Abstract
Vasopressin, a nonapeptide, signaling both as hormone in the blood and neuromodulator/neurotransmitter in the brain is considered to be causally involved in the pathological changes underlying anxiety and depression. In the present review we summarize experimental data obtained with Brattleboro rats as a model of congenital vasopressin-deficiency to test the hypothesis that central vasopressin signaling contributes to anxiety- and depression-like behavior. Male, female and lactating rats were studied. We focused on the paraventricular nucleus of the hypothalamus (PVN) and the septum, two brain areas in which vasopressin is proposed to control the endocrine and behavioral stress response, respectively. The presented data support the hypothesis that the behavioral changes seen in these rats are brought about by an altered vasopressin signaling at the brain level. Whereas vasopressin synthesized and released within the hypothalamus is primarily involved in endocrine regulation, vasopressin signaling in other brain areas may contribute to anxiety- and depression-like behavioral parameters. Further studies in this context might focus particularly on the interplay between extra-hypothalamic brain areas such as the septum and the medial amygdala.
Introduction
The nonapeptide arginine vasopressin (AVP) is synthesized in the mammalian brain primarily in distinct hypothalamic nuclei and controls diverse physiological parameters. AVP acts as a hormone and is known to sustain body water homeostasis and to influence the endocrine stress response by regulating the activity of the hypothalamo-pituitary-adrenocortical (HPA) axis. AVP released within the brain and acting as a neurotransmitter/neuromodulator has been proposed to modulate the stress-relevant generation of emotional behavior (including anxiety and depression) (Scott & Dinan, Citation1998; Zelena, Citation2012). Because of the signaling function of AVP in these two critical compartments (in blood as a hormone, in brain as a neurotransmitter/neuromodulator) it was hypothesized that this nonapeptide may provide a link to better understand the coordination of neuroendocrine and behavioral responses under stress conditions.
Vasopressin production
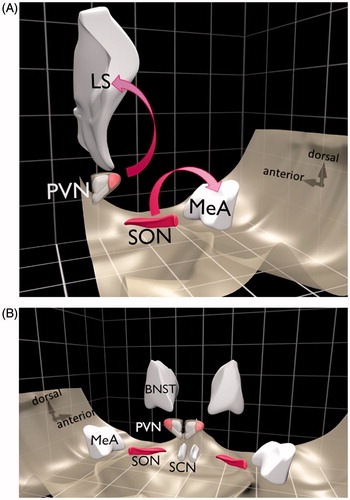
The vast majority of AVP is synthesized in magnocellular neurons residing in the paraventricular and supraoptic nuclei of the hypothalamus (PVN and SON, respectively) (). These magnocellular neurons compose the central part of the hypothalamic–neurohypophysial system and, as indicated above, are able to release AVP not only from axon terminals in the posterior pituitary into the circulating blood, but also into the extracellular fluid in the brain from their somata and dendrites (Ludwig et al., Citation2005). For the latter process the terms “intranuclear release” and “intra-hypothalamic release” were coined (Douglas et al.,Citation1994; Ludwig & Leng, Citation1998). Once released intranuclearly the nonapeptide may be able to reach other brain areas (e.g. septum, amygdala () via the extracellular fluid (volume transmission) thence to modulate neuronal activity, and thereby behavior under stress conditions (Albers, Citation2015; Engelmann et al., Citation2004; Landgraf & Neumann, Citation2004; Ludwig & Stern, Citation2015). In addition, AVP is also synthesized in parvocellular neurons of the PVN, the bed nucleus of the stria terminalis, medial amygdala and suprachiasmatic nucleus, whose projections remain within the brain to form the so-called central vasopressinergic system (using synaptic transmission) (Zelena, Citation2012) (). Some axon terminals from the parvocellular cells of the PVN reach the median eminence where AVP is released into the long hypothalamo-pituitary portal vessels and thereby reaches the anterior lobe of the pituitary and acts synergistically with corticotropin-releasing hormone (CRH) to trigger the release of adrenocorticotropin (ACTH).
