Abstract
We measured serum CORT elevation in wild-type and PACAP-deficient C57BL/6N male mice after acute (1 h) or prolonged (2–3 h) daily restraint stress for 7 d. The PACAP dependence of CORT elevation was compared to that of stress-induced hypophagia. Daily restraint induced unhabituated peak CORT elevation, and hypophagia/weight loss, of similar magnitude for 1, 2, and 3 h of daily restraint, in wild-type mice. Peak CORT elevation, and hypophagia, were both attenuated in PACAP-deficient mice for 2 and 3 h daily restraint. Hypophagia induced by 1-h daily restraint was also greatly reduced in PACAP-deficient mice, however CORT elevation, both peak and during recovery from stress, was unaffected. Thus, hypothalamic PACAPergic neurotransmission appears to affect CRH gene transcription and peptide production, but not CRH release, in response to psychogenic stress. A single exposure to restraint sufficed to trigger hypophagia over the following 24 h. PACAP deficiency attenuated HPA axis response (CORT elevation) to prolonged (3 h) but not acute (1 h) single-exposure restraint stress, while hypophagia induced by either a single 1 h or a single 3 h restraint were both abolished in PACAP-deficient mice. These results suggest that PACAP’s actions to promote suppression of food intake following an episode of psychogenic stress is unrelated to the release of CRH into the portal circulation to activate the pituitary-adrenal axis. Furthermore, demonstration of suppressed food intake after a single 1-h restraint stress provides a convenient assay for investigating the location of the synapses and circuits mediating the effects of PACAP on the behavioral sequelae of psychogenic stress.
Introduction
PACAP neurotransmission is important for the endocrine stress response, with respect to the two major stress output organs, the adrenal medulla and the adrenal cortex (Stroth & Eiden, Citation2010; Stroth et al., Citation2011a). Increased catecholamine secretion from the adrenal medulla elicited either by systemic or psychogenic stressors requires PACAP release at the adrenomedullary synapse (Hamelink et al., Citation2002; Stroth et al., Citation2013). Activation of the hypothalamo-pituitary-adrenal (HPA) axis leading to CORT elevation after stress depends on CRH release, at the median eminence, from PVN neurons of the basal hypothalamus. PACAPergic neurotransmission is also required for CORT elevation secondary to CRH stimulation of ACTH secretion from the anterior pituitary, but only after administration of psychogenic, and not systemic stressors (Hamelink et al., Citation2002; Lehmann et al., Citation2013; Tsukiyama et al., Citation2011). The latter finding raises two important questions about the role of PACAP in stress neurotransmission in the CNS. First, what distinguishes the role of PACAP in the CNS from that in the periphery with respect to the nature and quality of the stressor itself? Do systemic stressors differ in the intensity of stimulation of neuronal activity impinging on the CRH neurons of the PVN? Second, what distinguishes the intrinsic activity of PACAPergic neurotransmission in the CNS compared to that in the peripheral nervous system? PACAP signaling is classically thought to comprise calcium mobilization leading to partial depolarization and calcium influx through voltage-gated channels leading to secretion, as well as cyclic AMP elevation leading to changes in gene transcription (Arimura, Citation1992; Mustafa & Eiden, Citation2006; Vaudry et al., Citation2000). When both effects occur simultaneously, PACAP acts as a “stimulus-secretion-synthesis” coupler, as in its actions at the chromaffin cell (Jiang & Eiden, Citationin press). It is established that regulation of immediate-early gene expression, and CRH gene transcription, during either acute, prolonged, or chronic psychogenic stress requires PACAP (Lehmann et al., Citation2013; Stroth & Eiden, Citation2010; Stroth et al., Citation2011b; Tsukiyama et al., Citation2011), but the question of whether PACAP acts on CRH neurons at the level of both secretion and synthesis has not been explored. To investigate how PACAP signals to the CRH neuron to affect secretion, transcription, or both during HPA axis activation, we used the restraint-stress model as one in which a discrete “dose” of psychogenic stressor can be applied either acutely (1 h or less), in a prolonged fashion (greater than 1 h in a single exposure) or chronically (various periods of restraint over a period of several days).
We have previously shown that the HPA (CORT) response to prolonged (2–3 h) single-restraint stress, measured immediately after cessation of restraint, is PACAP-dependent, i.e. is attenuated in PACAP-deficient mice, and that chronic restraint stress, with as little as 2 h of restraint stress daily, triggers hypophagia, measured by weight loss consequent to decreased food consumption, throughout a three-week period (Mustafa et al., Citation2015). Furthermore, the chronic effects of PACAP deficiency were phenocopied in mice deficient in expression of the PAC1 PACAP receptor, indicating a potential therapeutic target for blunting the maladaptive behaviors emergent during chronic stress (Mustafa et al., Citation2015). Here, we have administered restraint stress for 1, 2, or 3 h over a period of 7 d, and monitored CORT elevation as an index of HPA axis (endocrine) activation, and decreased food consumption as an index of stress-dependent behavioral alteration. CORT elevation following stress was maintained with stress schedules comprising 1, 2, or 3 h of restraint per session, either in single or multiple sessions of stress, and resulted in significant suppression of feeding in all cases. Surprisingly, the PACAP dependence of CORT elevation, and the subsequent behavioral effect of stress on food intake, diverged in the 1-h restraint schedule. The implications of these results for the mechanism of PACAP action in the CNS during psychogenic stress, and the probable sites of action of PACAP in mediating behavioral versus endocrine responses to stress, are discussed.
