Abstract
Regular physical activity produces resistance to the negative health consequences of stressor exposure. One way that exercise may confer stress resistance is by reducing the impact of stress on diurnal rhythms and sleep; disruptions of which contribute to stress-related disease including mood disorders. Given the link between diurnal rhythm disruptions and stress-related disorders and that exercise both promotes stress resistance and is a powerful non-photic biological entrainment cue, we tested if wheel running could reduce stress-induced disruptions of sleep/wake behavior and diurnal rhythms. Adult, male F344 rats with or without access to running wheels were instrumented for biotelemetric recording of diurnal rhythms of locomotor activity, heart rate, core body temperature (CBT), and sleep (i.e. REM, NREM, and WAKE) in the presence of a 12 h light/dark cycle. Following 6 weeks of sedentary or exercise conditions, rats were exposed to an acute stressor known to disrupt diurnal rhythms and produce behaviors associated with mood disorders. Prior to stressor exposure, exercise rats had higher CBT, more locomotor activity during the dark cycle, and greater %REM during the light cycle relative to sedentary rats. NREM and REM sleep were consolidated immediately following peak running to a greater extent in exercise, compared to sedentary rats. In response to stressor exposure, exercise rats expressed higher stress-induced hyperthermia than sedentary rats. Stressor exposure disrupted diurnal rhythms in sedentary rats; and wheel running reduced these effects. Improvements in sleep and reduced diurnal rhythm disruptions following stress could contribute to the health promoting and stress protective effects of exercise.
Introduction
Disruptions in circadian/diurnal rhythms contribute to the development and symptom severity of mood disorders (Breslau et al., Citation2004; Emens et al., Citation2009; Germain & Nielsen, Citation2003; Hasler et al., Citation2010; Kyung Lee & Douglass, Citation2010; Ohayon & Roth, Citation2001; Papadimitriou & Linkowski, Citation2005; van Liempt et al., Citation2012). Depressed individuals, for example, have disrupted sleep/wake behavior (Hasler et al., Citation2010; Kyung Lee & Douglass, Citation2010; Ohayon & Roth, Citation2001) and circadian temperature rhythms (Avery et al., Citation1982; Hasler et al., Citation2010; Monk et al., Citation1994; Posener et al., Citation2000; Souetre et al., Citation1989). Disrupted sleep is an independent risk factor for the development of depression (Kronfeld-Schor & Einat, Citation2012; Mallon et al., Citation2000; Riemann et al., Citation2001; Roberts et al., Citation2000) and insomniacs, compared with controls, were more likely to develop depression at one-year follow-up (Ford & Kamerow, Citation1989). Given that disruptions in sleep/wake behavior and circadian physiology contribute to the development of stress-related mood disorders, identifying behavioral factors that can mitigate these stress-induced disruptions in sleep/wake behavior and diurnal/circadian rhythms will aid in reducing their burden on society.
One such behavioral factor is regular physical activity, which is effective at preventing and treating stress-related mood disorders including depression (Babyak et al., Citation2000; Blumenthal et al., Citation2007; Dunn et al., Citation2005), anxiety (Barbour et al., Citation2007; Newman & Motta, Citation2007), and post-traumatic stress disorder (Manger & Motta, Citation2005; Newman & Motta, Citation2007). Pre-clinical studies also demonstrate similar stress-protective effects of regular physical activity (Duman et al., Citation2008; Greenwood & Fleshner, Citation2011; Greenwood et al., Citation2003; Vollert et al., Citation2011). Regular physical activity is a behavioral factor that, when voluntary, will naturally align with the light/dark cycle (Verwey et al., Citation2013) and is an endogenous zeitgeber that can entrain sleep/wake behavior (Edgar & Dement, Citation1991). Prior data indicate that voluntary exercise can hasten the re-entrainment of the sleep/wake cycle in humans following a 4-d phase-shift (Yamanaka et al., Citation2010) and improve sleep in rodent models (Blanco-Centurion & Shiromani, Citation2006; Lancel et al., Citation2003). Importantly, exercise-induced increases in sleep quality are associated with a decreased suicide risk in depressed patients (Davidson et al., Citation2013). Taken together, these studies support the possibility that voluntary exercise may protect against stress-induced disruptions in diurnal physiological rhythms through reinforced entrainment of sleep/wake behavior and diurnal physiological rhythms.
The purpose of the current experiment was to investigate the impact of long-term voluntary exercise on diurnal rhythms of sleep/wake behavior and physiology and to examine whether this behavioral intervention could alleviate stress-induced disruptions to sleep/wake patterns and diurnal physiological rhythms. We hypothesized that prior wheel running would improve sleep and attenuate the disruptions in sleep/wake behavior and physiological rhythms following acute stressor exposure.
Methods
Animals
Adult male F344 rats (n = 16, Harlan Laboratories) weighing 200–230 g, postnatal day 50 at arrival; were housed with controlled temperature (22 °C) and humidity. We selected the F344 rodent strain for several reasons: (1) F344 rats are an inbred strain known to respond to stressors in a consistent fashion and allow us to minimize group size. (2) The F344 rat is also a consistently mid-high range voluntary wheel runner (Greenwood et al., Citation2003, Citation2005b; Speaker et al., Citation2014). The goal of this study was to explore the potential protective effects of exercise on stress-induced disruptions in sleep and physiology, thus by using the F344 rat, we were ensured that we would maximize the impact of stressor exposure and achieve consistent wheel running distances, thus allowing us to minimize group sizes and variability of both dependent and independent measures. The animals were maintained on a 12:12 h light/dark cycle (lights on 0400–1600 h). All rats were housed in Nalgene Plexiglas cages (45 × 25.2 × 14.7 cm) and were allowed to acclimate to the housing conditions for one week. Rats had ad libitum access to food and water and were weighed once per week. All experimental procedures were performed during the inactive cycle and animals were handled during the 1 week acclimation period. Animal discomfort was minimized during all procedures. Experimental protocols for these studies were approved by the University of Colorado Animal Care and Use Committee.
Exercise protocol
Rats in the running group had running wheels (1.1 m circumference; Mini Mitter, Bend, OR) mounted on the inside of their home cage. Wheels in the home cage were also locked during acclimatization and for one week (Week 4) following surgical implantation of the telemetry transmitters. All rats were allowed to exercise for 6 weeks prior to stressor exposure, not including the week recovering from surgical transmitter implant. The 6-week duration of exercise was chosen because previous studies demonstrate that 6 weeks of wheel running is necessary to prevent increased shock-elicited fear and deficits in shuttle box escape learning caused by exposure to inescapable tail shock (Greenwood et al., Citation2005a). Revolutions were automatically recorded by the Activity Wheel Monitor or Vital View software (Mini Mitter, Bend, OR) and distance was calculated by multiplying wheel circumference (1.081 m) by the number of wheel revolutions.