Figure 1. Main AVP synthesizing areas in the brain. Schematic diagram of a rat brain showing brain areas with cell populations synthesizing the nonapeptide arginine vasopressin (AVP). (A) The main axonal projections from the AVP magnocellular neurons in the SON and PVN are to the posterior pituitary gland (not shown). Intranuclearly released AVP may reach remote brain areas (e.g. LS or MeA). (B) The five main nuclei synthesizing AVP. Pink areas represent magnocellular cell groups. BNST: bed nucleus of the stria terminalis; LS: lateral septum; MeA: medial amygdala; PVN: paraventricular nucleus of the hypothalamus; SCN: suprachiasmatic nucleus; SON: supraoptic nucleus.
Vasopressin receptors
AVP exerts its effects through three types of plasma membrane receptors (Zelena, Citation2012). The V1a receptors (vascular) are widely distributed on vascular smooth muscle cells. This subtype is the most prominent in several nuclei of the brain, such as lateral septum, amygdala, neocortex, hippocampus and various hypothalamic areas. The V1b receptor subtype (pituitary) was initially described in the anterior lobe of the pituitary, but it was also detected at the periphery as well as in the septum, cortex, hippocampus and hypothalamus. The V2 subtype (renal) is located in the kidney effecting antidiuretic actions of AVP, while in the brain this receptor can be found only in the cerebellum.
Vasopressinergic signaling and affective behavior
The groups of Phillip Gold et al. (Gold et al., Citation1978) and David de Wied (de Wied, Citation1978) were the first to propose that AVP may play a role in stress-related diseases. These authors postulated that in animals, AVP deficiency produced complex behavioral changes that are also characteristic of affective illness, including alterations in memory, changes in pain sensitivity and desynchronization of biological rhythms. Moreover, these changes were diminished when AVP was replaced. They also showed that AVP-containing fibers are widely distributed throughout the brain, rendering AVP to be a suitable candidate for involvement in the control of complex behavioral performance. Since 1978 many studies, including those outlined below, have tested this hypothesis.
Clinical findings
Depressed patients
Patients with major depression were reported to have significantly elevated plasma AVP levels compared to healthy controls (van Londen et al., Citation1998). In a patient chronically elevated plasma AVP levels due to paraneoplastic AVP-production was associated with major depressive symptoms, which were ameliorated after resection of the tumor (Muller et al., Citation2000). However, it is important to be aware of the limited effect of AVP circulating in the blood directly on brain functions (Landgraf, Citation2006). Postmortem studies on brains of depressed patients have provided relevant information about the biological basis for depression. A threefold increase in the number of CRH neurons co-expressing AVP, double the number of AVP-immunopositive neurons in the PVN, and an increase in AVP mRNA in the SON may have all contributed to the elevated plasma AVP levels measured in depressed patients (Purba et al., Citation1996; van Londen et al., Citation2001). Depressed patients who attempted or committed suicide were found to have higher plasma AVP levels (Inder et al., Citation1997) and a higher number of AVP immunopositive neurons in the PVN (Merali et al., Citation2006), than depressed patients without suicidal attempts. Taken together, these findings suggest that greater activity of the hypothalamic vasopressinergic system paralleled the severity of the symptoms in depression.
Antidepressants
Studies testing the impact of antidepressants on vasopressinergic activity have provided evidence for the importance of AVP in stress-related disorders. It is of interest to note that treatment with fluoxetine, a selective serotonin reuptake inhibitor (SSRI) and potent antidepressant, decreased the level of AVP in the cerebrospinal fluid of depressive patients which was paralleled by improvement of the symptoms (De Bellis et al., Citation1993). Chronic fluoxetine administration has also been shown to reduce AVP secretion in vitro from rat hypothalamic organ cultures (Altemus et al., Citation1992). Thus, reduction of AVP signaling may be an important component of the antidepressant effect of SSRIs.
