ABSTRACT
Objectives: Fatigue is a common symptom in allogeneic-hematopoietic stem cell transplantation (allogeneic-HSCT) recipients. However, effects of severe fatigue on pulmonary functions, blood cells, dyspnea, muscle strength, exercise capacity, depression and quality of life (QOL) in allogeneic-HSCT recipients are still unknown. Therefore, to compare pulmonary functions, blood levels, dyspnea, muscle strength, exercise capacity, depression, and QOL between allogeneic-HSCT recipients according to fatigue severity and to determine predictors of severe fatigue were aimed in the current study.
Methods: Twenty-four severe-fatigued (Fatigue Severity Scale score ≥36) (40.08 ± 12.44years) and 25 non-severe-fatigued (36.20 ± 13.73years) allogeneic-HSCT recipients were compared. Blood levels, pulmonary functions (spirometer), dyspnea (Modified Medical Research Council Dyspnea scale), exercise capacity (6-minute walk test), depression (Beck Depression Inventory-II), QOL (European Organization for Research and Treatment of Cancer QOL Questionnaire), respiratory (mouth pressure device) and peripheral muscle strength (dynamometer) were evaluated.
Results: Symptom QOL-subscale and depression scores were significantly higher; peripheral muscle strength, global health status, and functional QOL-subscales scores were lower in severe-fatigued recipients (p < 0.05) whose exercise capacity was clinically (28.85 m) decreased. Blood levels, pulmonary functions, dyspnea, and respiratory muscle strength were similar in groups (p > 0.05). 42.4% of the variance in severe fatigue was explained by symptom QOL-subscale score and corticosteroid use after HSCT (p < 0.001).
Conclusions: Impairments in peripheral muscle strength, QOL, exercise capacity, and depression are more prevalent among severe-fatigued recipients. Moreover, poorer QOL and corticosteroid use after HSCT are most important predictors of severe fatigue. Effects of comprehensive exercise programs and psychosocial support for severe-fatigued recipients in late post-engraftment period should be investigated.
Introduction
Fatigue is the most complained side effect that may last for months or even years after treatment ends in patients with cancer [Citation1]. Cancer-related fatigue is observed in 80% of patients received chemotherapy and/or radiotherapy, yet its underlying mechanism is not still clearly explained [Citation2]. As for hematologic malignancies and treatments including allogeneic or autologous hematopoietic stem cell transplantation (HSCT), recipients are subject to lots of adverse effects such as deconditioning, immobility and muscle weakness along with fatigue [Citation3]. Of all treatment modalities, since allogeneic-HSCT is also highly associated with various complications such as mortality, morbidity, graft-versus-host disease (GvHD) [Citation4], recipients are required to remain close to the transplant center in the acute phase of transplantation, approximately 100 days [Citation5]. As a consequence, HSCT afflicts quality of life (QOL) in recipients [Citation6]. Given that fatigue exists before HSCT and further deteriorates during the first three weeks after HSCT, especially fatigue is a destructive symptom for recipients. Baseline fatigue perception continues until one year after HSCT, as well [Citation7].
Although fatigue has been one of the most intensely experienced and investigated symptoms by allogeneic-HSCT recipients, effects of severe fatigue on physical fitness, mood and systems have not been known yet. Therefore, to investigate differences in pulmonary and extra-pulmonary outcomes such as pulmonary functions, albumin-hemoglobin-white blood cell (WBC) levels, dyspnea, respiratory and peripheral muscle strength, submaximal exercise capacity, depression, and QOL between allogeneic-HSCT recipients according to fatigue perception severity was aimed in the current study. Secondary aim of this study was to determine predictors of severe fatigue in allogeneic-HSCT recipients.
Materials and methods
Forty-nine allogeneic-HSCT recipients who were at minimum 100 days status post-transplantation, between ages of 18 and 65, outpatients under standard medical treatment, transplanted at Bone Marrow Transplantation Unit of University Faculty of Medicine were included and compared according to fatigue severity level. If the level of fatigue perception severity evaluated using Fatigue Severity Scale is equal or above 36 scores, patient is accepted as severe-fatigued or if the score obtained from this scale is below 36 scores, patient is non-severe-fatigued [Citation8]. Fatigue severity of all recipients was identified according to this scoring. Twenty-four severe-fatigued and 25 non-severe-fatigued recipients were compared. Recipients had cognitive disorder, orthopedic or neurological disease with a potential to affect functional capacity, comorbidities such as chronic obstructive pulmonary disease (COPD), acute infections or pneumonia, problems which may prevent measurements such as visual problems and mucositis were excluded. This cross-sectional study was approved by local Ethics Committee and is in accordance with the current version of the Helsinki Declaration. Informed consent was obtained from all individual participants included in the study. Clinical trials number (NCT03448471) was obtained.
Measurements were done by physiotherapist between 2012 and 2013. The whole evaluation period of recipients for the current study was 1.5 hours. Demographic and clinical characteristics of recipients were recorded. Especially albumin-hemoglobin-WBC levels were selected to analyze from total blood count laboratory results but all total blood counts of recipients were checked while collecting data for safety of recipients.