Stress protocol
Rats were exposed to 100 inescapable tail shocks as previously described (Greenwood et al., Citation2005a; Thompson et al., Citation2013). On the day of exposure to tail shock, all rats were taken to a separate room, placed in Plexiglass restraining tubes (23.4 cm long and 7.0 cm in diameter) and exposed to 100, 5-s, 1.5 mA inescapable tail shocks. All rats were exposed to inescapable tail shocks at the same time on the day of stress exposure. Shocks were delivered at random with an average interval of 60-s between shocks and occurred during the inactive (light) cycle between 0800 and 1100 h. After exposure to inescapable tail shock all rats were immediately returned to their home cages where continuous uninterrupted biotelemetry recording continued for 6 d.
Biotelemetry surgeries
The F40-EET biotelemetry transmitters (Data Sciences International, St. Paul, MN) were implanted into animals as previously described (Greenwood et al., Citation2014; Thompson et al., Citation2014). Briefly, animals were fully anesthetized and unresponsive following ketamine (i.p. 75.0 mg/kg), and medetomidine (i.p. 0.5 mg/kg). Animals were shaved and prepped for surgery. A midline incision was made approximately 5.0 cm in length on the ventral abdominal wall. The F40-EET transmitter was placed on the intestines, the biopotential leads were passed through the ventral abdominal wall and then the F40-EET transmitter was sutured to the ventral abdominal wall. Once the transmitter was sutured into place, the ECG leads were positioned to measure cardiac electrical activity. Finally, the EEG leads were placed as previously described (Olivadoti & Opp, Citation2008). Briefly, insulated leads were passed subcutaneously to the base of the skull, where they were attached to pan head stainless steel screws (Plastics One Inc., Roanoke, VA) which served as electroencephalographic (EEG) recording electrodes. Using sterile technique, a hole was drilled to secure the screws in place at stereotaxic coordinates relative to bregma: anterior 2.0; lateral 2.5 and posterior −5.5; lateral 3.0. Once screws and leads were in place, they were embedded in dental acrylic to ensure the integrity of the recording signal. Immediately following surgery rats were given meloxicam (i.p. 1.0 mg/kg) for analgesia after which they recovered on a heating pad at 37 °C until ambulatory. Once ambulatory, rats were placed in their home cages and given one 2.0 mg rimadyl tablet (Bio-Serv, Plymouth Meeting, PA, product# MD150-2) and several fruity bites (Bio-Serv, Plymouth Meeting, PA, product# F6038). Animals were allowed to recover for one week following surgery where running wheels were locked for the exercise group.
Data acquisition and analysis
The F40-EET transmitter allows in vivo real-time measurement of locomotor activity (LA), heart rate (HR), core body temperature (CBT), and electroencephalogram (EEG) in freely behaving animals. Biotelemetry recordings were acquired/analyzed using Dataquest ART 4.3 Gold Acquisition/Analysis Software (Data Sciences International, St. Paul, MN) as previously described (Greenwood et al., Citation2014; Thompson et al., Citation2012, Citation2013, Citation2014). Locomotor activity, heart rate, and EEG were recorded at 500 Hz. Analysis of the sleep/wake cycles was performed using the automated Neuroscore 2.1.0 software (Data Sciences International, St. Paul, MN). The trace EEG signal was subjected to fast Fourier Transformation (FFT), yielding spectra between 0.5 and 30 Hz in 0.5-Hz frequency bins. The delta frequency band was defined at 0.5–4.5 Hz and the theta frequency band was defined as 6.0–9.0 Hz as previously described (Olivadoti & Opp, Citation2008). Arousal state was scored in 10-s epochs and classified as NREMS, REMS, or WAKE on the basis of state-dependent changes in multiple parameters, including the EEG, locomotor activity, heart rate, and CBT as previously described (Olivadoti & Opp, Citation2008). Briefly, wakefulness was defined on the basis of a low amplitude, mixed frequency (delta ≈ theta) EEG accompanied by body movements. Increases in CBT during wakefulness are associated with activity. NREM sleep was identified by increased absolute EEG amplitude with integrated values for the delta frequency band greater than those for the theta frequency and lack of body movements. CBT declines upon entry into NREM sleep until it reaches a regulated asymptote. REM sleep was characterized by a low amplitude EEG with integrated values for the delta frequency band less than those for the theta frequency band. Any epochs containing artifact or electrical noise were marked and excluded from subsequent spectral analysis. Time spent in NREM/REM/WAKE was calculated as a percentage (%) of time spent in a specific behavioral state per hour (i.e. % REM, % NREM, or % WAKE). Additionally, average bout durations of NREM/REM/WAKE were calculated as an average per hour. Finally, for NREM/REM/WAKE the total number of episodes per hour (#) was also calculated. All sleep/wake data were averaged into 20-min, 1-h, or 12-h intervals for statistical analysis. The sedentary and exercise groups had eight animals per group; however; two animals had to be eliminated from the exercise group due to animal destruction of the transmitter leads and a subsequent failure of accurate data collection from the transmitters.
Power analyses were also performed on the EEG after peak wheel running during the baseline period and for several hours following stress exposure and no significant differences were found (data not shown), which is consistent with the prior literature in young rats (Blanco-Centurion & Shiromani, Citation2006). Briefly, Fast Fourier Transformations were performed on the trace EEG signal in order to generate two different frequency bands (delta 0.5–4.5 Hz, theta 6.0–9.0 Hz) and obtain the absolute power for signals (Campbell, Citation2009). Average values for the absolute power were then compared via ANOVA to investigate potential differences due to exercise.
Experimental design
Rats were allowed to acclimate to housing conditions for one week before the running wheels were unlocked (). On day 0, 7 d after arrival to the colony, the wheels were unlocked and these rats were allowed to freely run on wheels for the remainder of the experiment (exercise group), except for 1 week following surgical implantation of the transmitters. The sedentary group was housed in sedentary cages with no access to running wheels. On experimental days 28 and 29, rats were implanted with biotelemetry devices and following recovery rats again either remained sedentary or were allowed access to their wheels for 2 weeks (days 35–49) during which time baseline measures (BL) were also captured in control conditions where rats were completely undisturbed in their home cages for 96 h (days 45–48) to measure diurnal/circadian rhythms and the day/night differences (i.e. diurnal differences for BL shown in ) were obtained by averaging these 4 d together. On day 50, all rats were exposed to acute stress (∼0800–1000). After stressor exposure, rats were immediately returned to their home cages and again completely undisturbed for 6 d (experimental days 51–56) in order to record real-time recovery from the stress-induced diurnal physiological rhythm disruptions.
