ABSTRACT
Objective: To investigate the association between the polymorphism of methylenetetrahydrofolate reductase (MTHFR) gene and the formation of lower extremities deep venous thrombosis, and to evaluate the etiology of deep venous thrombosis.
Methods: Polymorphisms of the 677th site C/T in MTHFR gene for 101 patients with lower extremities deep venous thrombosis (DVT group) and 120 healthy subjects (control group) were detected by polymerase chain reaction with sequence-specific primers. Genotype and allelic frequencies were compared between the two groups.
Results: Genotype frequencies of CC, CT and TT in MTHFR C677 T were 41.58, 25.74 and 32.67% in DVT group, and 58.33, 23.33 and 18.33% in control group, respectively. There was a significant difference in TT genotype frequency between the two groups (P <0.05).
Conclusion: The frequency of MTHFR 677TT genotype may be correlated with the morbility of DVT.
1. Introduction
5, 10- methylenetetrahydrofolate reductase (MTHFR) is a key enzyme with homocysteine metabolism pathway. It catalyzes the reduction of N5, N10- methylenetetrahydrofolate into N5 methylenetetrahydrofolate which is the most important methyl donor in the body with significant physiological functions. Goyette et al. successfully cloned MT HFR gene for the first time in 1994 and located it on chromosome 1p36.3. Then, they used DNA single-strand conformation polymorphism (SSCP) and DNA sequencing technology to find more than ten polymorphisms in the M TH FR gene, among of which, the most common one is C677 T polymorphism. Mutations of MTHFR gene may lead to the imbalance between vascular relaxing factor and vascular contraction factor, resulting in deep vein thrombosis (DVT). The purpose of this study was to investigate the relationship between the C677 T polymorphisms of MTHFR gene and DVT to provide a theoretical reference for the etiology, prevention and treatment of DVT.
2. Material and methods
2.1. Objects and groups
Lower extremity DVT group: 101 patients, diagnosed as lower extremity DVT by ultrasound or/and lower extremity deep vein angiography in the Second Affiliated Hospital of Nanchang University from February 2014 to May 2017. Forty-five cases of male, 56 cases of female; aged 28–62 (average 48.5) years of age; duration ≤15 days.
Control group: 120 cases, volunteers with normal physical examination results in the Second Affiliated Hospital of Nanchang University for the same period. There were 57 males and 63 females, aged 30–60 (average 42.5) years old. There was no significant difference between the two groups in gender and age (. All subjects had no blood relationship with each other, and both their parents are Han nationality.
Table 1. Comparison of general information between DVT group and control group.
2.2. Test method
2.2.1. DNA extraction
Fasting venous blood was drawn 3 ml from patients in DVT and control group, and anticoagulation with diethylamine tetrakis acetate-k2. Leukocytes were separated by a lymphocyte separation solution. Genomic DNA was extracted by conventional phenol–chloroform method, and the content of genomic DNA was determined by UV spectrophotometer. Keep the extraction at −20°C until using it.
2.2.2. Design and synthesis of primers
Selecting the reference sequence in the NCBI gene library according to the selected MTRR allele. The bioinformatics software VectrNTI was used to design the primer sequence, and the primers were synthesized by Shanghai ivirgen Bioengineering Company.
The upstream primer 1 is: 5-GAGAAGGTG TCT-GCGGGAGC-3;
the upstream primer 2 is: 5-GGAGAAGG T-G TCTGCGGGAG T-3;
the downstream primer is: 5-CGCTGTG-CAAGT TCTGGACC-3;
2.2.3. Polymerase chain reaction-sequence-specific primer
2.2.3.1. PCR reaction system
10 μl reaction system containing 10× PCR buffer 1 μl, 1U DNA polymerase 0.5 μl,
10 pmol/l Primer Mixture 0.5 μl, 200 μmol/l dNTP 1 μl,
10 ng genomic DNA 1 μl, add sterilized water to 10 μl.
2.2.3.2. The PCR reaction conditions
Pre-denaturation was performed at 95°C for 5 minutes on a 9600 PCR machine(The United States PE company), which was followed by 95°C for 30 seconds, 63°C for 50 seconds, 72°C for 30 seconds and was repeated for 30 cycles; 4°C constant temperature.
2.2.3.3. Gel electrophoresis analysis
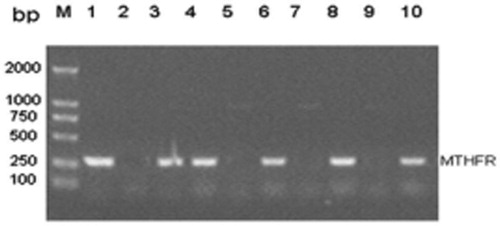
The PCR product of MTHFR allele was subjected to 1% agarose gel electrophoresis, then at voltage 100 V for 35 minutes. The result was observed in the UV-V (The United States BIORAD company) transmission instrument after the end of electrophoresis and taken photos to analyze genotypes.
