ABSTRACT
Objective: Immunoglobulin replacement therapy (IgRT) is increasingly used in secondary immunodeficiency (SID) related to hematological malignancies (HM) to prevent infections. Study's objective was to document prospectively the efficacy and safety of IgRT in patients with HM-associated SID.
Methods: Non-interventional, prospective French longitudinal study.
Results: One-hundred and sixty patients starting IgRT for HM-associated SID (myeloma: 54 cases, chronic lymphoid leukemia: 54, aggressive non-Hodgkin B-cell lymphoma: 19, indolent non-Hodgkin B-cell lymphoma: 29, and Hodgkin disease: 4. entered an observational, prospective, longitudinal study and were followed-up for 8.7 ± 4.0 months. Seventeen patients died (five within the context of sepsis). Compared to baseline, IgRT increased serum immunoglobulin levels by 3.4 ± 2.4 g/L and decreased frequency and severity of infections. Treatment was discontinued in 9% of patients, stopped for futility in 31%, temporally interrupted in 8%, suspended during summertime in 14% and pursued without interruption in 38% of patients.
Conclusion: Our data confirm the efficacy of IgRT in reducing the risk of infections in HM-associated SID therefore fulfilling physicians’ main expectations. They also illustrate the heterogeneity of management policies within the community setting.
Introduction
Hematological malignancies (HM) or their treatment represent the major causes of secondary immunodeficiencies (SID) [Citation1]. Mechanisms include neutropenia, T-cell, dendritic cell and natural killer cell disorders, and B-cell dysfunction leading to hypogammaglobulinemia (HG). High-dose chemotherapy, fludarabine, rituximab, stem cell transplantation, and radiotherapy have a major impact on the immune system and may cause HG [Citation1–5]. HM-associated SID increases the risk of infection which represents a major cause of morbidity and mortality [Citation1,Citation6]. As the defective immune response in HM patients is largely due to immunoglobulin (Ig) deficiency, Ig replacement therapy (IgRT) has been proposed as a strategy to reduce the infection risk. Both intravenous (IVIg) and subcutaneous (SCIg) routes are used for Ig administration [Citation1,Citation7–10].
Current guidelines promote the use of IgRT in chronic lymphocytic leukemia (CLL) patients with severe HG and recurrent infections [Citation11]. IgRT is also indicated in plateau phase multiple myeloma (MM) patients with HG and recurrent bacterial infections who have failed to respond to pneumococcal immunization and in patients with HG after allogeneic hematopoietic stem cell transplantation [Citation12]. New indications such as Hodgkin (HL) and non-Hodgkin lymphoma (NHL) are emerging. Poor response against pneumococcal vaccines increases the susceptibility of respiratory diseases in NHL patients and justifies the use of IgRT [Citation13]. We recently reported indications, modalities and physicians’ expectations when starting IgRT in HM-associated SID in real life setting [Citation14]. Beside CLL or MM, patients with NHL represented a large part of HM patients starting IgRT. The physicians’ decision-making process included but did not require low serum IgG levels and history of infections. We report here longitudinal data in order to better document efficacy and safety of IgRT with octagam® IV or gammanorm® in patients with HM-associated SID.
Material and methods
Study design and objectives
This was an observational, multicenter, prospective study conducted in France in real life conditions. Detailed methods have been extensively described elsewhere [Citation14]. Briefly, consecutive adult patients with HM-associated SID who were newly embarked on IgRT, regardless of brand name, route (IVIg or SCIg) or place (hospital or home) of administration were invited to participate in the study. Patients who had received IgRT at any time within the last 12 months could not enter the study. The study protocol prospectively planned that the subgroup of patients receiving octagam® IV or gammanorm® SC (Octapharma AG, Lachen, Switzerland) were eligible for a follow-up period of up to 12 months or until discontinuation of IgRT. The study aimed at describing the efficacy and safety of IgRT with octagam® IV or gammanorm® SC, patient adherence and satisfaction of physician expectations over the follow-up period.
Data collected
At enrollment, the following data were collected: demographics, vital signs, Eastern Cooperative Oncology Group performance status (ECOG-PS) score, type of HM, treatment modalities, antibiotic prophylaxis (if different from the association valaciclovir and trimethoprim/sulfamethoxazole), disease status, and history of infections over the last 12 months. Serum IgG levels, electrophoretic peak, and weight-based IgG quantitation were collected if available. Dose, frequency, route and place of IgRT were recorded. Physicians rated from 0 = none to 4 = very important their expectations about IgRT regarding the following items: secondary prevention of moderate and severe infections, improvement of survival, improvement of quality of life, decreased frequency of hospitalizations, decreased antibiotics consumption.