Materials and methods
Animals
All experiments were conducted using male C57BL/6N wild-type and C57BL/6N PACAP-deficient mice 8–12 weeks of age, generated through an in-house breeding program in accordance with NIH guidelines and standards and housed in cages containing 3–5 male siblings. All mice were individually housed for 7–10 d under a 12-h light/12-h dark cycle (lights off at 6 pm) with food and water ad libitum prior to experimental testing during the light phase. Mice were randomly assigned to experimental groups. All experiments were approved by the NIMH Institutional Animal Care and Use Committee (ACUC) and conducted in accordance with the NIH guidelines. Generation of PACAP knock-out mice have been described previously (Hamelink et al., Citation2002).
Acute, prolonged, and chronic restraint stress
Acute restraint stress was carried out as previously described (Stroth & Eiden, Citation2010). In brief, individually housed animals were transported to the test room in their home cages as described in the aforementioned methods. Mice were then restrained in a DecapiCone with all limbs positioned flat underneath the body to minimize physical pain. The large end of the tube was then rolled shut and secured with adhesive tape. The DecapiCones used were also previously perforated for adequate ventilation, with the tip of the cone opened to allow unobstructed breathing. The restrained mice were then placed back into the home cage for 1–3 h. Cages containing restrained mice were removed and visually examined every 15 min, to assure that restrained mice had neither escaped their restraint, nor were experiencing sufficient restraint to completely immobilize the animal or impede normal breathing. The control, unstressed cohort of mice was left undisturbed in the test room throughout the entire duration of the experiments. Blood was then collected from the tail according to the procedures described below, in a hood providing insulation of sound and odor from the rest of the room. Prior to experimentation, all mice were transported to the test room, allowed to acclimatize for at least 1 h and handled briefly (∼2 min) every day for 3 d, to achieve habituation to handling stress prior to restraint studies and blood sampling. Mice were restrained for 1–3 h on the days shown () as described above, released from the DecapiCone, and returned to the home cage, prior to having tail vein blood sampled within 5 min of release. Unstressed control counterparts were subjected to identical handling and habituation procedures and remained undisturbed in their home cages for 3 h prior to tail blood collection. All experiments were carried out between 11 am and 3 pm (between 5 and 9 h into the 12-h light phase).
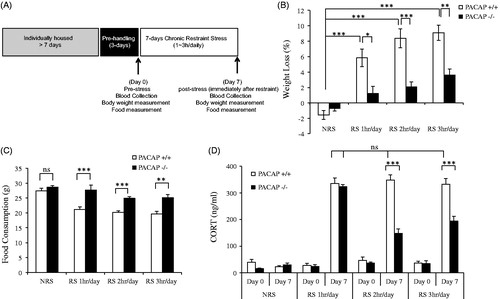
Figure 1. Effects of PACAP deficiency after chronic daily restraint stress for 1, 2, or 3 h per week. (A) Schematic representation of experimental design and timeline for 7-d chronic stress. Animals were single-housed for over a week and habituated to handling for 3 d prior to restraint stress. Chronic restraint stress (CRS) was administered for either 1-, 2-, or 3-h duration daily over seven consecutive days. Control cohort of animals was subjected to identical conditions except that they remained undisturbed in their home cages in the test room during the daily CRS period. Tail blood was systemically collected on days 0 and 7 for CORT assay. Body weight and food were measured on day 0 (pre-stress) and day 7 (post-stress). (B) Body weight loss during 7-d chronic restraint in wild-type and PACAP-deficient mice. Chronic restraint stress daily for either 1-, 2-, or 3 h, led to significant weight loss in wild-type restrained mice when measured on day 7 post-stress, but not in PACAP-deficient mice. Results are expressed as mean ± SEM (n = 8–15 for each group). Initial body weights for wild-type mice 24.75 ± 0.42 g (mean ± SEM, n = 41); for PACAP-deficient mice 24.71 ± 0.21 g (mean ± SEM, n = 42). Two-way ANOVA followed by Bonferroni’s post hoc test, *p < 0.05, **p < 0.01, ***p < 0.001. (C). Lack of weight loss after chronic restraint in PACAP−/− mice is due to reversal of decreased food intake. Measurement of food intake in PACAP+/+ mice subjected to stress demonstrated that weight loss was a direct consequence of hypophagia, and this stress-related decrease in food intake was not observed in PACAP−/− mice. Results are expressed as mean ± SEM (n = 8–19 per group). Two-way ANOVA followed by Bonferroni’s post hoc test, **p < 0.01; ***p < 0.001; ns, not significant. (D) CORT elevation during 7-d chronic restraint stress (CRS) in wild-type and PACAP-deficient mice. CORT was significantly elevated across 7 d for wild-type group upon CRS 1–3 h daily. PACAP deficit significantly reduced this elevation when restraint stress was applied 2 h or 3 h daily, but not 1 h daily. Results are expressed as mean ± SEM (n = 8–15 per group). Repeated measures two-way ANOVA followed by post hoc Bonferroni test, ***p < 0.001; ns: not significant.
