with the LMB-89 protocol. Pediatr Blood Cancer. 2004;43(5):580–586. doi: 10.1002/pbc.20116
- Barr RD. “Delays” in diagnosis. J Pediatr Hematol Oncol. 2014;36(3):169–172. doi: 10.1097/MPH.0000000000000108
- Jourdain A, Auperin A, Minard-Colin V, et al. Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “lymphomes malins B” protocols. A Societe Francaise des Cancers de l'Enfant study. Haematologica. 2015;100(6):810–817. doi: 10.3324/haematol.2014.121434
- Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa—report of the French-african pediatric oncology group (GFAOP). Pediatr Blood Cancer. 2008;50(6):1138–1142. doi: 10.1002/pbc.21452
- Guo J, Zhu YP, Gao J, et al. [Clinical and prognostic analysis of 43 children with mature B-cell non-Hodgkin's lymphoma/acute lymphoblastic leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24(1):72–79.
- The World Bank. https://data.worldbank.org/indicator/SI.POV.GINI?locations=MX. (Accessed January 15, 2018).
- Consejo Nacional de Poblacion. Proyecciones de la Poblacion 2010–2050. http://www.conapo.gob.mx/es/CONAPO/Proyecciones_Datos (Accessed January 15, 2018).
- Consejo Nacional de Evaluación de la Política de Desarrollo Social. Medicion de la Pobreza http://www.coneval.org.mx/Medicion/Paginas/PobrezaInicio.aspx (Accessed January 15, 2018).
- Rodriguez L, Olaya A, Gupta S, et al. Delivery of pediatric cancer care in Mexico: A national survey. J Glob Oncol. 2018;4:1–12.
- Estadisticas a proposito del dia del Nino, INEGI http://www.inegi.org.mx/saladeprensa/aproposito/2017/ni%C3%B1o2017_Nal.pdf (Accessed March 1, 2018).