Data collected at each routine visit during follow-up included number and type of infections since last visit, serum IgG levels when available, adverse events, compliance, and changes in modalities of administration. At the end of the study, every physician was asked to rate how much IgRT met his/her expectations.
Statistics
Descriptive statistics included the number of observations, mean and standard deviation, extreme values, and median for continuous variables and numbers and percentages for categorical variables. Percentages were calculated on non-missing observations. HM were classified as MM, CLL, aggressive NHL (aNHL), indolent NHL (iNHL), or HL. Aggressive NHL included T-cell lymphoma, T lymphoblastic lymphoma, Burkitt lymphoma, Richter syndrome, and angioimmunoblastic T-cell lymphoma. Indolent NHL included follicular lymphoma and Waldenström macroglobulinemia. Renal insufficiency was defined as creatinine clearance <60 mL/min (Modification of Diet in Renal Disease [MDRD] formula [Citation15]). Serum IgG levels were estimated after subtraction of the IgG monoclonal peak, if applicable. Change in serum IgG level was described in patients with IgG values at baseline and at last visit on IgRT. The annual incidence of infections was estimated by a Poisson regression model with the natural logarithm of follow-up duration as an offset term. The infections were classified by the physician according to the World Health Organization (WHO) grading with severe infections being WHO grade >2. Characteristics of patients were described and compared according to the route of IgRT using chi squared tests or Fisher’s exact tests for categorical variables and Student’s t tests for continuous variables. Statistical evaluation was performed using the software package SAS release 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics
The study was conducted under the French Regulations for non-interventional studies and in conformity with the principles of the original version of the Helsinki Declaration (1975), revised in 2008. The non-interventional nature of the research protocol was confirmed by the French ethics committee (Comité de Protection des Personnes Ile-de-France VI). This protocol was approved by the French Medical Research Data Processing Advisory Committee (CCTIRS) and the French Information Technology and Privacy Commission (CNIL).
Results
Patient disposition
From the seminal study [Citation14], which comprised 238 patients starting IgRT for HM-associated SID, 160 received either octagam® IV or gammanorm® SC and were followed-up. This sample is analyzed in the present study. Patients had a mean age of 67.3 ± 11.2 years and 61.9% (99/160) were males. Most of them (133/160, 83.1%) were fully active or only restrained by the disease during strenuous activities (ECOG-PS ≤1). Fifty-four (33.8%) patients were suffering from MM, 54 (33.8%) from CLL, 19 (11.9%) from aNHL, 29 (18.1%) from iNHL, and 4 (2.5%) from HL. HM had been diagnosed on average 5.2 ± 4.5 years before inclusion. Sixty (37.5%) patients were considered as in the relapsing or refractory phase (). Autoimmune cytopenia was present in 14 (8.8%) patients. Renal insufficiency was observed in 23 (15.0%) patients while 20 (12.5%) had diabetes mellitus. Serum IgG levels prior to IgRT initiation were available in 138 patients (86.3%). Conversely, they were not known for 22 patients including 10 with MM, 5 with CLL, 4 with aNHL and 3 with iNHL. Median values of serum IgG levels were 4.2 g/L. Serum IgG level was <5 g/L in 91 patients (65.9%) and <6 g/L in 111 (80.4%). Patients starting IgRT using the IV route were slightly older, had more frequently relapsing or refractory HM, had higher ECOG score. No significant differences were evidenced regarding IgG level or history of infections ().
Table 1. Hematological malignancy status and evolution during the follow-up period.
Table 2. Baseline characteristics of patients according to the route of IgRT.
Mean follow-up duration was 8.7 ± 4.0 months (median 10.8 months). During the follow-up period, HM remained stable in 87 (57.6%) patients, improved in 42 (27.8%), and worsened in 5 (3.3%). Seventeen patients died, 5 in a sepsis context.
Immunoglobulin replacement therapy
IgRT was initiated with IVIg in 50 patients (31.3%) and with SCIg in 110 (68.8%) patients. Mean duration of exposure (excluding periods when IgRT was temporarily interrupted) was 8.4 ± 4.0 months (median 8.8 months). From the 50 patients starting with IVIg, 4 (8.0%) changed to SCIg, 3 (6.0%) switched from hospital-based to home-based infusions but one (2.0%) switched back to hospital; on the other hand, from the 110 patients starting with SCIg, 4 (3.6%) switched to IVIg. SCIg started at hospital but 93 of 110 (84.6%) switched to home-based administration and 5 of the 93 switched back to hospital-based infusions.