New experimental tools
As the existing clinical studies failed to reveal a clear role that AVP signaling may play in the development, maintenance or relapse of affective disorders, recently developed technical approaches involving new genetic and imaging tools have been employed to shed more light on this issue. These studies revealed for instance that a single-nucleotide polymorphism (SNP) in the V1b receptor gene may reduce the chances of development of major depression, as this SNP was significantly over-represented in healthy controls compared to depressed patients (van West et al., Citation2004). The improvement of in vivo neuroimaging methods (in particular, functional magnetic resonance imaging) has allowed studies of the human brain with high spatial and temporal resolution. Such studies support the hypothesis that AVP acts as a modulator of the activity and connectivity in the cortical component of a medial prefrontal cortex-amygdala circuit implicated in emotional regulation (Zink et al., Citation2010). Further investigations will be necessary to determine the therapeutic potential of targeting the vasopressinergic system in the treatment of some types of mood and anxiety disorders.
Hypercortisolaemia
One of the best described biological abnormalities in depression is hypercortisolaemia (Gillespie & Nemeroff, Citation2005). Although a final proof as to whether this is the cause of, rather than a result of the depressive state remains to be convincingly provided, a number of studies have shown parallel changes in plasma levels of AVP and cortisol in patients with unipolar depression (Dinan & Scott, Citation2005). This leads to the proposal to classify depression into different subcategories, including “with above-normal plasma AVP characterized by a positive family history of depression”, and correlating plasma AVP and cortisol concentrations (Goekoop et al., Citation2009, Citation2011). Recent studies indicated that in patients with moderate depression lower levels of AVP-reactive autoantibodies were found than in controls, which was associated not only with altered mood, but also with a disturbed cortisol secretion as a result of physical exercise (Garcia et al., Citation2011). The abnormally high cortisol response to AVP administration in depressed patients further supported the link between alterations in AVP signaling and depression (Krahn et al., Citation1985). However, an alternative explanation for hypercortisolaemia suggested by Meller and coworkers is worth considering (Meller et al., Citation1987). They proposed that the key biological abnormality in major depressive disorder is the hypersecretion of CRH leading to downregulation of pituitary CRH receptors and a reduced ACTH release which finally results in an ACTH hyper-responsiveness of the adrenals. Thus, AVP-stimulated ACTH secretion may induce high cortisol secretion due to adrenal hyper-reactivity to ACTH.
In any case, it is clear that the defined mental illness is primarily the result of a dysfunction in brain signaling systems. As circulating AVP originates in the brain one may be tempted to extrapolate plasma concentrations of the nonapeptide to its signal intensity in the brain tissue. However, experimental data do not easily support this view (Engelmann et al., Citation1999). Therefore, the relevance of physiological parameters obtained in clinical studies should be critically considered on the background of the experimental data collected in animal studies which we provide below.