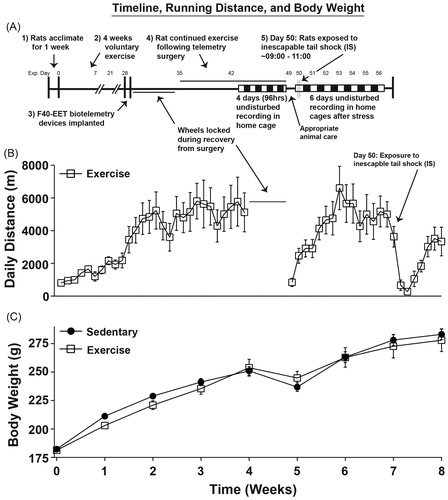
Figure 1. The experimental timeline, total daily running distances, and body weights are shown. (A) Animals were either sedentary or had access to running wheels (exercise) for 4 weeks prior to telemeter implantation. For the exercise group, the wheels were locked during recovery, but were unlocked for the remainder of the study following recovery from surgery. After stress exposure, all animals were allowed to recover from stress exposure completely undisturbed in their home cages for 6 subsequent days. (B) Total daily running distances across the entirety of the experiment are depicted. Wheels were locked during recovery from implantation of telemetry devices for animal safety. (C) Data are depicted in weekly increments demonstrating normal body weight gain across the experiment. Abbreviations are as follows: exp.: experimental; g: grams; m: meters.
Statistical analysis
All data were analyzed with repeated measures ANOVA either as 20-min blocks ( and ), 1-h blocks ( and ), as diurnal differences () or as weeks (), where the sedentary and exercise conditions were used as factors. The diurnal differences were calculated using the averages of the differences between the lights-on values and the lights-off values as previously described (Greenwood et al., Citation2014; Thompson et al., Citation2013, Citation2014). Additionally, during stress exposure the 20-min block data ( and ) were collapsed into pre-stress (4 h), during stress (2 h), or post-stress exposure (14 h). Finally, multiple linear regression analyses were performed to examine potential significant relationships between variables. When appropriate, post hoc analyses for multiple comparisons were performed using Fisher’s protected least significant differences (PLSD). Alpha was set to p < 0.05.
Figure 2. Physiological data are graphed in 1-hr blocks collected during 96 hours (experimental days 45–48) of completely undisturbed monitoring in order to assess the effects of regular voluntary physical activity on diurnal physiological rhythms when compared with sedentary rats. Rats preferred to run on the wheels (A) during the early nocturnal phase (p < 0.01) and (B) had increased locomotor activity at that time when compared with sedentary rats (p < 0.01). (C) There were no differences in the heart rate after six weeks of prior wheel running, but (D) wheel running produced increases in CBT (p < 0.05) during the peak and nadir of the diurnal temperature cycle (p < 0.01). Abbreviations are as follows: a.u.: arbitrary units; bpm: beats per minute; °C: degrees Celsius; m: meters.
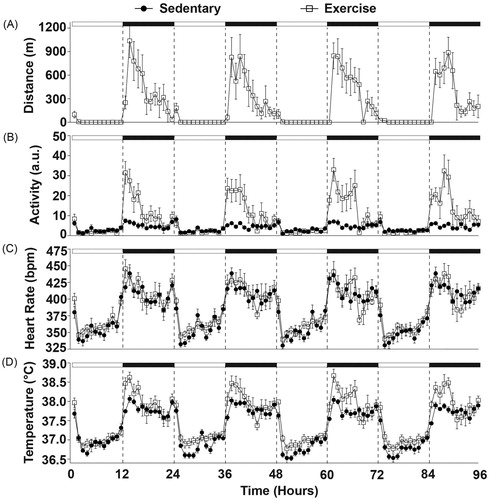
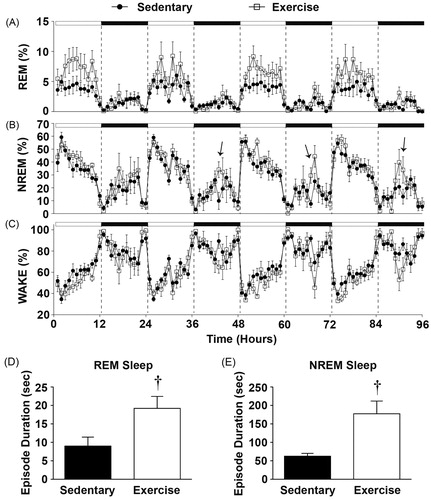
Figure 3. Sleep data graphed in 1-hr blocks revealing that (A) Physically active rats had a significant increase in the time spent in REM sleep during the light cycle. (B) There were no significant differences in the time spent in NREM. (C) There were no significant differences in the time spent in the WAKE state. Importantly, immediately following peak wheel running (mid-dark cycle) exercising rats had on an average (D) longer REM episode durations and E) NREM episode durations when compared with sedentary rats (see Results section for details). Arrows denote times when NREM sleep was significantly different in physically active versus sedentary rats. (†p < 0.05 exercise compared with sedentary).
Results
Experimental timeline and total running distance
delineates the experimental timeline (), total daily distance run (), and average body weights () throughout the experiment. Physically active rats increased total running distance prior to telemetry implantation (F(27, 135) = 8.137; p < 0.0001; main effect of time during weeks 0–4; ). Surgery temporarily reduced the daily distance run (F(22, 110) = 10.122; p < 0.0001; main effect of time during weeks 5–8; ), but running distance quickly recovered to pre-surgery levels before stress exposure. Prior to surgical implantation of the biotelemetry devices, there was a trend towards lower body weights in exercising rats across time (F(3, 36) = 2.47; p = 0.07; interaction between exercise and time during weeks 0–4; ). Following implantation, physically active rats weighed slightly more than sedentary rats initially (week 5), but as they continued exercise they weighed slightly less by week 8 (F(3, 36) = 4.528; p = 0.0086; interaction between exercise and time during weeks 5–8; ) post-hoc analysis revealed no main effects of exercise on body weight from weeks 5–8.
Voluntary exercise increases locomotor activity and shifts diurnal CBT curve higher
Diurnal variations in physiological parameters in physically active and sedentary rats are shown in and during a 96-h time period (experimental days 45–48) where rats remained completely undisturbed in their home cages. There was a significant effect of time on running wheel behavior in the physically active group (F(5, 95) = 6.135; p < 0.0001; ), indicating that rats with wheels in their cages voluntarily ran primarily during the dark cycle. This wheel running behavior was consistent with more overall locomotor activity in physically active rats when compared with sedentary rats (F(1, 12) = 26.985; p = 0.0002; ) and also occurred primarily during the dark cycle (F(95, 1141) = 5.996; p < 0.0001; interaction between exercise and time; ).
After 6 weeks of voluntary exercise there was no statistically significant effect of physical activity on heart rate () which is consistent with prior findings (Masini et al., Citation2011), however; several weeks earlier (experimental days 35–38; 96-h) physically active rats did have a larger increase in heart rate at the onset of the dark cycle when compared with sedentary rats (F(95, 1141) = 1.696; p < 0.0001; interaction between exercise and time; data not shown). These results raise the possibility that cardiovascular adaptations may have occurred between 4 and 6 weeks of voluntary wheel running.
Prior physical activity shifted the diurnal CBT curve higher (F(1, 12) = 6.351; p = 0.0269; main effect of exercise; ) and this effect was most evident during the peak and nadir of the diurnal temperature cycle (F(95, 1140) = 2.03; p < 0.0001; interaction between exercise and time; ).