Patient’s diaries documented a total of 398 IVIg and 3421 SCIg infusions. All but one IVIg infusion were performed at hospital while 92.6% of SCIg administrations were performed at home. The dose of IVIg was about 4-fold the dose of SCIg for each infusion but with a 4-fold decreased frequency of infusion (mean monthly dose of 387 ± 78 mg/kg for IVIg versus mean weekly dose of 97 ± 45 mg/kg for SCIg [resulting in a mean monthly dose of 388 mg/kg]). Compliance was excellent: only 4.4% of the patients missed one injection and no patient missed more than one injection. Sixty (37.5%) patients did not interrupt their treatment during the follow-up period whereas 23 (14.4%) suspended IgRT during summertime, 13 (8.1%) suspended IgRT regardless of the season and 49 (30.6%) definitely stopped IgRT for futility (the physician considered the IgRT was no longer useful i.e the patient was no longer at risk of infection or the infection risk had decreased significantly). Regarding their HM, these patients were composed of 16 (29.6%) patients with MM, 15 (27.8%) patients with CCL, 5 (26.3%) with aNHL, 12 (41.4%) with iNHL and 1 (25%) with HL. In addition, IgRT was stopped by 15 patients (9.4%) for personal convenience (N = 9), lack of efficacy (N = 3), tolerance concern (N = 1), organizational problem (N = 1). Early stoppers included 77 patients who suspended IgRT regardless of the season, or definitely stopped IgRT. Early stoppers had a similar history of infections before entry than those who pursued IgRT until the end of follow-up (N = 60) (data not shown). During follow-up, incidence rates of infections were always lower in early stoppers but differences did not reach statistical significance (data not shown). The proportion of early stoppers ranged from 0% to 100% in each center but numbers were often very small. The dose was never adapted during summertime and patients either received a full dose or suspended IgRT. Globally the IgRT dose was rarely adapted: six out of 160 patients changed IgRT dose at least once over the study.
Evolution of serum IgG levels
IgRT increased serum IgG levels by a mean of 3.4 ± 2.4 g/L from baseline to last visit on IgRT. A higher increment was observed following the SC route but the difference did not reach statistical significance (p = 0.10) (). The proportion of patients with serum IgG levels <5 g/L decreased from 69.2% at baseline to 15.9% at last visit whatever the route of administration (p = 0.14). Change in serum Ig level was not different between early stoppers and patients who pursued IgRT (p = 0.14).
Table 3. Evolution of serum IgG trough levels during the follow-up period.
History of infections
Eight patients (5%) received antibiotic prophylaxis (apart from the conventional association valaciclovir and trimethoprim/sulfamethoxazole) at least once during follow-up. Five patients (3.1%) died from infection. The annual incidence of sepsis (independent of the type or severity) decreased from 2.43 [95% confidence interval: 2.18–2.70] infectious episodes per patient*year within the 12 months prior to study entry to 1.90 [95% CI 1.46–2.49] during follow-up (p = 0.001). It decreased from 2.06 [95% CI 1.82–2.33] to 1.28 [95% CI 0.99–1.66] for infections that required antibiotics (p < 0.0001), from 0.45 [95% CI 0.36–0.57] to 0.27 [95% CI 0.19–0.39] (p = 0.09) for infections that required intravenous antibiotics, and from 0.58 [95% CI 0.45–0.73] to 0.31 [95% CI 0.22–0.44] (p = 0.04) for those that led to hospitalization. The annual incidence of World Health Organization (WHO) grade >2 infections was 0.51 [95% CI 0.39–0.67] prior to IgRT and 0.30 [95% CI 0.21–0.42] during the follow-up period (p = 0.09). Similar reduction in overall annual incidence of infections was observed in patients considered as relapsing or refractory and in those who were not relapsing or refractory to treatment. Only the rate of infections that required antibiotics tended to be more reduced in patients neither relapsing nor refractory (). Additionally, the annual incidence of infections during follow-up was independent of the previous management of HM: 1.92 [95% CI 0.75–4.94] for HM never treated, 1.99 [95% CI 1.32–2.99] for first-line treatment, and 1.88 [95% CI 1.29–2.73] for those receiving or having received more than one line of treatment.
Table 4. Annual incidence of infections.
Physician’ expectations
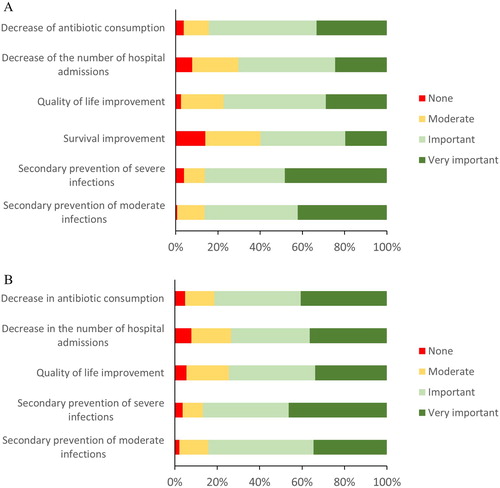
At IgRT initiation, physicians expected to prevent infections, to decrease hospitalization rates and to decrease antibiotic consumption. Improvement in quality of life was also anticipated (). During the study period, physician’ expectations were globally satisfied. Satisfaction regarding the prevention of moderate infections was rated “important” or “very important” by treating physicians for 117 out of 139 documented patients (84.2%). Prevention of severe infections was rated as “important” or “very important” for 86.8%, improvement of quality of life and improvement of survival rate for 74.5% of the 140 documented patients. The decrease in the number of hospitalizations and reduction of antibiotics use was also rated as “important” or “very important” for 73.6 and 81.4% of patients respectively ().