Pulmonary function tests
Pulmonary function test was performed using spirometer (Vmax 220 SensorMedics Corporation, Yorba Linda, CA) in sitting position according to American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines [Citation9]. Single breath diffusing capacity for carbon monoxide (DLCO) was performed with reference to ATS [Citation10]. Forced expiratory volume in first second (FEV1), forced vital capacity (FVC), FEV1/FVC, peak expiratory flow (PEF) and forced expiratory flow at 25–75% (FEF25–75%) were expressed as percentages of expected values. Reference values were used for comparison [Citation11].
Respiratory muscle strength
Respiratory muscle strength was assessed using a portable mouth pressure device (Micro Medical MicroRPM, England, UK) according to ATS/ERS guidelines [Citation12]. Maximal inspiratory pressure (MIP) was measured at residual volume, whereas maximal expiratory pressure (MEP) was measured at total lung capacity. The highest value measured for MIP and MEP were recorded and reference values were used for comparison [Citation13].
Peripheral muscle strength
Quadriceps femoris, elbow flexion, and shoulder abduction muscles strength were measured from the non-dominant side, using hand-held dynamometer (JTECH Commander, Salt Lake City, U.S.A.) [Citation14]. Test was repeated three times for each muscle and the highest value in Newton (N) was recorded. Reference values were used for comparison [Citation14]. Minimal clinically important difference (MCID) is 17.2 Nm for quadriceps femoris muscle strength [Citation15].
Functional exercise capacity
Recipients were walked along a 30-m unobstructed corridor during 6-minute according to ATS criteria [Citation16]. Recipients completed two encouraged 6-minute walk test (6-MWT) and rested minimum 30-minute between two tests. The highest distance was recorded and expressed as percentage of predicted values [Citation17]. MCID is 25 m for 6-MWT distance [Citation18]. Heart rate (Polar FT100, China), blood pressure, oxygen saturation (pulse oximeter, Spirodoc MIR, Italy), respiratory rate, dyspnea and fatigue perception (Modified Borg scale) were recorded before and after tests [Citation19].
Dyspnea
Modified Medical Research Council Dyspnea scale was used to evaluate the severity of dyspnea during daily living activities. Dyspnea is graded from zero (absence of dyspnea during strenuous exercise) to four (presence of dyspnea during all daily living activities) [Citation20]. This scale has been validated in pulmonary patients [Citation21].
Fatigue
Fatigue severity was measured using the Turkish version of Fatigue Severity Scale which is valid and reliable [Citation8]. Self-administered questionnaire is consisting of nine questions [Citation22]. Average score is determined on a seven-point scale. Patients mark a number from 1 to 7 for each nine question which indicates from strong disagreement to strong agreement, respectively [Citation22]. Cut-off fatigue severity score is 36 according to this scale [Citation8]. Scale is used in cancer patients [Citation23].
Depression
Depression was measured with the Turkish version of Beck Depression Inventory-II used to identify both the presence and severity of depressive symptoms [Citation24]. This valid and reliable instrument consists of 21 self-rating questions about depressive symptoms which are rated 0–3 scores, total score is 63. There are cut-off scores of Beck Depression Inventory-II for Turkish population and ≥13 of total score demonstrates from mild to severe depression [Citation24].
Quality of life
QOL was measured using the Turkish version of European Organization for Research and Treatment of Cancer QOL Questionnaire C30 version 3.0 (EORTCQLQ) which is widely used as health-related QOL questionnaire in cancer patients. This scale is also a valid and reliable tool for Turkish cancer patients [Citation25]. Cancer-specific questionnaire has 30 questions and incorporates five functional scales, three symptom scales, a global health status and several single items. All item scores are transformed to 0–100. Higher values indicate the higher functional/healthy level in functional scales, a higher QOL level in global health status and increased symptoms in symptom scales [Citation26].
Statistical analysis
Windows-based SPSS 15.0 statistical analysis program was used for statistical evaluation (SPSS Inc., Chicago, Illinois, U.S.A.). Variables were descriptively expressed as mean ± standard deviation (X ± SD), 95% confidence interval (95%CI), median (minimum–maximum), frequency and percentage. Variables were investigated using visual (histograms, probability plots) and analytical methods (Shapiro–Wilk’s test) to determine normally distribution. Normally distributed variables using Student’s t-test, undistributed variables using Mann–Whitney U test and nominal data using Chi-square test were compared. While investigating associations between normally and non-normally distributed and/or ordinal variables, correlation coefficients and their significance were calculated using Pearson and Spearman tests, respectively. A multiple linear regression model was used to identify independent predictors including corticosteroid use after HSCT, depression, quadriceps femoris and elbow flexion muscles strength, symptom, functional and global health status subscales scores of EORTCQLQ of severe fatigue. Model fit was assessed using appropriate residual statistics. Level of significance was set to p ≤ 0.05. Post hoc power analysis was done using quadriceps femoris muscle strength to detect 17.2Nm difference in quadriceps femoris muscle strength for an α value of 0.05 [Citation18].