Body weight and food consumption
All mice (control and stress groups) were weighed daily throughout the entire duration of experimental testing. Percentage weight loss was calculated as the weight of the mouse on the indicated day following initiation of the chronic restraint protocol, divided by the weight of the mouse on day 0 prior to initiation of the chronic restraint protocol, times 100. Food consumption of standard chow was determined daily, in some experiments, by weighing the food pellets (in grams) and determining the amount of food consumed by each mouse over a 24-h period.
Blood sampling and determination of plasma corticosterone levels
For tail vein blood sampling, sharp surgical scissors were used to remove ∼1–2 mm of the most distal portion of the tail while the mouse was loosely held but not restrained by the tail. The tail was gently massaged to stimulate blood flow, and blood (approximately 20 μl) was collected directly into heparinized 40 mm microhematocrit tubes (SafeCrit, IRIS Inc., Westwood, MA). Blood flow was stopped by applying gentle finger pressure, and then cauterizing the wound with silver nitrate applicator swabs (GF Health Products, Atlanta, GA) before returning the mouse to its home cage. Plasma was then collected by centrifugation (Micro-hematocrit centrifuge, IRIS, Inc., Westwood, MA) and stored at −80 °C. Corticosterone levels in serum were determined using the DetectX corticosterone ELISA kit (Arbor Assays, Ann Arbor, MI) according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed for statistical significance by two-way ANOVA followed by Bonferroni post-hoc test using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA). Repeated measures were conducted using STATISTICA (StatSoft, Tulsa, OK).
Results
PACAP-deficient mice previously showed a marked attenuation of CORT elevation, compared to wild-type mice, for either a single prolonged (>2 h) restraint stress, or for chronic restraint stress (2–3 h daily, for 7–21 d). Furthermore, prolonged restraint stress administered daily for one week or more resulted in weight loss secondary to hypophagia in wild-type, but not PACAP-deficient mice, suggestive of PACAP-dependent anhedonia or anxiety associated with chronic restraint stress (Mustafa et al., Citation2015). To examine the relationship between the duration of daily restraint stress and its endocrine and behavioral effects in a more quantitative fashion, we administered restraint stress daily, for 7 d, to groups of wild-type and PACAP-deficient mice for 1, 2, or 3 h daily (). Chronic restraint stress daily for either 1 h, or 2 h, or 3 h led to significant weight loss and reduction of food intake in wild-type mice, but not in PACAP-deficient mice (two-way ANOVA analysis indicated significant stress and genotype effects for body weight loss (, genotype effect: F(1, 88) = 46.542, p < 0.001; stress effect: F(3, 88) = 40.132, p < 0.001; genotype × stress: F(3, 88) = 10.322, p < 0.001) and feeding behavior (, genotype effect: F(1, 90) = 52.737, p < 0.001; stress effect: F(3, 90)=19.196, p < 0.001; genotype ×stress: F(3, 90) = 3.271, p = 0.025). Correlated with hypophagia, peak CORT levels were significantly elevated in wild-type mice across 7 d, but were attenuated in PACAP-deficient mice upon 2-h or 3-h restraint daily (, repeated measures two-way ANOVA followed by post hoc Bonferroni test, genotype effect, F(2, 77) = 35.24, p < 0.001; stress effect F(6, 154) = 53.02, p < 0.001; genotype × stress, F(6, 154) = 12.38, p < 0.001; PACAP+/+ versus PACAP−/−, p < 0.001). However, PACAP-deficient mice receiving only 1-h daily restraint showed a dissociation between CORT elevation (similar to wild-type mice, i.e. no effect of PACAP deficiency) () and hypophagia (reversal of the effect of restraint on cumulative feeding behavior, i.e. a significant effect of PACAP deficiency) ().
The somewhat surprising dissociation between effects of 1-h chronic restraint stress on CORT elevation and hypophagia raised two important questions. First, are peak CORT levels measured immediately after cessation of stress reflective of total CORT elevation occasioned by restraint stress, after either single or chronic administration of either acute or prolonged restraint? Second, can restraint stress be administered in a dose-dependent way, so that the neurochemical, endocrine, and behavioral effects of single administration, as well as the cumulative effects of chronic administration, can be distinguished?