Figure 1. Physicians’ expectations when prescribing immunoglobulin replacement therapy (A) and satisfaction during follow-up (B). Red bars = none; yellow bars = moderate; light green bars = important; dark green bars = very important. From the overall patient population (n = 160), data were missing for the following response: Secondary prevention of moderate infections (n = 6 in A and 21 in B), secondary prevention of severe infections (n = 7 in A and 24 in B), survival improvement (n = 3 in A), quality of life improvement (n = 1 in A and 15 in B), decrease in the number of hospital admissions (n = 5 in A and 20 in B) and decrease of antibiotic consumption (n = 1 in A and 15 in B).
Adverse events
IgRT was well tolerated. No serious adverse events were recorded during the study. Only 6 patients (3.7%) reported adverse events: pneumonia (IVIg), itching/swelling/redness (2 patients, SCIg), blood pressure increase (IVIg), and rash (SCIg), cold sensation/asthenia (SCIG). Furthermore, creatinine clearance did not markedly vary during the follow-up period independent of the IgRT route, from 83.17 ± 26.82 µmol/L prior to IgRT initiation to 79.92 ± 28.05 µmol/L after 12 months of IgRT.
Discussion
The study aimed at describing efficacy and safety of IgRT in patients with HM-related SID and, more generally, at evaluating if physicians’ expectations were satisfied. IgRT was preferentially initiated and pursued through the home-based subcutaneous route. The IgRT route at initiation determined the route of further infusions and switches of route of administration were rare. For both routes of administration, IgG doses were in accordance with current recommendations of about 400 mg/kg/month [Citation8,Citation9]. IgRT led to a mean increase in serum IgG of 3.4 g/L. Concomitantly, compared to the 12 months before IgRT initiation, the annual incidence of infections dropped from 2.43 to 1.90. IgRT was well tolerated. This study also found that IgRT was temporarily interrupted during summer in some patients or was stopped for futility when the physician considered it was no longer useful because he/she judged the patient was not at risk of infection anymore or the infection risk decreased significantly. Finally, physicians considered that their initial expectations regarding IgRT were globally met.
Route of IgRT
Similar performances of SCIg and IVIg in managing infection risks have been documented in SID patients [Citation16,Citation17]. Here, more than two thirds of the patients initiated IgRT via the home-based SC route, with almost the same monthly dose compared to patients on IVIg. Our results did not fall in line with the results of the SIGN registries showing that more than 90% of the German SID patients received lower doses of IgG when on IVIg compared to SCIg treatment (199 vs 343 mg/kg per 4 weeks, respectively) [Citation18]. An interesting feature of SCIg compared to IVIg is the lower incidence of systemic reactions [Citation19] but at the cost of a higher frequency of local reactions [Citation20,Citation21]. Furthermore, SCIg has been shown to provide better health-related quality of life, higher patient satisfaction [Citation22], and faster functional recovery with less time off work [Citation23,Citation24]. Additionally, improved autonomy is provided with SCIg as it can be easily self-administered at home [Citation25]. Our study confirmed the preferred home-setting for SCIg administration in France. Very few patients who received SCIg home-treatment switched back to IVIg or hospital-based SCIg treatment, suggesting that home-based SCIg are a viable alternative for patients with HM-associated SID. Similar results have already been described in patients suffering from PID [Citation26].
Efficacy of IgRT
Monitoring of serum IgG level over time is recommended since serum IgG levels are correlated with the incidence of infections in patients with PID receiving IgRT [Citation27,Citation28]. In our real-life study, monitoring of IgG levels was far from optimal. They were even unavailable at IgRT initiation for 22 patients, nearly half of whom had MM. This could be surprising as MM diagnosis requires serum Ig measurements. However, as IgRT could have been initiated any time after MM diagnosis, baseline serum Ig levels could have been missing. Serum IgG trough levels increased from baseline to last visit under IgRT. Overall, this increase was consistent with a previous report in HM-affected patients treated with SCIg (mainly CLL patients) that showed a mean increase in serum IgG of 2.4 g/L at steady state (after 6 months) [Citation20]. Of note, we found no statistically significant difference in serum IgG level increase between IVIg and SCIg, although several studies have reported that serum IgG trough levels were higher with the SCIg route than those with the IVIg route [Citation16,Citation17,Citation29]. This could be due to a lack of power.