2.3. Statistical analysis
Genotype frequency and allele frequency were calculated in DVT group and control group. Genotype comparison and genotype Hardy–Weinberg balance were detected by χ2 test with SAS 9.2 software package, and P < 0.05 was considered statistically significant.
3. Result
3.1. Genotype analysis of 1 MTHFR
Polymorphic site mutation at 677th locus of MTHFR gene Rs1801133, the fragment size of PCR product was 277 BP, and there were three genotypes: CC type, C type T and TT type, as shown in .
3.2. Detection frequency of C/T carrying frequency at 677th locus of MTHFR
The results of C/T carrying frequency at 677th locus of MTHFR in two groups were compared ().
Table 2. C/T frequency distribution of 677th loci in MTHFR between two groups of people.
3.3. Results of MTHFR sequencing in DVT group
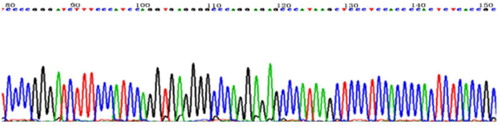
In DVT group, the 677th locus of MTHFR C/T mutation was partially sequenced, as shown in .
Figure 2. Partial sequencing of C/T mutation at 677th locus of MTHFR. 2.4 Hardy–Weinberg test of genotype distribution in two groups.
The distribution of genotypes in the two groups was consistent with the Hardy–Weinberg equilibrium (P > 0.05) by χ2 test, so it was representative of the population.
4. Discussion
DVT is a common disease affected by genetic factors and a variety of external factors, such as operation, fracture trauma and oral contraception. The genetic factors are more significant in DVT. There are also several risk factors in the process of the formation of DVT. In recent years, some studies have confirmed that the polymorphisms of coagulation factor VG1691A gene (FV Leiden mutation), prothrombin gene G20210A and MTHFR gene C677 T are closely related with DVT in white and Indian. The gene polymorphism of MTRR A66G is thought to be associated with DVT in southern India [Citation1]. A study in South Korea concluded that the gene polymorphism of MTRR A66G is a risk factor for hepatocellular carcinoma [Citation2]. A Brazilian study found that gene polymorphisms of MTHFR C677 T and MTRR A66G may lead to elevated levels of homocysteine and folic acid in children [Citation3]. In addition, several studies had investigated the association of the gene polymorphism of MTRR A66G with colorectal cancer, breast cancer, and microvascular complications of type 1 diabetes [Citation4–7].
Polymorphism refers to there are more than two genotypes in the same genetic loci in random mating population. In the population, the difference in the genetic nucleotide sequence in individuals is called genetic polymorphism. This kind of polymorphism can be divided into two categories, the DNA site polymorphism and DNA length polymorphism. In the past, genetic polymorphism analysis mostly used restriction fragment length polymorphism, SSCP, polymerase chain reaction (PCR), DNA sequencing, PCR oligonucleotide probe hybridization (PCR-SSO) method and sequence-specific oligonucleotide method (SSO) or other methods. The above method has the disadvantages of high technical requirements, complicated operation, expensive instruments, reagents required and long time for typing detection, which is not conducive to popularization in the laboratory. PCR-SSP utilizes Taq enzyme for the lack of 3′-5′ exonuclease activity. Sequence-specific primers (SSPs) and template DNAs are designed. The extension by Taq enzyme is blocked if bases are not complementary because of the failure in the formation of 3′, 5′ phosphodiester bond. then, the alleles of HLA were analyzed by gel electrophoresis amplification. The specificity of the duplicated product by the PCR-SSP method can be accurate to the difference in one base, and the amplified product can be analyzed by agarose gel electrophoresis. It has the characteristics of high resolution, strong specificity, simple technique, rapidness, easy application and promotion. Therefore, the PCR-SSP method was used for genetic polymorphism detection in this experiment.
The human MTHFR gene is located on the autosomal chromosome lp36.3, which is about 22 kb in length. Exchange of base C at position 677 of the MTHFR gene for base T resulted in the conversion of alanine to valine, and a new HnfI and TaqI restriction enzyme site was generated at the same time. Valine may be closely related to the active center of MTHFR, which greatly reduces the thermostability and activity of the enzyme. The mutation induced three genotypes: C/C (wild-type), C/T (heterozygous mutant) and T/T (homozygous mutant). The homozygous mutant (T/T) MTHFR had the lowest enzyme activity, about 45% lower than the wild-type (C/C) MTHFR [Citation8]. It has been found that the two most common gene mutation sites are C677 T and A1298C [Citation2,Citation3]. Defects in MTHFR will lead to the impaired conversion of homocysteine(Hcys) to methionine, inducing the accumulation of Hcys in the blood and eventually homocysteine results. An elevated level of plasma homocysteine cause homocysteine to oxidize itself to produce peroxides and oxygen free radicals mediated by metal ions, which damages the structure and function of endothelial cells. Injury in the vascular endothelial cells can increase the secretion of endothelin-1, decrease the secretion of endothelium-derived relaxing factor and prostaglandin, and leads to the imbalance of vasomotor factors in the patient, which triggers thrombosis and cardiovascular disease [Citation9]. The TT homozygote frequencies of normal populations in different countries and regions are different, ranging from 10.12% in Japan to 14.14% in Americans. The frequency of TT homozygote was 18.33% in the normal control group of this study, which was higher than that of the Japanese population and similar to the reported frequency of 18.18–20.15% in the Chinese population [Citation8]. Hyperhomocysteinemia has been reported to be an independent risk factor for venous thrombosis in Caucasians and Chinese Taiwanese [Citation4]. Valder et al. [Citation5] investigate a significant difference between Brazilian blacks and Turks, but no difference with Austrians [Citation6]. The results of studies in the Chinese mainland are not the same. Some studies have reported that MTHFR 677TT is associated with DVT [Citation7,Citation10], while others have reported no significant correlation between MTHFR 677TT and DVT [Citation11]. Wang et al. [Citation12] reported that hyperhomocysteinemia and folic acid deficiency are independent risk factors of thromboembolism, and MTHFR gene mutation may be one of the genetic factors resulting in folate deficiency. This further illustrates the complexity of the MTHFR C677 T distribution, which may be influenced by many factors such as race, region, ethnicity and geographical environment.