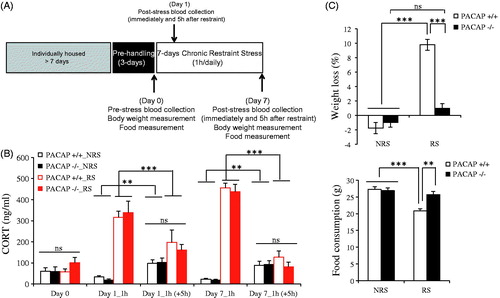
To address the first question, we repeated the chronic administration of 1-h restraint stress, which had resulted in cumulative (one week) effects on feeding behavior, without effects on cumulative peak CORT elevation, but now measured not only peak CORT elevation immediately following stressor administration, but also 5 h after the cessation of restraint on day 1 and day 7 in the chronic restraint paradigm (). On both day 1 and day 7 of chronic administration, peak CORT levels, as well as CORT levels 5 h after cessation of restraint, were indistinguishable between wild-type and PACAP-deficient mice (, repeated measures two-way ANOVA indicated no significant genotype effect, F(5, 12) = 0.61, p = 0.693; significant stress effect, F(5, 12) = 77.27, p < 0.001; and significant genotype × stress, F(5, 12) = 0.528, p = 0.751). However, as demonstrated previously, weight loss due to decreased feeding (hypophagia) was significantly greater in stressed compared to non-stressed wild-type mice, but absent in stressed compared to non-stressed PACAP-deficient mice (, two-way ANOVA analysis on body weight, genotype effect: F(1, 19) = 31.792, p < 0.001; stress effect: F(1, 19) = 90.063, p < 0.001; genotype × stress: F(1, 19) = 45.394, p < 0.001; two-way ANOVA analysis on food consumption, genotype effect: F(1, 19) = 7.473, p = 0.015; stress effect: F(1, 19) = 22.354, p < 0.001; genotype × stress: F(1, 19) = 10.222, p = 0.006). The data show a clear dissociation between the PACAP dependence of CORT elevation as a consequence of 1-h restraint, and the PACAP dependence of hypophagia as a consequence of 1-h restraint.
Figure 2. Peak CORT elevation and decay across 1-h chronic daily restraint stress for one week. (A) Schematic representation of experimental design and timeline for 7-d chronic stress, with two times of blood collection on day 1 and day 7. Animals were single-housed for over a week and habituated to handling for 3 d prior to restraint stress. Chronic restraint stress (CRS) was administered for 1-h duration daily over seven consecutive days. Control cohort of animals was subjected to identical conditions except that they remained undisturbed in their home cages in the test room during the daily CRS period. Tail blood was collected twice (immediately and 5 h after restraint) on days 0, 1, and 7 for CORT assay. Body weight and food were measured on day 0 (pre-stress) and day 7 (post-stress). (B). CORT elevation on day 1 and day 7 during 7-d restraint stress in wild-type and PACAP-deficient mice. CORT was significantly elevated on days 1 and 7 for both wild-type and PACAP-deficient group immediately after 1-h restraint. CORT level dropped to a similar level to unstressed animals (NRS) when blood was collected 5 h later. Results are expressed as mean ± SEM (n = 5 for each group). Repeated measures two-way ANOVA followed by post hoc Bonferroni test, **p < 0.01, ***p < 0.001; ns, not significant. (C) Weight loss (upper panel) and food consumption (lower panel) during 7-d restraint-effect of PACAP deficiency. Chronic restraint stress daily for 1 h, led to significant weight loss and decreased food consumption in wild-type restrained mice when measured on day 7 post-stress, but not in PACAP-deficient mice. Results are expressed as mean ± SEM (n = 5 for each group). Two-way ANOVA followed by Bonferroni’s post hoc test, **p < 0.01, ***p < 0.001; ns: not significant. Initial body weights for wild-type mice 26.57 ± 0.41 g (mean ± SEM, n = 10); for PACAP-deficient mice 24.95 ± 0.43 g (mean ± SEM, n = 10).
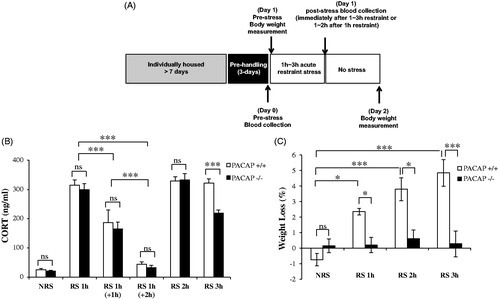
To address the second question, and to be certain that there were no intermediate times following restraint stress at which a differential effect on CORT elevation could account for PACAP-dependent behavioral effects of acute (1 h) restraint stress, we designed a set of experiments in which a single restraint stress was administered acutely (1 h) or for a prolonged period (2–3 h), and peak CORT elevation, as well as CORT levels after the peak, but prior to return to baseline, were measured (). As shown in , neither peak CORT elevation immediately after cessation of 1-h restraint, nor intermediate CORT elevation 1 h following cessation of 1-h restraint, nor baseline CORT levels 2 h following cessation of 1-h restraint, were significantly different between wild-type and PACAP-deficient mice (). Controls for restraint stress administered for 2 and 3 h showed significantly elevated peak CORT in both wild-type and PACAP-deficient mice at both times, with peak CORT identical in wild-type and PACAP-deficient mice at 2 h, and peak CORT significantly greater in wild-type than in PACAP-deficient mice at 3 h (, two-way ANOVA indicated no significant genotype effect: F(1, 124) = 5.322, p = 0.023; significant stress effect: F(5, 124) = 142.426, p < 0.001 and genotype × stress: F(5, 124) = 3.429, p = 0.006; Bonferroni’s post hoc test showed no difference between wild-type and PACAP-deficient mice for 1-h or 2-h restraint, but significant attenuation in PACAP-deficient mice for 3-h restraint).