The medically-relevant objective pursued with IgRT is to reduce the frequency of infections. Rates of severe infections (requiring antibiotics, IV antibiotics, leading to hospitalization, WHO grade >2) were reduced after IgRT initiation. Our results are consistent with several studies showing that prophylactic IVIg decreases bacterial infection rates in patients with HM [Citation30–35]. Hence, a meta-analysis estimated that IVIg were associated with a reduced risk ratio (RR) of 0.45 for major infections in HM-associated HG [Citation36]. A retrospective study comparing hospitalizations due to infections and use of antibiotics showed a decrease in antibiotics consumption and a reduction in hospital admissions in SID patients treated with SCIg [Citation20]. Similar observations were made in a monocenter retrospective analysis of 61 patients with HG secondary to B-cell lymphoproliferative disorders. Results showed that the annual incidence of infectious episodes with SCIg decreased from 0.46 to 0.11, with a parallel decrease in antibiotic consumption [Citation16]. More recently, in SID patients mostly affected by HM, IgRT decreased the overall infection rate from 82% to 21% of patients during one year [Citation18]. Nonetheless, a recent retrospective study addressing patients with MM during the post-hematopoietic stem cell transplant period showed that after a period of 12 months, incidence of infections was not statistically significant (P = 0.631) between patients receiving IVIg and patients not receiving IVIg. Incidences of infections were 31.3% and 34.8%, respectively. No difference was also observed regarding infections requiring hospitalization and multiple episodes of infections [Citation37]. Furthermore, as pointed out by Windegger et al., the impact of HM stage on the efficacy of IgRT may be important and remains to be addressed [Citation38]. Here, we observed that IgRT reduced the annual incidence of infections whether or not patients were relapsing or refractory suggesting that IgRT efficacy may be independent of the HM status.
Tolerance of IgRT
Systemic reactions were infrequent and occurred with SCIg as well as with IVIg. This observation was consistent with the well-established good tolerability profile of IgRT. Local reactions were rare and observed only with SCIg. Local reactions with SCIg are well-known to be of mild intensity and to resolve within a day [Citation39]. Systemic reactions are usually more frequently reported with IVIg [Citation40].
Creatinine levels and creatinine clearance did not markedly vary during the follow-up period independent of the route of IgRT administration. Although rare, renal impairment has been reported to be a significant concern in IgRT. Typically, renal insufficiency may arise within 10 days post IVIg initiation, with common manifestations such as hematuria, mild to moderate proteinuria and serum creatinine level peaking from day 3 to 8 [Citation41]. Several cases resulting from IVIg, but not SCIg, have been reported in the literature, as recently reviewed [Citation40]. The presence of sucrose in some IVIg preparations has been associated with most of these adverse outcomes [Citation42]. Both IVIg and SCIg preparations used in our study were sucrose-free, markedly alleviating the risk for renal impairment.
Satisfaction of physicians’ expectations
Initiation of IgRT was mainly driven by the expectation of reducing moderate and severe infections in HM patients. This has been well documented [Citation16,Citation30–35,Citation43]. In light of the marked reduction in the incidence of infections in our study, physicians’ expectations were largely satisfied. Consequently, their expectations regarding the decrease in hospitalizations and antibiotics consumption were also largely met. Physicians also expected an improvement in patients’ quality of life. During this study quality of life was not evaluated and, thus, we were unable to assess if IgRT has fulfilled this need.
Interestingly, some physicians’ expectations were beyond evidence-based medicine data as for the perspective of survival improvement. Currently available data do not support IgRT-mediated survival increase in patients with CLL or MM or in patients undergoing bone marrow transplantation [Citation36,Citation44,Citation45]. Of note, in two meta-analyses [Citation36,Citation45] only the one-year or two-year survival rates were registered and this may explain that there was no significant effect on mortality [Citation18]. Nevertheless, physicians largely considered that IgRT may improve patients survival. The decrease in the rate of infection episodes may support this global expectation.
Interruption of IgRT
Another interesting finding of this study was that only 37.5% of patients did not interrupt IgRT during the follow-up. Twenty-three patients (14.4%) stopped IgRT during the summer and resumed the treatment at autumn, winter and spring. There are no clear guidelines on the benefits of pursuing IgRT without interruption even during summertime when the risk of infections spontaneously decreases. Recently, Reiser et al observed that IgRT was interrupted in every fourth patient. The period of interruption encompassed summer months but on average extended over 10 months. However, as acknowledged by the authors, the question of effectiveness of the seasonal treatment could not be addressed [Citation18].