Results
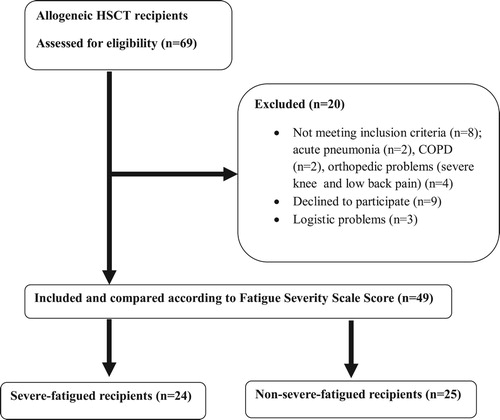
Sixty-nine allogeneic-HSCT recipients were screened and 20 recipients were excluded due to acute pneumonia (n = 2), COPD (n = 2), severe pain in the knee and low back (n = 4), declined to participate (n = 9) and logistic problems (n = 3) (). Of all 69 recipients, 49 were allocated to severe-fatigued (n = 24) and non-severe-fatigued (n = 25) groups and analyzed. Baseline characteristics of Fatigue Severity Scale questions for severe-fatigued and non-severe-fatigued groups were given in . All recipients were receiving optimal medical therapy including immunosuppressive agents, antibiotics and other drugs. Demographic characteristics, smoking-performance status, transplantation age, disease-hospitalization duration, time elapsed both from diagnosis to transplantation and from transplantation to physiotherapy assessment, donor type, stem cell source, total body irradiation, GvHD presence, corticosteroid use before HSCT, number of chemotherapy cycles before HSCT and distribution of diagnosis were similar in groups except conditioning regimen and corticosteroid use after HSCT (, p > 0.05).
Table 1. Baseline characteristics of Fatigue Severity Scale Questions in severe-fatigued and non-severe-fatigued groups.
Table 2. Baseline demographic and transplantation characteristics of severe-fatigued and non-severe-fatigued groups.
Pulmonary functions
No statistically significant differences were observed in FEV1%, FVC%, FEV1/FVC, PEF%, FEF25–75%% and DLCO% between groups (, p > 0.05). Obstructive type pulmonary abnormalities in 4% non-severe-fatigued and 12.5% severe-fatigued recipients; restrictive type pulmonary abnormalities in 8% non-severe-fatigued and 16.7% severe-fatigued recipients were present.
Total blood count
No statistically significant differences were observed in albumin-hemoglobin-WBC levels between groups (, p > 0.05).
Inspiratory and expiratory muscle strength
No statistically significant differences were observed in MIP, MEP and their percentage values between groups (, p > 0.05). Three (12%) recipients’ MIP in the non-severe-fatigued group was lower than 80% of predicted values (p = 0.235). Two (8%) recipients’ MEP in the non-severe-fatigued group and 4 (16.7%) in the severe-fatigued group were lower than 80% of predicted values (p = 0.417). Nine (37.5%) severe-fatigued recipients’ MIP was lower than the lower limit of 95%CI (102.33–124.79) of non-severe-fatigued recipients.
Peripheral muscle strength
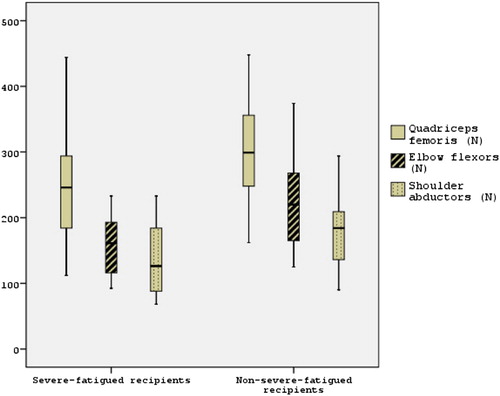
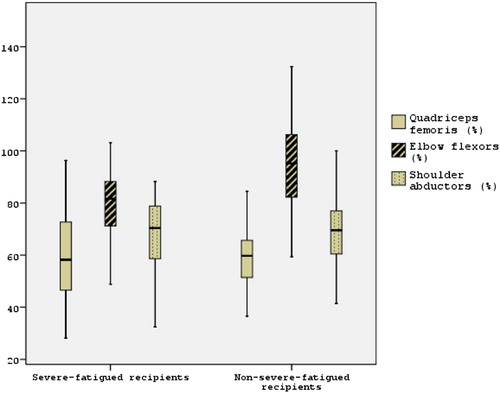
Quadriceps femoris, shoulder abductors and elbow flexors muscle strength was significantly lower in the severe-fatigued group than non-severe-fatigued group (–, , p < 0.05). Besides, mean difference of groups for quadriceps femoris muscle strength (56.82 Nm) was higher than 17.2 Nm. Twenty-two (88%) recipients’ quadriceps femoris muscle strength in the non-severe-fatigued group and 21 (87.5%) in the severe-fatigued group were lower than 80% of predicted values (p = 1.000). Fifteen (68.2%) severe-fatigued recipients’ quadriceps femoris muscle strength was lower than the lower limit of 95%CI (268.98–330.29) of non-severe-fatigued recipients. Six (24%) recipients’ elbow flexors muscle strength in the non-severe-fatigued group and 10 (41.7%) in the severe-fatigued group were lower than 80% of predicted values (p = 0.187). Twenty (80%) recipients’ shoulder abductors muscle strength in the non-severe-fatigued group and 18 (75%) in the severe-fatigued group were lower than 80% of predicted values (p = 0.675). Post hoc power according to quadriceps femoris muscle strength value was (1 − β) = 71.2% and effect size was 0.74.