Voluntary exercise alters sleep/wake behavior in a diurnal specific manner
Regular physical activity also entrained sleep/wake behavior. Voluntary exercise resulted in significantly more time spent in the REM sleep state during the light cycle (F(95, 1140) = 1.787; p < 0.0001; interaction between exercise and time; ), but there was only a trend towards a greater number of REM bouts across the same time period (F(1, 12) = 3.856; p = 0.0731; main effect of exercise; data not shown). There were no differences in the average duration of REM bouts across the 96-h period between sedentary and physically active rats.
Six weeks of physical activity had less impact on the percent of time spent in NREM sleep (), however, exercise altered sleep architecture. Immediately following the peak of voluntary physical activity (mid-dark cycle), the exercise group had longer average NREM bout lengths for each of the four dark cycles of the 96-h baseline period (F(95, 1140) = 1.592; p = 0.0004; interaction between exercise and time; data for NREM bout length across hours not shown) and had a greater number of NREM bouts (F(95, 1140) = 2.036; p < 0.0001; interaction between exercise and time; data not shown) when compared with the sedentary group.
A more in depth analysis of sleep architecture revealed that exercising rats had longer NREM bouts when compared with sedentary rats for the first hour immediately following peak wheel running, which was consistently 6 h after lights off or mid-dark cycle (; see arrows). depicts the average of the first hour immediately after peak wheel running for each of the days of baseline recording for NREM bouts. These results demonstrate that physically active rats had longer average NREM bout durations at this time when compared to sedentary rats (F(1, 12) =14.012; p = 0.0028; a main effect of exercise; ), thus revealing greater consolidation of NREM sleep immediately following voluntary exercise. Although there were no overall statistical differences in average REM bout durations across the 96-h undisturbed recording period as stated previously, it should be noted that exercising rats also had longer REM bouts immediately following cessation of peak wheel running which was also consistent with the 96-h undisturbed recording period (F(1, 12) = 6.667; p = 0.0240; main effect of exercise; ).
Finally, there was a trend towards reduced percent of time spent in WAKE across the 96-h period in physically active animals although this did not reach significance (F(95, 1140) = 1.217; p = 0.0843; ).
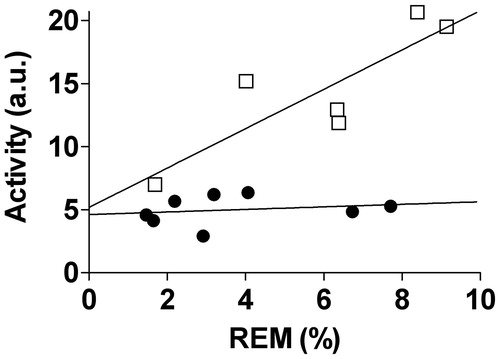
One intriguing finding, based on stepwise multiple regression analysis, was that higher nocturnal locomotor activity levels in exercising animals were positively correlated (y = 0.617x + (−1.61)) with more time spent in the REM sleep state (F(1, 5) = 18.966; p = 0.012; , open squares) during the lights on period. Although there was a similar linear relationship between the average distance run (meters) during the dark cycle and time spent in the REM sleep state during the light cycle, this did not reach statistical significance (F(1, 5) = 2.729; p = 0.1739; data not shown). In contrast to the exercise group, there was no such relationship between nocturnal locomotor activity and time spent in REM sleep during the light cycle in the sedentary group (, closed circles).
Figure 4. Multiple linear regression analysis revealed that exercising animals (open squares) with greater amounts of exercised induced locomotor activity spent more time in REM sleep throughout the 96-hour baseline period. The adjusted r2 = 0.782; p < 0.05 suggesting that the exercise induced variance in locomotor activity can account for 78% of the variance in REM sleep using the following equation: y = 0.617x + (−1.61). In contrast, the sedentary animals (closed circles) had no such relationship. Abbreviations are as follows: a.u.: arbitrary units; REM: rapid eye movement.
Exercise alters physiological responses and sleep/wake behavior in the hours following acute stress exposure
and depict the effects of the interaction between stress and exercise on physiology and sleep/wake behavior for the remaining light cycle (∼5 h) following stress exposure on experimental day 50. These data are depicted in twenty-minute blocks obtained in the home cage immediately prior to stressor exposure (pre-stress), during exposure to stress, and upon return to the home cage (post-stress). The averaged values of each time (pre-stress, stress, and post-stress) are shown in the graph insets. There were no statistical differences between groups in any measure immediately prior to stressor exposure (pre-stress). Struggling in the tail shock tubes can be accurately represented by locomotor activity during stress (Thompson et al., Citation2014). Struggling behavior during stress did not differ between groups (; during stress). Physically active rats, however, had increased locomotor activity following stress exposure when compared with the sedentary rats (F(13, 156) = 3.745; p < 0.0001; ), which was consistent with increased wheel running following acute stress in the physically active animals (F(5, 13) = 3.967; p < 0.0001; ).
Figure 5. Physiological data on the day of exposure to stress are graphed in 20-min blocks (or averaged, insets) before stress in the home cage, during stress and immediately after stress upon return to the home cage. There were no differences between groups 1 h prior to stressor exposure. (A) Wheel running and (B) locomotor activity were increased upon return to the home cage following stress, but there were no differences in locomotor activity during stress exposure. (C) Although stress increased heart rate, it did so equally and did not differ between groups upon return to the home cage following stress. (D) CBT was also elevated as a result of stress exposure, but CBT was higher in animals with prior access to running wheels during stress. CBT remained slightly elevated after return to the home cage following stress, but was slightly higher in exercising animals which may have been a result of the increased wheel running immediately following stress exposure. Abbreviations are as follows: a.u.: arbitrary units; bpm: beats per minute; °C: degrees Celsius; m: meters. (*p < 0.05 compared with pre-stress values; #p < 0.05 compared with during stress values; †p < 0.05 exercise compared with sedentary).
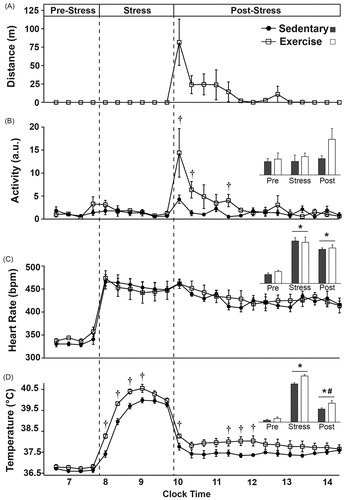
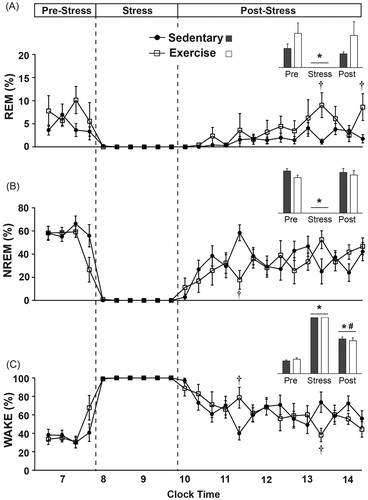
Figure 6. Sleep/wake behavior on the day of exposure to stress is graphed in 20-minute blocks (or averaged, insets) before stress in the home cage, during stress and immediately after stress upon return to the home cage. There were no differences between groups in the hour prior to stress exposure. (A) Stress exposure eliminated REM sleep in both groups, which initially remained reduced following stress, but was not reduced on average (inset). Several hours after stress exposure, exercising rats spent slightly more time in REM sleep when compared to sedentary rats. (B) Stress exposure also eliminated NREM sleep in both groups. Immediately following stress exposure, exercising rats spent slightly less time in NREM sleep and had a trend towards increased NREM sleep several hours later. (C) During stress exposure time spent in WAKE was increased to a maximum. Immediately following stress, exercising rats initially spent slightly more time in WAKE, but several hours later spent slightly less time in WAKE (*p < 0.05 compared with pre-stress values; #p < 0.05 compared with during stress values; †p < 0.05 exercise compared with sedentary).