More surprisingly, IgRT was stopped in 49 patients (30.6%) for futility according to the physician's opinion. This means that the treating physician considered the risk of infection to have returned to normal after several weeks or months. To our knowledge, there is no data supporting that the infectious risk will decrease over such a short period of time. Of note, termination of treatment for futility does not seem to be related to a particular HM in our study. It raises the question about the definition of futility and when to consider the patient is no longer at risk of infection. Unfortunately, our study did not capture the detailed elements retained by physicians to conclude the futility of keeping the treatment in place.
Of note, 38 (77.6%) of these 49 patients were recruited by the same center. This proportion may result from therapeutic habits of a single physician. Characteristics of early stoppers regarding the history of infections and infectious risk during the study were not different than those of patients who pursued IgRT.
Limitations of the study
Limitations are inherent to the observational nature of the study. Large amounts of data were lacking on serum IgG levels as centers did not monitor IgG levels at regular intervals. Moreover, centers may measure serum IgG levels preferentially in patients at higher risk for infection and this could partly explain the variability observed. The longitudinal follow-up involved only the patients receiving octagam® IV or gammanorm® SC. Therefore, the present results cannot be generalized to the entire inception cohort of 238 patients.
Conclusion
The 160 patients who started IgRT with octagam® or gammanorm® were mainly affected by MM, CLL, and to a lesser extent by iNHL and aNHL. IgRT was preferentially given through the SC route. Frequency and severity of infections decreased during IgRT while serum IgG levels increased between baseline and last dosage under IgRT, independent of the IgRT route. Switches of administration route were infrequent and IgRT schedules were heterogeneous among centers.
IgRT was well tolerated. Physicians’ expectations were largely met during the follow-up period in terms of prevention of moderate or severe infections, decrease in the number of hospitalizations for sepsis and antibiotic consumption.
Acknowledgments
The authors would like to thank all participants who included patients in the longitudinal part of the EPICURE study: Dr Vivianne Dubruille (CHU Nantes); Dr Faress Husseini (Hôpital Pasteur de Colmar); Dr Michel Maigre (Hôpital Louis Pasteur); Dr Miguel Carreiro (CH de Montauban); Dr Lina Aljassem (CHI Meulan); Dr Olivier Décaux (Hôpital de Rennes Sud); Dr Frédéric Bauduer (CH de la Côte Basque); Pr Brigitte Dreyfus (CH La Miletrie); Dr Emmanuel Fleck (Centre Hospitalier de La Rochelle); Dr Stéphane Lepretre (Centre Henri Becquerel); Dr Bruno Royer (CHU d'Amiens Sud); Dr Alain Saad (CH de Béziers); Dr Laurent Sutton (CH Victor Dupouy); Dr Loic Fouillard (CH Meaux); Dr Gwenaëlle Leyral (HIA Begin); Dr Amélie Servetaz (CH Robert Debré); Dr Laurent Voillat (CH William Morey); Dr Claudine Sohn (CHI Toulon-La Seyne-sur-Mer); Dr Florence Lachenal (Centre Hospitalier Pierre Oudot). They also thank François Carron (Soladis Clinical Studies) for site monitoring.
Disclosure statement
JFV has served as a consultant for Amgen and NOVARTIS and participated on advisory boards and/or as a speaker at medical education events supported by Amgen, SHIRE, GSK, CSL-Behring, Octapharma and Novartis and declares no competing financial interest. FB participated on advisory boards and/or as a speaker at medical education events supported by Novartis, BMS and Genzyme and declares no competing financial interest. BR participated on advisory boards supported by Amgen, Janssen, Celgene and Octapharma and declares no competing financial interest. OD participated on advisory boards and/or as a speaker at medical education events supported by Amgen, Celgene, Janssen, Novartis, Octapharma, Sebia, Siemens, Takeda, The Binding Site and declare no competing financial interest. YF and PC work as independent statisticians and have no conflict of interest to declare. JCC works as an employee of Octapharma in France.