Functional exercise capacity
No statistically significant differences were observed in 6-MWT distance (−28.85 m, 95%CI = −78.97 to 21.27), its percentage, values of before 6-MWT, after 6-MWT and differences in after and before 6-MWT measurements between groups (–, p > 0.05). Mean difference of groups for functional exercise capacity (28.85 m) was higher than 25 m. Thirteen (52%) recipients’ functional exercise capacity in the non-severe-fatigued group and 13 (54.2%) in the severe-fatigued group were lower than 80% of predicted values (p = 0.879). Eleven (45.8%) severe-fatigued recipients’ 6-MWT distance was lower than the lower limit of 95%CI (547.61–617.28) of non-severe-fatigued recipients.
Table 3. Comparison of pulmonary functions, DLCO, albumin, hemoglobin and WBC levels, dyspnea, exercise capacity, muscle strength, depression and QOL in severe-fatigued and non-severe-fatigued allo-HSCT recipients.
Table 4. Comparison of 6-MWT values in severe-fatigued and non-severe-fatigued allo-HSCT recipients.
Dyspnea
No statistically significant difference was observed in Modified Medical Research Council Dyspnea score between groups (, p > 0.05). Dyspnea during daily living activities and exertional dyspnea during 6-MWT existed by 44.9 and 51% of our all allogeneic-HSCT recipients, respectively.
Depression
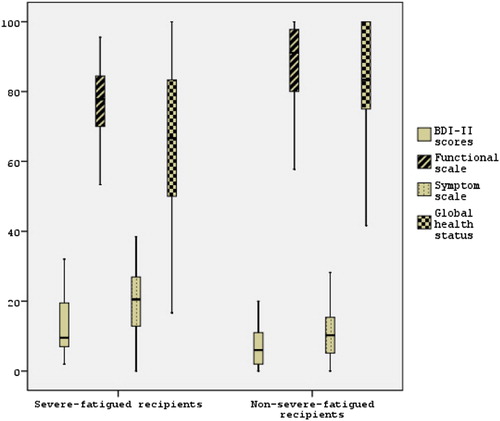
Depression scores were significantly higher in the severe-fatigued group than non-severe-fatigued group (Figure , , p < 0.05). Totally 32.7% of allogeneic-HSCT, 5 (20%) in the non-severe-fatigued group and 11 (45.8%) in severe-fatigued recipients had mild to severe depression (p = 0.054).
Quality of life
Symptom subscale score of EORTCQLQ was significantly higher, global health status and functional subscales scores of EORTCQLQ were lower in severe-fatigued group than non-severe-fatigued group (Figure , , p < 0.05).
Correlations
Severe fatigue was statistically significantly and negatively correlated with depression (r = −0.370, p = 0.009), symptom subscale score of EORTCQLQ (r = −0.508, p < 0.001), corticosteroid use after HSCT (r = −0.321, p = 0.028) and disease duration (r = −0.274, p = 0.056); positively correlated with conditioning regimen (r = 0.285, p = 0.047), quadriceps femoris (r = 0.376, p = 0.009), elbow flexors (r = 0.409, p = 0.004), shoulder abductors muscle strength (r = 0.295, p = 0.040), functional (r = 0.462, p = 0.001) and global health status subscale scores of EORTCQLQ (r = 0.373, p = 0.008).
Severe fatigue was not statistically correlated with age, transplantation age, time elapsed from diagnosis to transplantation, duration of hospitalization, time elapsed from transplantation to physiotherapy assessment, presence of total body irradiation and number of chemotherapy cycles before HSCT, donor type, stem cell source, acute and chronic GvHD, corticosteroid use before HSCT, albumin-hemoglobin-WBC levels, pulmonary functions, dyspnea, MIP, MEP and 6-MWT distance (p > 0.05).
Based on multiple linear regression analysis 42.4% of the variance in severe fatigue (R2 = 0.424, p < 0.001) was explained by symptom subscale score of EORTCQLQ (R2 = 0.226, p < 0.001) and corticosteroid use after HSCT (R2 = 0.198, p = 0.001).