In the present study, the frequencies of MTHFR 677TT genotypes and T alleles in DVT group were significantly higher than those in the control group. This suggests that the MTHFR 677TT mutation may be related to the occurrence of DVT, and the TT genotype may be a genetic risk factor for DVT. Due to the limited sample size in this study, the conclusion needs to be further confirmed by increasing the sample size. The frequency of mutations of these suspected pathogenic genes is not high. And the synergistic effects between the microphenotypic accumulation effects generated by polymorphisms of various genes and environmental factors have made their role in the development of DVT become more complicated. Therefore, the development of a unified principle, standardized testing methods, multi-center cooperation, and large-scale epidemiological and clinical research would help to better explore the intrinsic links between genotypes and phenotypes. Whether MTHFR can be used as a biomarker for predicting thrombosis requires a larger sample to carry out more in-depth study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Coulam CB, Wallis D, Weinstein J, et al. Comparison of thrombophilic gene mutation s among patients ex periencing recurrent miscarriage and deep vein thrombosis. Am J Reprod Immunol. 2008;60(5):426–431. doi: 10.1111/j.1600-0897.2008.00640.x
- De Mattia E, Toffoli G. C677t and A1298C MT HF R polymorphisms, a challenge for antifolate and fluoropyrimidine-based therapy personalisation. Eur J Cancer. 2009;45(8):1333–1351. doi: 10.1016/j.ejca.2008.12.004
- Spiroski I, Kedev S, Antov S, et al. Methylenetetrahydrofolate reductase (M TH FR-677 and MT HFR-1298)genotypes and haplotypes and plasma homocysteine levels in patients with occlusive artery disease and deep venous thrombosis. Acta Biochim Pol. 2008;55(3):587–594.
- Hsu TS, Hsu LA, Chang CJ, et al. Importance of hyperhomoeysteinemia as a risk factor for venous thromboembolismin Taiwanese population. A case-control study. Thromb Res. 2001;102:387–395. doi: 10.1016/S0049-3848(01)00262-6
- Valder RA, Paula M, Luiz C, et al. The mutation Ala667Val in the methylene tetrahydrofolate reductase gene: a risk factor for arterial disease and venous thrombosis. Thromb Haemost. 1997;77:818–821. doi: 10.1055/s-0038-1656059
- Lalouschek W, Aull S, Serles W, et al. C677t MTHFR mutation in patients with TIA/min or stroke: a case control study. Thromb Res. 1999;93:61–69. doi: 10.1016/S0049-3848(98)00154-6
- Haidong Y, Hong Z, Hua Q, et al. Correlation between homocysteine metabolic enzyme gene polymorphism and deep venous thrombosis. Chin J Med Genetics. 2006;23(6):635–639.
- Chenhong G, Qiongxing G, Yaoqin G, et al. Mutation of methylenetetrahydrofolate reductase gene C677T in deep venous thrombosis in Shandong Han population. Chin J Med Genetics. 2002;19(4):295–297.
- Yufang W. Mechanism of atherosclerosis induced by homocysteine. Chin J Arteriosclerosis. 1998;6(3):259–263.
- Hong Z, Ying H, Hui C, et al. Association of clotting factor gene mutations and MTHFR/C677T gene polymorphisms with deep vein thrombosis. Chin J Hematol. 2006;27(3):197–118.
- Shouqi L, Xianhui C, Qicai L. Polymorphism analysis of methylenetetrahydrofolate reductase gene C677T in patients with deep venous thrombosis. J Shanxi Coll Traditional Chin Med. 2009;10(1):45–49.
- Wang MT, Li Q, Han FL, et al. Plasma folic acid, homocysteine levels, methylenetetrahydrofolate reductase gene mutation and venous thromboembolism. Zhonghua Int Med. 2004;43(8):591–594.