Figure 3. Effects of PACAP deficiency on weight loss and total CORT elevation following a single administration of restraint stress for 1, 2, or 3 h. (A) Schematic representation of experimental design and timeline for acute or one time prolonged restraint stress. Animals were single-housed for over a week and habituated to handling for 3 d prior to restraint stress. Restraint stress (ARS) was administered for 1 ∼ 3-h duration. Control cohort of animals was subjected to identical conditions except that they remained undisturbed in their home cages in the test room during the restraint session. Tail blood was collected immediately after 1–3-h restraint, or 1 ∼ 2 h after 1-h restraint) for CORT assay. Body weight was measured on day 1 (pre-stress) and day 2 (post-stress). (B) Effect of PACAP deficiency on CORT elevation after one time 1 ∼ 3-h restraint stress. CORT was significantly elevated after 1 ∼ 3-h restraint stress in wild-type mice. PACAP deficiency significantly reduced this elevation only after 3-h restraint. Results are expressed as mean ± SEM (n = 4–17 per group). Two-way ANOVA followed by Bonferroni’s post hoc test, ***p < 0.001; ns, not significant. (C) Effect of PACAP deficiency on weight loss after single (1–3 h) restraint stress. Body weight was significantly decreased after 1–3 h restraint stress in wild-type mice, but not in PACAP-deficient mice. Results are expressed as mean ± SEM (n = 4–10 per group). Two-way ANOVA followed by Bonferroni’s post hoc test, *p < 0.05, ***p < 0.001; ns, not significant. Initial body weights for wild-type mice 25.26 ± 0.49 g (mean ± SEM, n = 24); for PACAP-deficient mice 24.83 ± 0.37 g (mean ± SEM, n = 25).
We further examined whether feeding behavior was affected after a single restraint stress administration, as occurred cumulatively after one week of chronic stress, and if the behavior impact of a single acute or prolonged episode of restraint stress correlated with CORT elevation following restraint. Although decreased food intake 24 h after single restraint stress could not be accurately measured, there was a significant decrease in body weight 24 h after a single restraint for either 1, 2, or 3 h (, two-way ANOVA followed by Bonferroni’s post hoc test, genotype effect: F(1, 48) = 29.322, p < 0.001; stress effect: F(3, 48) = 8.002, p < 0.001; genotype x stress: F(3,48) = 6.607, p < 0.001; PACAP+/+ versus PACAP−/−, p < 0.05 after 1-h or 2-h restraint, p < 0.001 after 3-h restraint). Furthermore, there was no significant positive correlation between magnitude of CORT elevation and weight loss in wild-type mice restrained for 1, 2, or 3 h, and no significant negative correlation between CORT levels and body weight in PACAP-deficient mice ().
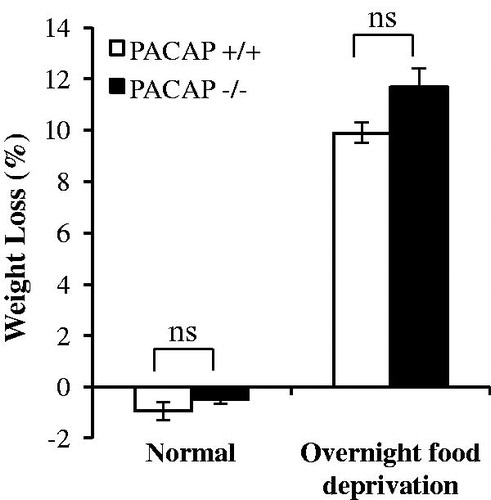
We wished to determine whether or not the resistance to weight loss in PACAP-deficient mice following stress administration might be secondary to a generalized resistance to weight loss in these mice. Accordingly, we deprived both wild-type and PACAP-deficient mice to food for a 24-h period, and measured weight loss in both groups. In this experiment, we chose cohorts of wild-type and PACAP-deficient mice of similar age, and these two cohorts of mice were of similar body weights (24.13 ± 0.91 g versus 24.61 ± 0.43 g). Wild-type and PACAP-deficient groups showed a similar loss of body weight after food deprivation (, two-way ANOVA followed by Bonferroni’s post hoc test indicated significant fast effect: F(1, 26) = 390.683, p < 0.001; but no genotype effect: F(1, 26) = 3.732, p = 0.066; genotype × fast effect: F(1, 26) = 1.27, p = 0.271; PACAP+/+ versus PACAP−/− in food deprivation, p = 0.086). Thus, we conclude that resistance to weight loss triggered over a 24-h period by a single restraint stress in PACAP-deficient mice is a specific resistance to stress-induced hypophagia/anorexia, rather than a metabolic thrift response. This is consistent with the close relationship between weight loss and decreased food intake in C57BL/6N mice, and resistance to weight loss and maintenance of food intake in PACAP-deficient C57BL/6N mice, after one or several weeks of chronic restraint stress.
Figure 4. Weight loss after 24-h food deprivation. Animals were housed in normal cages supplied with food or from which food was removed for a 24-h period. There is no significant difference in weight loss due to fasting, between wild-type mice and PACAP-deficient mice. Results are expressed as mean ± SEM (n = 4–10 per group). Two-way ANOVA followed by Bonferroni’s post hoc test, ns, not significant. Initial body weights for wild-type mice 24.13 ± 0.91 g (mean ± SEM, n = 14); for PACAP-deficient mice 24.61 ± 0.43 (mean ± SEM, n = 13).