Stressor exposure increased heart rate to the same extent in physically active and sedentary rats and it remained elevated above pre-stress levels upon return to the home cage for several hours (F(2, 24) = 86.067; p < 0.0001; main effect of stress; see inset for results of post hoc analysis). Similarly, CBT increased during stress and remained elevated following stress; although CBT following stress was not as elevated as during stress (F(2, 24) = 478.375; p < 0.0001; main effect of stress; see inset for results of post hoc analysis).
The exercise group reached a higher CBT during stress when compared with the sedentary group (F(1, 12) = 15.576; p = 0.0019; main effect of exercise; see for results of post hoc analysis) although both groups had similar CBT’s by the end of stressor exposure (F(5, 60) = 4.499; p = 0.0015; interaction between exercise and time; ). Physically active rats had higher CBT’s for several hours following acute stress (F(1, 12) = 5.237; p < 0.041; main effect of exercise; see for results of post hoc analysis).
Stress exposure reduced the time spent in REM sleep (F(2, 24) = 15.741; p < 0.0001; main effect of stress; see inset for results of post hoc analysis) and NREM sleep (F(2, 24) = 124.953; p < 0.0001; main effect of stress; see inset for results of post hoc analysis) to a minimum, but increased time spent in WAKE to a maximum (F(2, 24) = 373.845; p < 0.0001; main effect of stress; see inset for results of post hoc analysis).
Following stress, exercising rats spent slightly more time in REM sleep (F(13, 156) = 2.495; p = 0.004; interaction between exercise and time; see for results of post hoc analysis). In contrast, exercising rats spent less time in NREM sleep immediately following stress (F(13, 156) = 2.289; p = 0.0085; interaction between exercise and time; see for results of post hoc analysis). Consistent with the sleep data, exercising rats initially spent more time in WAKE immediately following stress exposure when compared with the sedentary rats but spent significantly less time in WAKE several hours later (F(13, 156) = 2.167; p = 0.013; interaction between exercise and time; see for results of post hoc analysis).
Voluntary exercise protects against stress-induced flattening of the diurnal rhythms of sleep and temperature
Stress exposure reduced the nightly running distances for post-stress days 1–4, which returned to pre-stress levels by post-stress day 5 (F(5, 25) = 7.205; p = 0.0003; main effect of stress; see for results of post hoc analysis). Exercising rats had greater levels of locomotor activity both before and after stress exposure (F(1, 12) = 29.943; p = 0.0001; main effect of exercise; see for results of post hoc analysis). Stress exposure significantly flattened the diurnal rhythm of locomotor activity in both groups for post-stress days 1–3 (F(5, 60) = 21.587; p < 0.0001; main effect of stress; see for results of post hoc analysis), but this effect lasted longer in the exercise group (F(5, 60) = 12.2294; p < 0.0001; interaction between exercise and stress; see for results of post hoc analysis) likely due to the large exercise effect on the diurnal difference of locomotor activity at baseline. Stress exposure also flattened the diurnal rhythm of heart rate in both groups for post-stress day 1 (F(5, 60) = 20.242; p < 0.0001; main effect of stress; see for results of post hoc analysis). Flattening of diurnal rhythm of heart rate persisted longer in the sedentary relative to the physically active group (F(5, 60) = 2.689; p = 0.0294; interaction between exercise and stress; see for results of post hoc analysis). Stress exposure significantly flattened the diurnal rhythm of CBT in both groups for post-stress days 1 and 2, but this effect persisted until post-stress day 3 in the sedentary group (F(5, 60) = 50.110; p < 0.0001; main effect of stress; see for results of post hoc analysis). Exercise, however, attenuated the stress-induced flattening of the diurnal rhythm of CBT (F(5, 60) = 4.117; p = 0.0028; interaction between exercise and stress; see for results of post hoc analysis).
Figure 7. Data are graphed as diurnal differences in order to identify whether exercise attenuated the stress-induced flattening of diurnal physiological rhythms and sleep/wake behavior. (A) Stress exposure reduced nightly wheel running for post-stress days 1–4, but returned to pre-stress levels by post-stress day 5. (B) Stress exposure significantly flattened the diurnal rhythms of locomotor activity in both groups for post-stress days 1–3, but this effect lasted until post-stress day 4 in the exercise group. Both groups regained their pre-stress diurnal differences in locomotor activity by post-stress day 5 and the diurnal difference in locomotor activity between the exercise and sedentary groups was again evident at this time. (C) Stress exposure flattened the diurnal rhythms of heart rate in both groups for post-stress day 1, but this effect lasted until post-stress day 3 in the sedentary group. (D) Stress exposure flattened the diurnal rhythms in CBT in both groups until post-stress day 2, but this effect lasted until post-stress day 3 in the sedentary groups. Exercise attenuated the impact of stress on flattening of the diurnal rhythm of CBT on post-stress day 1 only, when compared with sedentary rats. (E) Stress exposure flattened the diurnal rhythm of REM sleep in sedentary rats only on post-stress day 1, but exercise reduced the stress-induced flattening of the diurnal rhythm in REM sleep. The differences in the diurnal rhythm of REM sleep between exercise and sedentary groups were evident both before stress and until post-stress day 3. (F) Stress exposure flattened the diurnal rhythms of NREM sleep in both groups until post-stress day 2. Exercise attenuated the impact of stress on the flattening of the diurnal rhythm of NREM sleep on post-stress day 1 only, when compared with sedentary rats. Although the effects of stress on flattening the diurnal rhythm of NREM sleep had dissipated in both groups by post-stress day 3, exercising rats had a greater diurnal rhythm in NREM sleep when compared to sedentary rats on post-stress day 3. (G) Consistent with the sleep data, stress exposure flattened the diurnal rhythm of WAKE until post-stress day 2. Exercise also reduced the impact of stress on flattening the diurnal rhythm of WAKE on post-stress day 1 only, when compared with the sedentary group. Abbreviations are as follows: a.u.: arbitrary units; BL: pre-stress baseline; bpm: beats per minute; °C: degrees Celsius; Diff.: diurnal difference; m: meters. (*p < 0.05 effect of stress compared with baseline values; †p < 0.05 exercise compared with sedentary).