Discussion
The present study demonstrates for the first time in the literature that upper and lower extremity muscle strength, depression and QOL are more impaired, exercise capacity is clinically (−28.85 m, 95%CI = −78.97 to 21.27) decreased in allogeneic-HSCT recipients with severe fatigue. On the other hand, dyspnea perception, pulmonary functions, respiratory muscle strength and albumin-hemoglobin-WBC levels do not alter according to fatigue severity in survived HSCT recipients. As predicted, poorer QOL and corticosteroid use after HSCT are predictors of severe fatigue. The presence of received myeloablative conditioning regimen, corticosteroid use after HSCT and also longer disease duration afflict severity of fatigue in HSCT recipients during the late post-engraftment period.
Both restrictive and obstructive pulmonary function abnormalities are prevalent in almost one-fifth of recipients during the post-transplantation process [Citation27]. Severe-fatigued and non-severe-fatigued recipients had similar dynamic lung volumes and pulmonary diffusion capacity in the current study. In addition obstructive and restrictive type pulmonary abnormalities also existed in a minority of our recipients. No relationship between pulmonary functions and level of severe fatigue perception was present, either. In contrast to our study, Breukink et al. demonstrated that two physical dimensions of fatigue (reduced activity and reduced motivation) are related to only FEV1, but its other dimensions (general, physical and mental fatigue) are not related to any other dynamic pulmonary parameters in COPD patients [Citation28]. In the present study, pulmonary function impairments were not common problem in allogeneic-HSCT recipients in contrast to COPD patients, since it is a lung disease. Large airway obstruction, inactivity and fatigue may be a destructive cycle for COPD patients [Citation28]. Further studies investigating effects of abnormal pulmonary functions on fatigue in recipients are needed.
Both groups had similar albumin, hemoglobin and WBC levels, also no relation between selected blood parameters and severe fatigue was demonstrated in the present study. In addition, all our recipients were normal-over weighted according to body mass index values. That is, severe fatigue occurred independently from albumin, hemoglobin and WBC levels in allogeneic-HSCT recipients. A study conducted with various cancer patients showed that hypoalbuminaemia and higher C-reactive protein level which are responsible for systemic inflammation and amount of lean tissue are associated with higher mortality rates [Citation29]. It is known that the presence of fatigue seen with eating disorders, weakness, lack of energy and weight loss – physical dimensions of fatigue – often appear together [Citation30]. In contrast to our findings, it is stated that hemoglobin and WBC levels are also hematologic indicators/causative elements of cancer-related fatigue [Citation31]. However, majority of our recipients’ albumin (93.9%), hemoglobin (57.1%) and WBC (73.5%) values were in normal ranges. These similarities between groups may be due to blood levels in normal ranges that our recipients have.
Dyspnea perception during these activities was similar in groups, besides dyspnea was not related with severe fatigue in the present study. In contrast to our study, Breukink et al. suggested that dyspnea perception during symptom-limited exercise test in COPD patients is substantially related to reduced motivation [Citation28], a subscale dimension of Fatigue Index-20 which is used in cancer patients [Citation32]. Whereas nearly half of allogeneic-HSCT recipients had dyspnea complaint, its severity was not affected from severe fatigue in the current study. In other words, severe dyspnea may not be a main complaint for severe-fatigued allogeneic-HSCT recipients, unlike COPD patients. Consistent with our outcome, Seo et al. [Citation31] stated that dyspnea is not one of physical factors (pain, nausea, vomiting) of cancer-related fatigue. We used subjective methods to evaluate dyspnea in recipients. However, there is a golden standard method to evaluate reasons and contact of fatigue and dyspnea which is cardiopulmonary exercise testing. Reduced peak oxygen uptake in cancer outpatients with clinically important unexplained dyspnea was previously shown [Citation33]. Therefore, oxygen uptake should be measured to determine connection between severe fatigue and dyspnea in further studies.
It was previously reported that inspiratory muscle weakness exists by 52% of all allogeneic-HSCT recipients [Citation34]. In the current study, respiratory muscle strength was similar in both groups besides it was not related to severe fatigue. Additionally, inspiratory muscle weakness was present by 12.2% of all allogeneic-HSCT recipients. Presence of respiratory muscle weakness was lower in our recipients compared to 52% which was finding of Kovalszki et al. [Citation34]. It was reported in advance that spirometry and lung volumes are well preserved nearly two years after HSCT [Citation35]. Therefore respiratory muscle strength may have been less affected from process of HSCT via well preserved pulmonary functions. In line with our study, Breukink et al. displayed that physical and mental fatigues are not related to respiratory muscle weakness in COPD patients [Citation28]. In further studies, severe fatigue should be investigated in allogeneic-HSCT recipients with respiratory muscle weakness.