Experimental findings
V1b receptor
Chronic stress is a widely used model of experimental depression and the switch from primarily CRH- to AVP-controlled ACTH secretion under chronic stress conditions found in rodents (Aguilera, Citation1994; Dallman, Citation1993) turned attention to AVP as a possible drug target. The V1b receptor in particular, considered to be the most important vasopressin receptor in HPA axis regulation, was addressed (Griebel et al., Citation2003). Indeed, a V1b receptor antagonist, SSR149415 produced anxiolytic- and antidepressant-like effects in numerous preclinical tests (Griebel et al., Citation2002) in both rats and mice (Rotzinger et al., Citation2010). Importantly its antidepressive-like effect in the forced swim (FS) test was present also in hypophysectomized rats, suggesting an intracerebral target. However, another V1b receptor antagonist, V1B-30N, was completely ineffective in the FS test (Hodgson et al., Citation2014), and V1b-receptor knockout (KO) mice did not show an anxiolytic- or antidepressive-like phenotype (Wersinger et al., Citation2002). Moreover, the V1b receptor antagonist SSR149415 had anxiolytic-like effects in the elevated plus-maze test (EPM) when it was injected into the basolateral amygdala (Salome et al., Citation2006), but not in the lateral septum (Stemmelin et al., Citation2005). However, in the FS test an antidepressive-like effect was observed in both cases. Both the site of injection and the treatment duration are important for the characteristics of the effect(s) observed, for example, in an olfactory bulbectomy model of depression SSR149415 was effective only after chronic treatment (Breuer et al., Citation2009), while in the FS test a single dose effectively reduced depressive-like behavior (Rotzinger et al., Citation2010). Thus, the available data with antagonists concerning affective behavior are contradictory. Nevertheless, animal studies with V1b receptor KO mice support the hypothesis of an involvement of V1b receptor signaling in the effect of clinically used antidepressants, such as the tricyclic antidepressant desipramine or the SSRI fluoxetine (Stewart et al., Citation2008). Moreover, the antidepressant-induced HPA axis changes were also observed in V1b KO animals, reinforcing the idea of an intracerebral rather than a hypophysial target. Indeed, in male rats the more anxious phenotype was accompanied by enhanced V1b receptor expression in the hypothalamus, which was reduced by citalopram, another SSRI (Kokras et al., Citation2011).
V1a receptor
In line with intracerebral action of AVP, male mice with knockout of the V1a receptor gene (most abundant AVP receptor in the brain) displayed significantly less anxiety-related behavior than wildtype mice in several tasks (Bielsky et al., Citation2004; Egashira et al., Citation2007). This fits well with the observation that over-expression of the V1a receptor gene in the lateral septum significantly increased anxiety-related behavior (Bielsky et al., Citation2005). Application of the V1a receptor antagonist into the septum and amygdala also had antidepressant-like effects (Ebner et al., Citation1999, Citation2002). Low anxiety behavior (LAB) rats and mice, selected from their behavior on the EPM test, have AVP “deficiency” in their hypothalamus and behave in a similar fashion to rats that had received anxiolytic treatment, further supporting the role of AVP in anxiety (Keck et al., Citation2003; Landgraf et al., Citation2007). Conversely, administration of a V1a receptor antagonist decreased anxiety- and depression-like behavior in high anxiety behavior (HAB) rats (Wigger et al., Citation2004).
Endophenotypes
Currently, there is a shift away from traditional pharmacological animal studies towards research dealing with a so-called “endophenotype-style” approach (Cryan & Slattery, Citation2007). It is understood that this provides insight into how subtle genetic and epigenetic changes may lead to behavioral alterations. In this respect, in HAB rats, a SNP was detected in the promoter of the AVP gene that resulted in an increase in transcription of AVP mRNA in the PVN of these rats, which may have contributed to the high-anxiety-like phenotype (Landgraf et al., Citation2007). However, in LAB mice another SNP in the AVP signal peptide resulted in a deficit in bioavailable AVP (Landgraf et al., Citation2007). Another molecular approach involving epigenetic studies also provided a new insight into a possible contribution of AVP signaling in affective disorders (Murgatroyd & Spengler, Citation2011; Veenema, Citation2009). Epigenetic marking of the AVP gene (through hypomethylation) by early-life stress in mice underpinned a sustained increased AVP expression and HPA axis activity, and also triggered a reduced stress-coping ability and memory deficits (Murgatroyd et al., Citation2010).
Septum as a possible target site
The idea that AVP signaling may play a significant role in affective disorders was further supported by data obtained with injections targeting specific brain areas and receptors. Anxiety-related behavior measured on the EPM was significantly reduced with a septal infusion of antisense oligonucleotide to the V1a mRNA (Landgraf et al., Citation1995) as well as local administration of a V1a antagonist (Liebsch et al., Citation1996) without causing significantly altered locomotion. Interestingly, Appenrodt and coworkers found in separate, but similar experimental setups, that both intraseptal and intraperitoneal application of AVP influenced the behavior of the rat on the EPM, which was interpreted as an indication for a possible action of the synthetic nonapeptide outside the septum (Appenrodt et al., Citation1998).