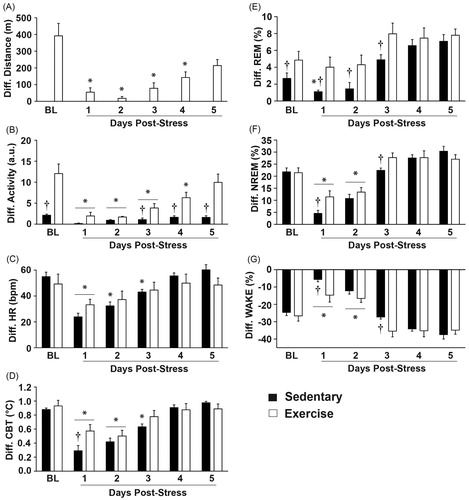
Stress exposure flattened the diurnal rhythm of REM sleep in the sedentary group, but stress had little effect on the diurnal rhythm of REM sleep in the exercise group (F(5, 60) =27.629; p < 0.0001; main effect of stress; see for results of post hoc analysis). This result was likely due to the pre-stress differences in the diurnal rhythms of REM sleep between exercising and sedentary groups (F(1, 12) = 5.160; p = 0.042; main effect of exercise; see for results of post hoc analysis). Stress exposure also flattened the diurnal rhythm of NREM sleep in both groups until post-stress day 2 (F(5, 60) = 67.542; p < 0.0001; main effect of stress; ) and voluntary exercise also attenuated the magnitude of the stress-induced flattening of the diurnal rhythm of NREM sleep (F(5, 60) = 3.302; p = 0.010; interaction between exercise and stress; see for results of post hoc analysis). Consistent with the NREM data, stress exposure also disrupted the diurnal rhythm of WAKE until post-stress day 2 (F(5, 60) = 69.303; p < 0.0001; main effect of stress; see for results of post hoc analysis) and voluntary exercise attenuated the disruption of the diurnal rhythm of WAKE following stress exposure (F(5, 60) = 3.063; p = 0.015; interaction between exercise and stress; see for results of post hoc analysis).
Discussion
The results from this study support the hypothesis that prior wheel running improves sleep and attenuates the disruptions in sleep/wake behavior and physiological rhythms following acute stressor exposure. By using in vivo continuous wireless biotelemetry measurement of LA, HR, CBT and EEG in freely behaving animals, we found a significant linear relationship between increased nocturnal physical activity and increased time spent in REM sleep. Although prior studies have reported increased REM sleep following exercise (Blanco-Centurion & Shiromani, Citation2006; Gambelunghe et al., Citation2001; Hanagasioglu & Borbely, Citation1982; Lancel et al., Citation2003), our results suggest a direct relationship between increased locomotor activity for animals with access to a running wheel, and increased REM sleep. Thus increased physical activity is associated with changes in sleep architecture prior to stressor exposure.
Stressor exposure substantially disrupts REM sleep in sedentary rats which is consistent with earlier findings (Cheeta et al., Citation1997; Greenwood et al., Citation2014; Kant et al., Citation1995; Papale et al., Citation2005; Pawlyk et al., Citation2008; Rampin et al., Citation1991), but we show here for the first time that this effect was attenuated in exercising rats. Sufficient REM sleep appears to be important for recovery from stressful events or situations, especially in those with stress-related disorders (Germain & Nielsen, Citation2003; Kyung Lee & Douglass, Citation2010). In fact, victims of traffic accidents that slept more and had more consolidated REM sleep episodes did not go on to develop post-traumatic stress disorder (Mellman et al., Citation2007), which highlights the importance of sufficient REM sleep following aversive events. This idea is consistent with the finding that rats who lost REM rebound after stressor exposure to a traumatic event expressed the greatest stress sensitization (Greenwood et al., Citation2014; Thompson et al., Citation2014). In our study, wheel running attenuated the stress-induced diurnal disruption of REM sleep. It may be possible that exercise induced improvements in REM sleep underlie, in part, the preventative benefits of physical activity in affective disorders.
We also discovered that 6 weeks of wheel running in freely behaving animals improves NREM sleep quality in a diurnally dependent manner. A similar pre-clinical study found that both young and old F344 rats had displayed slightly better NREM sleep quality after 8 weeks of exercise, although they used forced exercise in a slowly rotating wheel for only 50 min per night (Blanco-Centurion & Shiromani, Citation2006). In another study using forced rota-rod running, light physical exercise for 45 min also resulted in improved NREM sleep immediately after exercise, which was the only time sleep was measured in this study (Gambelunghe et al., Citation2001). The current results and prior studies are also consistent with the clinical literature where it has been found that trained individuals have higher levels of NREM sleep compared with untrained controls. For example, female swimmers spent more time in NREM sleep at sleep onset following increased training as the swim season progressed (Taylor et al., Citation1997), suggesting a link between increased physical activity and increased need for NREM sleep especially at sleep onset. Another clinical study in females demonstrated that physical activity with concomitant increases in external temperatures also significantly increased time spent in NREM sleep (Horne & Moore, Citation1985), which reveals the potential connection between temperature and activity induced changes in NREM sleep. Thus the current results are in agreement with previous studies but also extend our understanding of wheel running, NREM sleep patterns, and how they relate to each other in a diurnally dependent manner. One current hypothesis of the function of NREM sleep is that it may help reorganize and strengthen synapses in response to daily activities (Tononi & Cirelli, Citation2012) and it is believed that during NREM sleep slow wave oscillations are occurring between the thalamus and the cortex (de Andres et al., Citation2011), however, the specific purpose of NREM sleep remains poorly understood. Interestingly, there may be a connection between increased temperature and the induction of NREM sleep that could involve the pre-optic area of the hypothalamus (McGinty & Szymusiak, Citation2001), which could account for the finding that physically active rats had better NREM sleep immediately after wheel running.
Stressor exposure also produced a disruption in the diurnal rhythm of NREM sleep in sedentary rats but this effect was attenuated in exercising rats. Our results are the first to demonstrate that prior wheel running can attenuate the stress-induced disruption of the diurnal rhythm of NREM sleep following stress exposure, thus based on previous work and our current findings, exercise both produces better quality of NREM sleep (Blanco-Centurion & Shiromani, Citation2006; Gambelunghe et al., Citation2001) and protects NREM from stress-induced disruptions. Interestingly, Dang-Vu et al. (Citation2015) recently reported that better NREM quality may decrease vulnerability to stress-induced sleep disruptions (Dang-Vu et al., Citation2015). It is well known that disruptions of diurnal rhythms are prevalent in those with stress-related disorders (Cole et al., Citation1990; Emens et al., Citation2009; Hasler et al., Citation2010; Scott et al., Citation1997) and that reducing their disruption may benefit health.