It is previously known that peripheral muscle weakness is present by 75% of allogeneic-HSCT recipients [Citation34]. Our study is the first to show upper and lower extremity muscle strength were more decreased in severe-fatigued allogeneic-HSCT recipients in the current literature, to our knowledge. Peripheral muscle weakness existed in almost all of our recipients. Finally, mean difference of groups for quadriceps femoris muscle strength (56.82 Nm) was higher than 17.2 Nm and post hoc power to detect differences in quadriceps femoris muscle strength indicates that our study had an adequate sample size. Consistently, Breukink et al. displayed that physical and mental fatigue is substantially related with upper and lower extremity muscle weakness [Citation28]. In consistent with our study, studies about the process of HSCT also showed respiratory and peripheral muscle weakness, impaired functional exercise capacity, physical inactivity and the presence of severe fatigue. In addition to these outcomes, the relationship between decline in these physical functions using of high corticosteroid dose and evident fatigue was demonstrated [Citation34,Citation36–38]. Corticosteroid use after HSCT is one of two predictors of severe fatigue in our recipients. Corticosteroid use after HSCT was higher in severe-fatigued recipients than non-severe-fatigued recipients and it was also related with quadriceps femoris muscle strength in the present study. Moreover, GvHD prophylaxis consisted of cyclosporine and methotrexate for myeloablative conditioning regimen (high-dose) was higher (79.2%) in severe-fatigued recipients. Acute GvHD was related with all muscle strength and exercise capacity as time elapsed from diagnosis to transplantation was related to elbow flexor muscle strength in our recipients. It is known that myeloablative regimen has higher treatment-related mortality but serves better malignant disease control after HSCT [Citation39]. For these reasons, peripheral muscle strength may be affected by more use of high dose conditioning regimen and corticosteroids in severe-fatigued allogeneic-HSCT recipients. Routine measurement of fatigue and muscle strength should be taken into account in severe-fatigued recipients. Cancer-related fatigue is both alleviated and explained by daily living activities, physical activity, regular exercise and usual activity level [Citation31]. Therefore rehabilitation programs including upper and lower extremity resistance training exercises, especially eccentric exercises which facilitate mesenchymal stem cell appearance in skeletal muscle [Citation40], should be applied during all transplantation process [Citation2].
In the current study, exercise capacity was similarly reduced in both groups besides it was not related to severe fatigue. However, exercise capacity was clinically decreased in severe-fatigued allogeneic-HSCT recipients versus non-severe-fatigued (6-MWT distance mean difference: 28.85 m). Braam et al. showed that remarkable impairments in maximum oxygen consumption and maximal workload evaluated during maximal exercise test are evident in severe-fatigued sarcoidosis patients whose pulmonary functions were in normal limits [Citation41]. Breukink et al. indicated that fatigue dimensions are not related with exercise capacity (maximum workload of exercise test), in consistent with our study [Citation28]. In fact, that study showed reduced motivation (a dimension of fatigue scale) is related to Borg dyspnea and fatigue perception at maximum workloads. Morishita et al. demonstrated that recipients treated with myeloablative conditioning regimen have more decreased 6-MWT distance rather than muscle weakness in short-term after HSCT compared with recipients treated with reduced intensity conditioning regimen despite physical therapy interventions during the process [Citation38]. Much as recipients given high dose corticosteroids immediately after HSCT to prevent and heal GvHD are related with massive physical function declines included especially muscle strength, but not decline in exercise capacity [Citation38]. As for our results, number of recipients given corticosteroids after HSCT and treated with myeloablative conditioning regimen were higher in severe-fatigued recipients, in line with results of Morishita et al. [Citation38]. Corticosteroid use after HSCT is also one of predictors of severe fatigue in our study. Consequently submaximal exercise capacity may be less affected than peripheral muscle strength in severe-fatigued allogeneic-HSCT recipients. Routine follow-up of exercise capacity and aerobic exercise interventions for recipients are substantial from the time of initial diagnosis for hematological malignancy to HSCT process before fatigue becomes evident.
Depressive symptoms are experienced by 15% of HSCT recipients whose post-transplantation disease durations are 1–3 years [Citation42]. In the present study, totally 32.7% of allogeneic-HSCT, 45.8% of severe-fatigued and 20% of non-severe-fatigued recipients had from mild to severe depression, which was higher than previously reported [Citation42]. In addition, depressive symptoms were remarkably higher in severe-fatigued recipients than others and severe fatigue was related with depression in the current study. In a newly published study, adult HSCT survivors with persistent fatigue which is more severe than occasional tiredness of daily living have more anxiety and depression than healthy controls with occasional tiredness have been shown [Citation43]. Moreover, survivors have higher interleukin-6 and tumor necrosis factor-α (TNF-α) levels as well as lower red blood cell and hemoglobin levels [Citation43]. Significant pathway for severe fatigue via chronic inflammation may play a significant role in allogeneic-HSCT recipients, as well [Citation43]. Therefore this pathway via chronic inflammation and the impact of psychosocial support on severe fatigue should be investigated.