Discussion
PACAP is expressed throughout the hypothalamus and extended amygdala in rodent and primate brain (Piggins et al., Citation1996; Vigh et al., Citation1991), and PACAP-expressing neurons are also found in other areas of the brain, including the hippocampus and prefrontal cortex (Arimura et al., Citation1991; Hannibal, Citation2002), that are involved in interceptive and exteroceptive processing of stressful stimuli. PACAPergic synapses densely innervate CRH-expressing neurons of the PVN of the hypothalamus (Legradi et al., Citation1998), and PACAP infusion into the brain increases CRH mRNA expression (Grinevich et al., Citation1997) and immediate–early gene activation in PVN, and stress-like behavioral effects (Agarwal et al., Citation2005), which are observed also upon direct infusion of PACAP into the PVN itself (Norrholm et al., Citation2005). PACAPergic synapses are also found on CRH-expressing neurons in the BNST of the rat (Kozicz et al., Citation1997), an area in which PACAP expression is up-regulated by both stress and CORT (Lezak et al., Citation2014), in which PACAP infusion causes anxious behavior (Hammack et al., Citation2009), and in which infusion of the PAC1 receptor antagonist PACAP (6–38) antagonizes both anorexia and CORT elevation in response to chronic stress (Roman et al., Citation2014). The BNST is also a site of PACAP-dependent regulation of both c-Fos and Fos-B expression as a result of chronic stress (Gaszner et al., Citation2012; Lehmann et al., Citation2013). PACAP infusion into the central amygdala decreases active coping in response to stress (Legradi et al., Citation2007), consistent with PACAPergic innervation patterns of this brain area (Piggins et al., Citation1996; Schafer et al., Citation2010). Understanding how PACAP functions as a mediator of the stress response at each of these various synaptic locations has the potential to reveal important features of how brain stress circuitry functions (Hammack & May, Citation2015; Hammack et al., Citation2010; Herman & Cullinan, Citation1997; Herman et al., Citation2005; Radley, Citation2012; Radley et al., Citation2006; Ulrich-Lai & Herman, Citation2009) and will be a necessary prelude to translational efforts to target PACAPergic synapses for pharmacological interventions in stress-associated psychiatric illness (Ressler et al., Citation2011).
We have previously shown that CORT elevation and suppressed eating following either a single prolonged (>2 h) restraint stress, or chronic daily prolonged restraint stress, is significantly lower in PACAP-deficient, compared to wild-type mice (Mustafa et al., Citation2015). Likewise, PACAP deficiency blunts both CORT elevation and the emergence of stress-related anxious and depressive behaviors during chronic social defeat in mice (Lehmann et al., Citation2013). These results are consistent with a dual role for PACAP neurotransmission in both the endocrine and behavioral effects of psychogenic stress, and raise the question of whether behavioral effects of restraint stress are secondary to, or occur in parallel with, CORT elevation. Here, we attempted to calibrate more finely the relationship between the duration of daily restraint stress and its effects on HPA axis activation (CORT elevation) and suppression of feeding behavior. We also examined the PACAP dependence of each, by comparison of wild-type with PACAP-deficient mice, in order to gain further insight into the likely mechanism of action of PACAP at multiple presumed sites within the brain, including the PVN neurons of the hypothalamus responsible for CRH release, and downstream pituitary-adrenal axis activation, manifested as increased plasma CORT levels.
Administration of restraint stress for 1, 2, or 3 h, daily, produced a non-habituating elevation of CORT levels measured immediately after cessation of stress, on day 7, that was equivalent for all three daily stress durations. There was also significant weight loss after 7 d with all three stress schedules, in all cases corresponding to decreased food consumption. These data suggest that weight loss was secondary to psychogenic, rather than metabolic, effects of chronic stress. This paradigm also provided an avenue for examination of the effects of discrete doses of psychogenic stress administered via acute, prolonged, and chronic psychogenic schedules, and correlating surrogate read-outs for HPA activation (CORT elevation) with behavioral effects (hypophagia) during and following stressor administration. The application of this stress paradigm has allowed us to draw two major conclusions about the role of PACAP in stress responding in the CNS.