Prior to stressor exposure, voluntary exercise increased CBT body temperature both during the active (dark) and inactive (light), cycles. These results are consistent with prior literature demonstrating an effect of exercise on CBT (Blanco-Centurion & Shiromani, Citation2006; Masini et al., Citation2011). Similar to the effect of stress on sleep/wake diurnal rhythms, stressor exposure flattened the diurnal rhythm of CBT in sedentary rats, and exercise attenuated this effect. Interestingly, this effect of regular physical activity on CBT could also contribute to many of the protective effects of exercise on the negative health consequences of stress, including mood disorders. For example, increasing body temperature alone can alleviate symptoms of depression in depressed patients (Hanusch et al., Citation2013; Koltyn et al., Citation1992) and depressed individuals have deficits in thermoregulatory cooling (Ward & Doerr, Citation1986). Additionally, individuals who are exercise trained activate thermoregulatory cooling mechanisms more efficiently than untrained controls (Cramer et al., Citation2012; Ichinose-Kuwahara et al., Citation2010). Thus it seems plausible that the antidepressant-like effects of exercise may depend, in part, on alterations in thermoregulation; however, the potential mechanisms remain poorly understood.
One possibility is that repeated low-intensity exercise bouts, such as that in voluntary wheel running, repeatedly increased CBT thus stimulating thermoregulatory centers in the brain. Two thermoregulatory centers that could be involved are the serotonergic neurons in the dorsal raphé nucleus and the pre-optic area of the hypothalamus. Lowry and colleagues have hypothesized that temperature sensitive serotonergic neurons in the dorsal raphé nucleus may contribute to antidepressant effects (Hale et al., Citation2013) and it is known that serotonergic neurons in the dorsal raphé nucleus are important for mediating the antidepressant-like effects of voluntary free wheel running (Greenwood et al., Citation2003, Citation2005b). Voluntary exercise can also activate preoptic neurons responsible for thermoregulation (Hasegawa et al., Citation2005) and activation of warm-sensitive neurons in the preoptic area can promote sleep onset (Kumar et al., Citation2011; McGinty et al., Citation1994). In fact, the preoptic area is important in both thermoregulation and sleep regulation as lesions of the preoptic area can decrease NREM sleep and increase REM sleep (Mendelson, Citation1998). It could be possible that repeated increases in locomotor activity led to repeated increases in CBT, subsequently activating thermosensitive brain regions like the preoptic area (McGinty & Szymusiak, Citation2001), thus improving sleep/wake behavior and facilitating a stress-protective phenotype.
Conclusions
Regular physical activity can both prevent and reduce the severity of stress-related disorders. Given the link between disruptions in sleep, diurnal rhythms and stress-related disorders (Breslau et al., Citation2004; Emens et al., Citation2009; Germain & Nielsen, Citation2003; Hasler et al., Citation2010; Kyung Lee & Douglass, Citation2010; Ohayon & Roth, Citation2001; Papadimitriou & Linkowski, Citation2005; van Liempt et al., Citation2012), it may be possible that the impacts of exercise on sleep and diurnal rhythms, contribute to these effects. Despite these associations, a thorough understanding of the role of sleep recovery in response to stressful situations remains elusive (Sanford et al., Citation2014); however, our data add that exercise protects against the stress-induced flattening of diurnal rhythms of NREM/REM sleep and body temperature which could reduce the negative consequences of stress exposure and/or enhance recovery following aversive events. More research is needed to understand the specific patterns of sleep recovery following aversive events that are beneficial to physical and mental well-being.
Acknowledgements
This study was generously supported by The National Institutes of Health (NIH RO1 MH068283) and by the Department of Defense Enabling Stress Resistance initiative (DARPA W911NF-10-1-0050).
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Avery DH, Wildschiodtz G, Rafaelsen OJ. (1982). Nocturnal temperature in affective disorder. J Affect Disord 4:61–71.
- Babyak M, Blumenthal JA Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, et al. (2000). Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 62:633–8.
- Barbour KA, Edenfield TM, Blumenthal JA. (2007). Exercise as a treatment for depression and other psychiatric disorders. Rev J Cardiopulm Rehabil Prev 27:359–67.
- Blanco-Centurion CA, Shiromani PJ. (2006). Beneficial effects of regular exercise on sleep in old F344 rats. Neurobiol Aging 27:1859–69.
- Blumenthal JA, Babyak MA Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, et al. (2007). Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 69:587–96.
- Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. (2004). Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry 61:508–16.
- Campbell IG. EEG recording and analysis for sleep research. Current Protocols Neurosci 49:10.2:10.2.1–10.2.19.
- Cheeta S, Ruigt G, van Proosdij J, Willner P. (1997). Changes in sleep architecture following chronic mild stress. Biol Psychiatry 41:419–27.
- Cole RJ, Loving RT, Kripke DF. (1990). Psychiatric aspects of shiftwork. Occup Med 5:301–14.
- Cramer MN, Bain AR, Jay O. (2012). Local sweating on the forehead, but not forearm, is influenced by aerobic fitness independently of heat balance requirements during exercise. Exp Physiol 97:572–82.
- Dang-Vu TT, Salimi A, Boucetta S, Wenzel K, O'Byrne J, Brandewinder M, Berthomier C, Gouin JP. (2015). Sleep spindles predict stress-related increases in sleep disturbances. Front Hum Neurosci 9:68.
- Davidson CL, Babson KA, Bonn-Miller MO, Souter T, Vannoy S. (2013). The impact of exercise on suicide risk: examining pathways through depression, PTSD, and sleep in an inpatient sample of veterans. Suicide Life Threat Behav 43:279–89.
- de Andres I, Garzon M, Reinoso-Suarez F. (2011). Functional anatomy of non-REM sleep. Front Neurol 2:70.
- Duman CH, Schlesinger L, Russell DS, Duman RS. (2008). Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res 1199:148–58.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. (2005). Exercise treatment for depression: efficacy and dose response. Am J Prev Med 28:1–8.
- Edgar DM, Dement WC. (1991). Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol 261:R928–33.
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. (2009). Circadian misalignment in major depressive disorder. Psychiatry Res 168:259–61.
- Ford DE, Kamerow DB. (1989). Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? J Am Med Assoc 262:1479–84.
- Gambelunghe C, Rossi R, Mariucci G, Tantucci M, Ambrosini MV. (2001). Effects of light physical exercise on sleep regulation in rats. Med Sci Sports Exerc 33:57–60.
- Germain A, Nielsen TA. (2003). Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biol Psychiatry 54:1092–8.
- Greenwood BN, Fleshner M. (2011). Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev 39:140–9.
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. (2005a). The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res 1033:164–78.
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. (2005b). Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry 57:559–68.
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. (2003). Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci: Offic J Soc Neurosci 23:2889–98.
- Greenwood BN, Thompson RS, Opp MR, et al. (2014). Repeated exposure to conditioned fear stress increases anxiety and delays sleep recovery following exposure to an acute traumatic stressor. Front Psychiatry 5:146.
- Hale MW, Raison CL, Lowry CA. (2013). Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol Ther 137:108–18.
- Hanagasioglu M, Borbely AA. (1982). Effect of voluntary locomotor activity on sleep in the rat. Behav Brain Res 4:359–68.
- Hanusch KU, Janssen CH, Billheimer D, et al. (2013). Whole-body hyperthermia for the treatment of major depression: associations with thermoregulatory cooling. Am J Psychiatry 170:802–4.