Among HSCT survivors QOL impairments due to some worries about fertility, future employment status and having different physical appearance are unfortunately present [Citation42]. Impaired QOL was one of the predictors of severe fatigue and severe fatigue was related with QOL in the present study. In parallel with our study, Cohen et al. showed that symptom severity including fatigue, dyspnea, and pain effects QOL in HSCT recipients [Citation44]. Moreover, recipients received myeloablative conditioning regimen have severe symptoms and poorer QOL [Citation44]. Inal-Ince et al. reported that severe fatigue has larger negative impact on QOL in COPD patients, consistent with findings of the present study [Citation45]. As presented in the study of Cohen et al., a number of recipients received myeloablative conditioning regimen was higher in severe-fatigued recipients than others in our study [Citation44]. Due to adverse early and late effects of myeloablative conditioning regimen on many body systems and organs, QOL may be more impaired in severe-fatigued allogeneic-HSCT recipients [Citation39]. Therefore, it should be alleviated with proper interventions such as inspiratory muscle training [Citation46] and moderate exercise training [Citation47].
Fatigue is a destructive symptom for all allogeneic-HSCT candidates and recipients. Thus, fatigue severity of recipients during HSCT process should be evaluated routinely. Peripheral muscle weakness, depression, poorer QOL, and functional exercise capacity impairments are more common among severe-fatigued allogeneic-HSCT recipients. In conclusion, impacts of exercise training and psychosocial support on severe fatigue should be investigated.
To best investigate factors resulted in severe fatigue, the cardiopulmonary exercise test is the golden standard. However, due to technical issues, we could not perform the cardiopulmonary exercise test which is the most prominent limitation of this study. Another important limitation of the study, prior sample size analysis could not be calculated because of lack of studies about this topic. However, post hoc power calculation analysis showed relatively higher power with large effect size. Further studies are needed with higher number of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Clinical Trials Number: NCT03448471
ORCID
Gülşah Barğı http://orcid.org/0000-0002-5243-3997
References
- Rüffer J, Flechtner H, Tralls P, et al. Fatigue in long-term survivors of Hodgkin‘s lymphoma; a report from the German Hodgkin Lymphoma Study Group (GHSG). Eur J Cancer. 2003;39(15):2179–2186. doi: 10.1016/S0959-8049(03)00545-8
- Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–1039. doi: 10.6004/jnccn.2015.0122
- Paul KL. Rehabilitation and exercise considerations in hematologic malignancies. Am J Phys Med Rehabil. 2011;90(5):S88–S94. doi: 10.1097/PHM.0b013e31820be055
- Papadopoulos EB, Jakubowski AA. Novel approaches in allogeneic stem cell transplantation. Curr Oncol Rep. 2006;8(5):325–336. doi: 10.1007/s11912-006-0054-0
- Wulff-Burchfield E, Jagasia M, Savani B. Long-term follow-up of informal caregivers after allo-SCT: a systematic review. Bone Marrow Transplant. 2013;48(4):469–473. doi: 10.1038/bmt.2012.123
- Poloméni A, Lapusan S, Bompoint C, et al. The impact of allogeneic-hematopoietic stem cell transplantation on patients’ and close relatives’ quality of life and relationships. Eur J Oncol Nurs. 2016;21:248–256. doi: 10.1016/j.ejon.2015.10.011
- Frödin U, Lotfi K, Fomichov V, et al. Frequent and long-term follow-up of health-related quality of life following allogeneic haematopoietic stem cell transplantation. Eur J Cancer Care (Engl). 2015;24(6):898–910. doi: 10.1111/ecc.12350
- Armutlu K, Korkmaz NC, Keser I, et al. The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. Int J Rehabil Res. 2007;30(1):81–85. doi: 10.1097/MRR.0b013e3280146ec4
- Quanjer PH, Tammeling G, Cotes J, et al. Lung volumes and forced ventilatory flows. Eur Respiratory Soc. 1993;6:5–40. doi: 10.1183/09041950.005s1693
- Society AT. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique-1995 update. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796
- Society AT. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202
- European RS, Society AT. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518. doi: 10.1164/rccm.166.4.518
- Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54(10):1348–1359.
- Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78(1):26–32. doi: 10.1016/S0003-9993(97)90005-8
- Knols RH, Aufdemkampe G, De Bruin ED, et al. Hand-held dynamometry in patients with haematological malignancies: measurement error in the clinical assessment of knee extension strength. BMC Musculoskelet Disord. 2009;10(1):31. doi: 10.1186/1471-2474-10-31
- Laboratories ACoPSfCPF. Statement AT: guidelines for the Six-Minute Walking-Test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102
- Gibbons WJ, Fruchter N, Sloan S, et al. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil Prev. 2001;21(2):87–93. doi: 10.1097/00008483-200103000-00005
- Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221–225. doi: 10.1016/j.apmr.2009.10.017
- Wilson RC, Jones P. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin Sci. 1989;76(3):277–282. doi: 10.1042/cs0760277
- Stenton C. The MRC breathlessness scale. Occup Med. 2008;58(3):226–227. doi: 10.1093/occmed/kqm162
- Fletcher CM, Elmes PC, Fairbairn AS, et al. Significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257. doi: 10.1136/bmj.2.5147.257
- Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022
- Stone P, Hardy J, Huddart R, et al. Fatigue in patients with prostate cancer receiving hormone therapy. Eur J Cancer. 2000;36(9):1134–1141. doi: 10.1016/S0959-8049(00)00084-8
- Kapci EG, Uslu R, Turkcapar H, et al. Beck Depression Inventory II: evaluation of the psychometric properties and cut-off points in a Turkish adult population. Depress Anxiety. 2008;25(10):104–110. doi: 10.1002/da.20371
- Cankurtaran E, Ozalp E, Soygur H, et al. Understanding the reliability and validity of the EORTC QLQ-C30 in Turkish cancer patients. Eur J Cancer Care (Engl). 2008;17(1):98–104.