ORCID
Sylvain Choquet http://orcid.org/0000-0002-7791-0470
Yann Fardini http://orcid.org/0000-0002-6800-1777
Additional information
Funding
References
- Friman V, Winqvist O, Blimark C, et al. Secondary immunodeficiency in lymphoproliferative malignancies. Hematol Oncol. 2016;34(3):121–132. doi: 10.1002/hon.2323
- Mouthon L, Fermand J-P, Gottenberg J-E. Management of secondary immune deficiencies: what is the role of immunoglobulins? Curr Opin Allergy Clin Immunol. 2013;13:S56–S67. doi: 10.1097/01.all.0000433132.16436.b5
- Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2012;13(2):106–111. doi: 10.1016/j.clml.2012.11.011
- De Angelis F, Tosti ME, Capria S, et al. Risk of secondary hypogammaglobulinaemia after rituximab and fludarabine in indolent non-Hodgkin lymphomas: a retrospective cohort study. Leuk Res. 2015;39(12):1382–1388. doi: 10.1016/j.leukres.2015.10.013
- Dhalla F, Misbah SA. Secondary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2015;15(6):505–513. doi: 10.1097/ACI.0000000000000215
- Hallek M. Chronic lymphocytic leukemia: 2015 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90(5):446–460. doi: 10.1002/ajh.23979
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v78–v84. doi: 10.1093/annonc/mdv303
- EMA. Guideline on core SmPC for human normal immunoglobulin for intravenous administration (IVIg) EMA/CHMP/BPWP/94038/2007 Rev. 4 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/12/WC500136433.pdf.
- EMA. Core summary of product characteristics for human normal immunoglobulin for subcutaneous and intramuscular administration EMA/CHMP/BPWP/410415/2011 rev 1 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184870.pdf.
- Lachance S, Christofides AL, Lee JK, et al. A Canadian perspective on the use of immunoglobulin therapy to reduce infectious complications in chronic lymphocytic leukemia. Curr Oncol. 2016;23(1):42. doi: 10.3747/co.23.2810
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for diagnosis, indications for treatment, response assessment and supportive management of chronic lymphocytic leukemia. Blood. 2018 Mar 14. DOI:10.1182/blood-2017-09-806398. PubMed PMID: 29540348.
- Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3):S1–S46. DOI:10.1016/j.jaci.2016.09.023.
- de Souza KJ, Ferro RS, Prestes-Carneiro LE, et al. Infectious diseases and immunological markers associated with patients with non-Hodgkin lymphoma treated with rituximab. Immunopharmacol Immunotoxicol. 2018 Feb;40(1):13–17. DOI:10.1080/08923973.2017.1392562 PubMed PMID: 29094629.
- Benbrahim O, Viallard J-F, Choquet S, et al. A French observational study describing the use of human polyvalent immunoglobulins in hematological malignancy-associated secondary immunodeficiency. Eur J Haematol. 2018;101(1):48–56. DOI:10.1111/ejh.13078.
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. PubMed PMID: 16908915. doi: 10.7326/0003-4819-145-4-200608150-00004
- Compagno N, Cinetto F, Semenzato G, et al. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: a single-center experience in 61 patients. Haematologica. 2014;99(6):1101–1106. doi: 10.3324/haematol.2013.101261
- Shankar T, Gribowicz J, Crespo M, et al. Subcutaneous IgG replacement therapy is safe and well tolerated in lung transplant recipients. Int Immunopharmacol. 2013;15(4):752–755. doi: 10.1016/j.intimp.2013.02.021
- Reiser M, Borte M, Huscher D, et al. Management of patients with malignancies and secondary immunodeficiencies treated with immunoglobulins in clinical practice: long-term data of the SIGNS study. Eur J Haematol. 2017 Aug;99(2):169–177. DOI:10.1111/ejh.12900. PubMed PMID: 28467615.
- Gardulf A, Nicolay U. Replacement IgG therapy and self-therapy at home improve the health-related quality of life in patients with primary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2006 Dec;6(6):434–442. PubMed PMID: 17088648. doi: 10.1097/01.all.0000246619.49494.41
- Hammarstrom L, Samuelsson J, Grimfors G. Subcutaneous gammaglobulin for patients with secondary hypogammaglobulinaemia. Lancet. 1995 Feb 11;345(8946):382–383. PubMed PMID: 7531264.
- Kobrynski L. Subcutaneous immunoglobulin therapy: a new option for patients with primary immunodeficiency diseases. Biologics. 2012;6:277–287. PubMed PMID: 22956859.
- Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006 Jan;26(1):65–72. PubMed PMID: 16418804. doi: 10.1007/s10875-006-8905-x
- Abolhassani H, Sadaghiani MS, Aghamohammadi A, et al. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta analysis. J Clin Immunol. 2012 Dec;32(6):1180–1192. PubMed PMID: 22730009. doi: 10.1007/s10875-012-9720-1
- Shapiro RS. Why I use subcutaneous immunoglobulin (SCIG). J Clin Immunol. 2013 Jan;33(Suppl 2):S95–S98. PubMed PMID: 23264027. doi: 10.1007/s10875-012-9853-2
- Misbah S, Sturzenegger MH, Borte M, et al. Subcutaneous immunoglobulin: opportunities and outlook. Clin Exp Immunol. 2009 Dec;158 Suppl 1:51–59. PubMed PMID: 19883424. doi: 10.1111/j.1365-2249.2009.04027.x
- Bienvenu B, Cozon G, Hoarau C, et al. Does the route of immunoglobin replacement therapy impact quality of life and satisfaction in patients with primary immunodeficiency? Insights from the French cohort “visages”. Orphanet J Rare Dis. 2016 Jun 22;11(1):83. PubMed PMID: 27334100. doi: 10.1186/s13023-016-0452-9
- Orange JS, Grossman WJ, Navickis RJ, et al. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137(1):21–30. doi: 10.1016/j.clim.2010.06.012
- Lucas M, Lee M, Lortan J, et al. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125(6):1354–1360. doi: 10.1016/j.jaci.2010.02.040
- Hoffmann F, Grimbacher B, Thiel J, et al. Home-based subcutaneous immunoglobulin G replacement therapy under real-life conditions in children and adults with antibody deficiency. Eur J Med Res. 2010 Jun 28;15(6):238–245. PubMed PMID: 20696632.
- Boughton BJ, Jackson N, Lim S, et al. Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol. 1995 Mar;17(1):75–80. PubMed PMID: 7621634. doi: 10.1111/j.1365-2257.1995.tb00322.x
- Bunch C. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia: a randomized, controlled clinical trial. N Engl J Med. 1988;319(14):902–907. doi: 10.1056/NEJM198810063191403
- Chapel H, Dicato M, Gamm H, et al. Immunoglobulin replacement in patients with chronic lymphocytic leukaemia: a comparison of two dose regimes. Br J Haematol. 1994;88(1):209–212. doi: 10.1111/j.1365-2141.1994.tb05002.x
- Chapel HM, Lee M, Hargreaves R, et al. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. The UK group for immunoglobulin replacement therapy in multiple myeloma. Lancet. 1994 Apr 30;343(8905):1059–1063. PubMed PMID: 7909099. doi: 10.1016/S0140-6736(94)90180-5
- Griffiths H, Brennan V, Lea J, et al. Crossover study of immunoglobulin replacement therapy in patients with low-grade B-cell tumors. Blood. 1989 Feb;73(2):366–368. PubMed PMID: 2492832.
- Molica S, Musto P, Chiurazzi F, et al. Prophylaxis against infections with low-dose intravenous immunoglobulins (IVIG) in chronic lymphocytic leukemia. Results of a crossover study. Haematologica. 1996;81(2):121–126.
- Raanani P, Gafter-Gvili A, Paul M, et al. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis. Leuk Lymphoma. 2009;50(5):764–772. doi: 10.1080/10428190902856824
- Park S, Jung CW, Jang JH, et al. Incidence of infection according to intravenous immunoglobulin use in autologous hematopoietic stem cell transplant recipients with multiple myeloma. Transpl Infect Dis. 2015;17(5):679–687. DOI:10.1111/tid.12424.
- Windegger TM, Lambooy CA, Hollis L, et al. Subcutaneous immunoglobulin therapy for hypogammaglobulinemia secondary to malignancy or related drug therapy. Transfus Med Rev. 2017;31(1):45–50. doi: 10.1016/j.tmrv.2016.06.006
- Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies – a prospective, multi-national study. J Clin Immunol. 2006 Mar;26(2):177–185. PubMed PMID: 16758340. doi: 10.1007/s10875-006-9002-x
- Cherin P, Marie I, Michallet M, et al. Management of adverse events in the treatment of patients with immunoglobulin therapy: a review of evidence. Autoimmun Rev. 2016;15(1):71–81. doi: 10.1016/j.autrev.2015.09.002
- Itkin YM, Trujillo TC. Intravenous immunoglobulin-associated acute renal failure: case series and literature review. Pharmacotherapy. 2005;25(6):886–892. doi: 10.1592/phco.2005.25.6.886
- Epstein JS, Zoon KC. Important drug warning: immune globulin intravenous (human)(IGIV) products. Neonatal Netw. 2000;19(2):60.
- Jurlander J, Geisler CH, Hansen MM. Treatment of hypogammaglobulinemia in chronic lymphocytic leukaemia by low-dose intravenous gammaglobulin. Eur J Haematol. 1994;53(2):114–118. doi: 10.1111/j.1600-0609.1994.tb01874.x
- Visentin A, Compagno N, Cinetto F, et al. Clinical profile associated with infections in patients with chronic lymphocytic leukemia. Protective role of immunoglobulin replacement therapy. Haematologica. 2015;100(12):e515–e518. DOI:10.3324/haematol.2015.126763.
- Raanani P, Gafter-Gvili A, Paul M, et al. Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2008;27(5):770–781. doi: 10.1200/JCO.2008.16.8450