The first conclusion is that PACAP acts functionally at the CRH neuronal ensemble of the PVN to support prolonged and chronic CRH secretion indirectly, through CRH gene activation, but not by stimulating CRH secretion directly. PACAP deficiency, as expected, reduced peak CORT elevation in response to chronic stress with 2-h and 3-h daily restraint schedules for 7 d, but not in response to 1-h daily restraint for 7 d. Furthermore, these results on peak CORT levels were recapitulated after a single administration of restraint stress for 1, 2, or 3 h. CORT measurement following peak elevation during restraint also showed a decay in CORT elevation following stress that was PACAP-dependent with prolonged stress, but PACAP-independent following acute stress. PACAP deficiency had little or no effect on either peak activation or decay of CORT elevation (i.e. total CORT secretion) following an episode of acute (1 h) restraint stress. We interpret CORT elevation as a read-out of the functional patency of the stress transduction response at the level of the CRH neuron of the PVN in these experiments, especially as previous investigations have demonstrated that PACAP-deficiency, in the mouse, does not affect either the responsivity of the pituitary to release ACTH in response to infusion of CRH, or the responsivity of the adrenal cortex to release CORT in response to ACTH (Tsukiyama et al., Citation2011). Thus, our results strongly suggest that PACAPergic neurotransmission within the hypothalamus does not directly affect secretion of CRH into the portal circulation. In contrast, regulation of CRH biosynthesis appears to be strongly regulated by PACAP at the level of the PVN: elevation of CRH mRNA in PVN after initiation of restraint stress is prompt, reversible upon cessation of stress, maintained throughout application of both acute and prolonged restraint, and wholly abolished in PACAP-deficient mice (Stroth et al., Citation2011b). Elevation of serum CORT after initiation of restraint stress is also prompt, also reversible upon cessation of stress, and also maintained throughout application of both acute and prolonged restraint. However, the requirement for PACAPergic transmission in the elevation of serum CORT after initiation of restraint stress is not immediate, but rather emerges only after secretion has been prolonged, indicating that PACAP controls CRH transcription, but not secretion, at the level of the PVN, indirectly allowing maintenance of CRH secretion during prolonged and chronic stress but not affecting stress-induced CRH secretion immediately and directly. This is unlike the effects of PACAP at the adrenomedullary synapse, where both secretion and biosynthesis of catecholamines are controlled by PACAP release (Hamelink et al., Citation2002; Stroth et al., Citation2013). However it is highly consistent with our earlier observations on the effects of PACAP deficiency on CORT elevation following social defeat. There is no PACAP dependence of CORT elevation after 1 d of social defeat, and the PACAP dependence of CORT elevation associated with social defeat emerges only after several days, perhaps reflecting the gradual lengthening of the duration of subjective psychogenic stress perceived by the subject animals as social defeat is repeated on a daily basis (Lehmann et al., Citation2013).
The second conclusion is that PACAP clearly has two separate functional sites of action in CNS for endocrine and behavioral stress responding. At the level of the PVN, PACAPergic neurotransmission supports hypothalamopituitary activation, and CORT secretion, by maintaining CRH biosynthesis during periods of prolonged stress. At a second site or sites, PACAP promotes behavioral responses to psychogenic stress. Thus, administration of a single restraint stress, at graded durations of 1, 2, or 3 h, revealed a pronounced uncoupling between the PACAP dependence of HPA axis activation (CORT elevation), and suppression of feeding, strongly suggesting that PACAP’s actions to enable the behavioral effects of both acute and prolonged stress does not occur primarily at the level of the CRH neurons of the PVN projecting to the median eminence.
Where might PACAP act to suppress feeding as a consequence of psychogenic stress? There is a plethora of anatomical reports suggesting loci at which activation of PACAPergic synapses might plausibly affect feeding behavior, including brain stem (Das et al., Citation2007; Dun et al., Citation1996; Hannibal, Citation2002), PVN (i.e. CRH-positive neurons projecting to brain stem rather than median eminence; Geerling et al., Citation2010), and an additional complement of sites at which injection of PACAP suppresses feeding, including the ventromedial nuclei of the hypothalamus (Resch et al., Citation2014), and the BNST (Kocho-Schellenberg et al., Citation2014). However, it is noteworthy that feeding behavior in the absence of stress is apparently normal in PACAP-deficient mice, and that PACAP-deficient mice respond metabolically normally to food withdrawal (this report). PACAP-dependent c-Fos gene regulation following psychogenic stress occurs at a number of cortical and sub-cortical sites (Gaszner et al., Citation2012; Tsukiyama et al., Citation2011), and induction of fosB gene expression following chronic social defeat stress appears to be held in check by PACAP expression in the BNST and pre- and infralimbic cortices (Lehmann et al., Citation2013). A rapidly emerging literature suggests that PACAP may act at the level of the extended amygdala (Dore et al., Citation2013; Hammack & May, Citation2015; Hammack et al., Citation2009, Citation2010; Seiglie et al., Citation2015) as an anxiogenic neurotransmitter that promotes altered behavior, including anhedonia, decreased social interaction, and decreased exploration, following psychogenic stress. Dore et al. have suggested that the anxiogenic and anhedonic, but not the anorectic or endocrine effects of PACAP, in the rat, are mediated via CRHR1 receptors within the brain (Dore et al., Citation2013). Thus, PACAP may influence multiple behaviors emerging during stress, each at separate sites in the brain. PACAP dependence of altered immediate–early gene expression in medial prefrontal cortex (mPFC) (Lehmann et al., Citation2013) suggests a site of action, in addition to the extended amygdala, of particular relevance given the broad regulatory effects of the mPFC in psychogenic stress responding in mammals.
Both conclusions converge to illuminate the clear dissociation between PACAPergic signaling to the CRH neurons of the PVN that project to the median eminence, modulating the endocrine effects of psychogenic stress, and PACAPergic signaling at multiple additional sites within the CNS, mediating multiple stress-induced behaviors. In addition to emphasizing the independence of endocrine and behavioral sequelae of both acute and chronic stress, these findings offer the possibility of detailed examination of the PACAP receptor requirements for each, in an experimentally tractable stress model. Should these also be distinguishable, pharmacological agents that suppress PACAP-dependent behavioral sequelae of psychogenic stress, without affecting the physiological regulation of the HPA axis, might be sought.