- Fayers PM, Aaronson NK, Bjordal K, et al. EORTC QLQ-C30 scoring manual. 2001.
- Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. CHEST J. 1996;109(4):1066–1077. doi: 10.1378/chest.109.4.1066
- Breukink S, Strijbos J, Koorn M, et al. Van der Schans C. Relationship between subjective fatigue and physiological variables in patients with chronic obstructive pulmonary disease. Respir Med. 1998;92(4):676–682. doi: 10.1016/S0954-6111(98)90517-0
- McMillan DC, Elahi MM, Sattar N, et al. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41(1–2):64–69. doi: 10.1080/01635581.2001.9680613
- Walsh D, Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer. 2006;14(8):831–836. doi: 10.1007/s00520-005-0899-z
- Seo YM, Oh HS, Seo WS, et al. Comprehensive predictors of fatigue for cancer patients. J Korean Acad Nurs. 2006;36(7):1224–1231. doi: 10.4040/jkan.2006.36.7.1224
- Smets E, Garssen B, Bd B, et al. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-O
- Travers J, Dudgeon DJ, Amjadi K, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol. 2008;104(1):57–66. doi: 10.1152/japplphysiol.00653.2007
- Kovalszki A, Schumaker G, Klein A, et al. Reduced respiratory and skeletal muscle strength in survivors of sibling or unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(11):965–969. doi: 10.1038/bmt.2008.15
- Prince DS, Wingard JR, Saral R, et al. Longitudinal changes in pulmonary function following bone marrow transplantation. Chest. 1989;96(2):301–306. doi: 10.1378/chest.96.2.301
- Morishita S, Kaida K, Ikegame K, et al. Impaired physiological function and health-related QOL in patients before hematopoietic stem-cell transplantation. Support Care Cancer. 2012;20(4):821–829. doi: 10.1007/s00520-011-1156-2
- Danaher EH, Ferrans C, Verlen E, et al. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncol Nurs Forum. 2006;33(3):614–624. doi: 10.1188/06.ONF.614-624
- Morishita S, Kaida K, Yamauchi S, et al. Relationship between corticosteroid dose and declines in physical function among allogeneic hematopoietic stem cell transplantation patients. Support Care Cancer. 2013;21(8):2161–2169. doi: 10.1007/s00520-013-1778-7
- Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344–353. doi: 10.1182/blood-2014-02-514778
- Valero MC, Huntsman HD, Liu J, et al. Eccentric exercise facilitates mesenchymal stem cell appearance in skeletal muscle. PLoS One. 2012;7(1):e29760. doi: 10.1371/journal.pone.0029760
- Braam A, De Haan S, Vorselaars A, et al. Influence of repeated maximal exercise testing on biomarkers and fatigue in sarcoidosis. Brain Behav Immun. 2013;33:57–64. doi: 10.1016/j.bbi.2013.05.006
- Mosher CE, DuHamel KN, Rini C, et al. Quality of life concerns and depression among hematopoietic stem cell transplant survivors. Support Care Cancer. 2011;19(9):1357–1365. doi: 10.1007/s00520-010-0958-y
- Hacker ED, Fink AM, Peters T, et al. Persistent fatigue in hematopoietic stem cell transplantation survivors. Cancer Nurs. 2017;40(3):174–183. doi: 10.1097/NCC.0000000000000405
- Cohen MZ, Rozmus CL, Mendoza TR, et al. Symptoms and quality of life in diverse patients undergoing hematopoietic stem cell transplantation. J Pain Symptom Manage. 2012;44(2):168–180. doi: 10.1016/j.jpainsymman.2011.08.011
- Inal-Ince D, Savci S, Saglam M, et al. Fatigue and multidimensional disease severity in chronic obstructive pulmonary disease. Multidiscip Respir Med. 2010;5(3):162. doi: 10.1186/2049-6958-5-3-162
- Bosnak-Guclu M, Arikan H, Savci S, et al. Effects of inspiratory muscle training in patients with heart failure. Respir Med. 2011;105(11):1671–1681. doi: 10.1016/j.rmed.2011.05.001
- Mock V, Atkinson A, Barsevick A, et al. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park, NY). 2000;14(11A):151–161.