- Hasegawa H, Ishiwata T, Saito T, Yazawa T, Aihara Y, Meeusen R. (2005). Inhibition of the preoptic area and anterior hypothalamus by tetrodotoxin alters thermoregulatory functions in exercising rats. J Appl Physiol 98:1458–62.
- Hasler BP, Buysse DJ, Kupfer DJ, Germain A. (2010). Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res 178:205–7.
- Horne JA, Moore VJ. (1985). Sleep EEG effects of exercise with and without additional body cooling. Electroencephalogr Clin Neurophysiol 60:33–8.
- Ichinose-Kuwahara T, Inoue Y, Iseki Y, Hara S, Ogura Y, Kondo N. (2010). Sex differences in the effects of physical training on sweat gland responses during a graded exercise. Exp Physiol 95:1026–32.
- Kant GJ, Pastel RH, Bauman RA, Meininger GR, Maughan KR, Robinson TN III, Wright WL, Covington PS. (1995). Effects of chronic stress on sleep in rats. Physiol Behav 57:359–65.
- Koltyn KF, Robins HI, Schmitt CL, Cohen JD, Morgan WP. (1992). Changes in mood state following whole-body hyperthermia. Int J Hyperthermia: Offic J Eur Soc Hyperthermic Oncology, N Am Hyperthermia Group 8:305–7.
- Kronfeld-Schor N, Einat H. (2012). Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology 62:101–14.
- Kumar D, Kumar VM, Mallick HN. (2011). Warm sensitive neurons of the preoptic area regulate ambient temperature related changes in sleep in the rat. Indian J Physiol Pharmacol 55:262–71.
- Kyung Lee E, Douglass AB. (2010). Sleep in psychiatric disorders: where are we now? Can J Psychiatry. Revue Canadienne De Psychiatrie 55:403–12.
- Lancel M, Droste SK, Sommer S, Reul JM. (2003). Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. Eur J Neurosci 17:2171–9.
- Mallon L, Broman JE, Hetta J. (2000). Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr/IPA 12:295–306.
- Manger TA, Motta RW. (2005). The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. Int J Emerg Mental Health 7:49–57.
- Masini CV, Nyhuis TJ, Sasse SK, Day HE, Campeau S. (2011). Effects of voluntary wheel running on heart rate, body temperature, and locomotor activity in response to acute and repeated stressor exposures in rats. Stress 14:324–34.
- McGinty D, Szymusiak R. (2001). Brain structures and mechanisms involved in the generation of NREM sleep: focus on the preoptic hypothalamus. Sleep Med Rev 5:323–42.
- McGinty D, Szymusiak R, Thomson D. (1994). Preoptic/anterior hypothalamic warming increases EEG delta frequency activity within non-rapid eye movement sleep. Brain Res 667:273–7.
- Mellman TA, Pigeon WR, Nowell PD, Nolan B. (2007). Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress 20:893–901.
- Mendelson WB. (1998). Effects of parenterally administered triazolam on sleep in rats with lesions of the preoptic area. Pharmacol Biochem Behav 61:81–6.
- Monk TH, Buysse DJ, Frank E, Kupfer DJ, Dettling J, Ritenour AM. (1994). Nocturnal and circadian body temperatures of depressed outpatients during symptomatic and recovered states. Psychiatry Res 51:297–311.
- Newman CL, Motta RW. (2007). The effects of aerobic exercise on childhood PTSD, anxiety, and depression. Int J Emerg Mental Health 9:133–58.
- Ohayon MM, Roth T. (2001). What are the contributing factors for insomnia in the general population? J Psychosom Res 51:745–55.
- Olivadoti MD, Opp MR. (2008). Effects of i.c.v. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience 153:338–48.
- Papadimitriou GN, Linkowski P. (2005). Sleep disturbance in anxiety disorders. Int Rev Psychiatry 17:229–36.
- Papale LA, Andersen ML, Antunes IB, Alvarenga TA, Tufik S. (2005). Sleep pattern in rats under different stress modalities. Brain Res 1060:47–54.
- Pawlyk AC, Morrison AR, Ross RJ, et al. (2008). Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev 32:99–117.
- Posener JA, DeBattista C, Williams GH, Chmura Kraemer H, Kalehzan BM, Schatzberg AF. (2000). 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry 57:755–60.
- Rampin C, Cespuglio R, Chastrette N, Jouvet M. (1991). Immobilisation stress induces a paradoxical sleep rebound in rat. Neurosci Lett 126:113–18.
- Riemann D, Berger M, Voderholzer U. (2001). Sleep and depression-results from psychobiological studies: an overview. Biol Psychol 57:67–103.
- Roberts RE, Shema SJ, Kaplan GA, Strawbridge WJ. (2000). Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry 157:81–8.
- Sanford LD, Suchecki D, Meerlo P. (2014). Stress, arousal, and sleep. Curr Top Behav Neurosci 25:379–410.
- Scott AJ, Monk TH, Brink LL. (1997). Shiftwork as a risk factor for depression: a pilot study. Int J Occup Environ Health 3:S2–9.
- Souetre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, Ardisson JL, Darcourt G. (1989). Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res 28:263–78.
- Speaker KJ, Cox SS, Paton MM, et al. (2014). Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats. Brain Behav Immun 39:87–98.
- Taylor SR, Rogers GG, Driver HS. (1997). Effects of training volume on sleep, psychological, and selected physiological profiles of elite female swimmers. Med Sci Sports Exerc 29:688–93.
- Thompson RS, Christianson JP, Maslanik TM, Maier SF, Greenwood BN, Fleshner M. (2013). Effects of stressor controllability on diurnal physiological rhythms. Physiol Behav 112–113:32–9.
- Thompson RS, Strong PV, Clark PJ, Maslanik TM, Wright KP Jr, Greenwood BN, Fleshner M. (2014). Repeated fear-induced diurnal rhythm disruptions predict PTSD-like sensitized physiological acute stress responses in F344 rats. Acta Physiol 211:447–65.
- Thompson RS, Strong PV, Fleshner M. (2012). Physiological consequences of repeated exposures to conditioned fear. Behav Sci (Basel) 2:57–78.
- Tononi G, Cirelli C. (2012). Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plasticity 2012:415250.
- van Liempt S, Arends J, Cluitmans PJ, Westenberg HG, Kahn RS, Vermetten E. (2012). Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology 38:155–65.
- Verwey M, Robinson B, Amir S. (2013). Recording and analysis of circadian rhythms in running-wheel activity in rodents. J Vis Exp (71):e50186. doi:10.3791/50186.
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, et al. (2011). Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res 224:233–40.
- Ward NG, Doerr HO. (1986). Skin conductance. A potentially sensitive and specific marker for depression. J Nerv Ment Dis 174:553–9.
- Yamanaka Y, Hashimoto S, Tanahashi Y, Nishide SY, Honma S, Honma K. (2010). Physical exercise accelerates reentrainment of human sleep-wake cycle but not of plasma melatonin rhythm to 8-h phase-advanced sleep schedule. Am J Physiol Regul Integr Comp Physiol 298:R681–91.