Acknowledgements
We acknowledge support from the National Institute of Health (NIMH) Intramural Research Program (1ZIAMH002386); and David Huddleston for assistance with management of mouse colonies and genotyping.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Agarwal A, Halvorson LM, Legradi G. (2005). Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res 138:45–57.
- Arimura A. (1992). Pituitary adenylate cyclase-activating polypeptide (PACAP): discovery and current status of research. Regul Peptides 37:287–303.
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. (1991). Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129:2787–9.
- Das M, Vihlen CS, Legradi G. (2007). Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J Comp Neurol 500:761–76.
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V. (2013). CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology 38:2160–9.
- Dun NJ, Miyazaki T, Tang H, Dun EC. (1996). Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: implication of sensory and autonomic functions. Neuroscience 73:677–86.
- Gaszner B, Kormos V, Kozicz T, Hashimoto H, Reglodi D, Helyes Z. (2012). The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience 202:283–99.
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. (2010). Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 518:1460–99.
- Grinevich V, Fournier A, Pelletier G. (1997). Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res 773:190–6.
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee H-W, Eiden LE. (2002). Pituitary adenylate cyclase activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA 99:461–6.
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. (2009). Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34:833–43.
- Hammack SE, May V. (2015). Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol Psychiatry 78:167–77.
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. (2010). Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci 42:327–40.
- Hannibal J. (2002). Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 453:389–417.
- Herman JP, Cullinan WE. (1997). Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84.
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29:1201–13.
- Jiang SZ, Eiden LE. (in press). PACAPergic synaptic signaling and circuitry mediating mammalian responses to psychogenic and systemic stressors. In: Reglodi D, Tamas A, editors. Pituitary adenylate cyclase activating polypeptide – PACAP. New York: Springer Nature.
- Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, Edwards S, et al. (2014). PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology 39:1614–23.
- Kozicz T, Vigh S, Arimura A. (1997). Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Res 767:109–19.
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. (2007). Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plasticity 2007:79102–13.
- Legradi G, Hannibal J, Lechan RM. (1998). Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett 246:145–8.
- Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE. (2013). PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology 38:702–15.
- Lezak KR, Roman CW, Braas KM, Schutz KC, Falls WA, Schulkin J, May V, Hammack SE. (2014). Regulation of bed nucleus of the stria terminalis PACAP expression by stress and corticosterone. J Mol Neurosci 54:477–84.
- Mustafa T, Eiden LE. (2006). The secretin superfamily: PACAP, VIP and related peptides. In: Lim R, editor. Handbook of neurochemistry and molecular neurobiology: XIII neuroactive peptides and proteins. Heidelberg: Springer. p. 1–36.
- Mustafa T, Jiang SZ, Eiden AM, Weihe E, Thistlethwaite I, Eiden LE. (2015). Impact of PACAP and PAC1 receptor deficiency on the neurochemical and behavioral effects of acute and chronic restraint stress in male C57BL/6 mice. Stress 18:408–18.
- Norrholm SD, Das M, Legradi G. (2005). Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN). Regul Pept 128:33–41.
- Piggins HD, Stamp JA, Burns J, Rusak B, Semba K. (1996). Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol 376:278–94.
- Radley JJ. (2012). Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front Behav Neurosci 6:7.
- Radley JJ, Arias CM, Sawchenko PE. (2006). Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci 26:12967–76.
- Resch JM, Maunze B, Phillips KA, Choi S. (2014). Inhibition of food intake by PACAP in the hypothalamic ventromedial nuclei is mediated by NMDA receptors. Physiol Behav 133:230–5.
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, et al. (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470:492–7.
- Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, Hammack SE, May V. (2014). PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 47:151–65.
- Schafer MK, Mahata SK, Stroth N, Eiden LE, Weihe E. (2010). Cellular distribution of chromogranin A in excitatory, inhibitory, aminergic and peptidergic neurons of the rodent central nervous system. Regul Pept 165:36–44.
- Seiglie MP, Smith KL, Blasio A, Cottone P, Sabino V. (2015). Pituitary adenylate cyclase-activating polypeptide induces a depressive-like phenotype in rats. Psychopharmacology (Berl) 232:3821–31.
- Stroth N, Eiden LE. (2010). Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience 165:1025–30.
- Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. (2011a). PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann N Y Acad Sci 1220:49–59.
- Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. (2013). PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology 154:330–9.
- Stroth N, Liu Y, Aguilera G, Eiden LE. (2011b). Pituitary adenylate cyclase-activating polypeptide (PACAP) controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. J Neuroendocrinol 23:944–55.
- Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, MoritaY, Tanida M, et al. (2011). PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress 14:368–75.
- Ulrich-Lai YM, Herman JP. (2009). Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409.
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. (2000). Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Revs 52:269–324.
- Vigh S, Arimura A, Koves K, Somogyvari-Vigh A, Sitton J, Fermin CD. (1991). Immunohistochemical localization of the neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP), in human and primate hypothalamus. Peptides 12:313–18.