Summary of background information
Taken together, the available evidence supports the hypothesis that significant alterations in AVP signaling contribute to the development of abnormal affective behavior in humans. This was simulated in animal models, although the cause and effect relationship is not always unequivocal. It seems that not only the regulatory role played by AVP on the HPA axis via the V1b receptor, but also, and maybe more importantly, AVP action via the V1a receptors in the lateral septum and/or amygdala explains its causal involvement in the reported behavioral alterations. It should be noted that some of the reported data in the field are contradictory. For instance, the finding that the V1b antagonist SSR149415, in the same dose, found to be ineffective on EPM performance, was effective in the FS test, suggests an involvement of AVP signaling via V1b receptors in the development of depressive-, but not anxiety-like behavior (Salome et al., Citation2006). Indeed, clinical studies with SSR149415 failed to confirm its effectiveness in generalized anxiety disorder, but with some positive results in major depression (Griebel et al., Citation2012).
Nevertheless, endogenous AVP may exert its behavioral effects via both V1a and b receptor subtypes, thus targeting AVP signaling in general seems to provide a more complex insight. It was hypothesized that AVP released intra-hypothalamically, into the PVN, in response to different stressful stimuli may reach the septum and alters behavior. This hypothesis could be tested in rats using the microdialysis technique, which, unfortunately, is only of limited application in mice (Wotjak et al., Citation2008). In addition, it remains difficult to extract general conclusions from mutant mice studies as mixing the genetic material from different strains has consequences for an individual’s stress response. To circumvent these limitations we have used a spontaneous mutated rat strain, the AVP-deficient Brattleboro rat model. This has allowed us to investigate in more detail the role that AVP signaling plays in controlling behavioral performance from a perspective different from clinical findings (i.e. diminished AVP signaling versus excessive AVP signaling), mutually reinforcing the main conclusions. We would expect that “normalisation” of neuropeptide signaling would not only heal the diabetes insipidus, but would also directly affect the behavior of the AVP deficient rats.
The Brattleboro rat as a model for studying the relevance of AVP signaling for anxiety and depression
In the early 1960s, a substrain of Long-Evans rats was identified with hereditary diabetes insipidus. Animals of this substrain were originally bred in Brattleboro (Vermont, USA), and hence subsequently named “Brattleboro rats” (Sawyer et al., Citation1964; Valtin & Schroeder, Citation1964). Later research revealed that this recessively heritable genetic diabetes insipidus was caused by a point mutation, leading to the inability of properly processing the AVP precursor (Ivell et al., Citation1986; Schmale & Richter, Citation1984). One of the interesting features of this strain is the finding that AVP production is blocked at the brain level, but seems to remain intact in peripheral tissues (Friedmann et al., Citation1993) indicating that it is not a “whole body AVP KO” animal model (indeed “whole body AVP KO” mice are not viable). In the Brattleboro rat AVP is synthesized in peripheral tissues such as the adrenal gland (Nussey et al., Citation1984), but in contrast to their lack of circulating AVP of hypothalamic origin, peripherally synthesized AVP is reactive to hemorrhagic challenges (Somova et al., Citation1986). Initially, Brattleboro rats were extensively used in different lines of neuroendocrine, behavioral and stress research (Sokol & Valtin, Citation1982). However, the use of Brattleboro rats in neuroendocrine research has continually declined since the mid-1980s.
In Budapest, a line of Brattleboro rats was maintained until recently and used for several types of study. A characterization of the general phenotype of these rats revealed that the congenital lack of AVP resulted in greatly elevated water consumption, compensating for the increased urine volume, with reduced food intake and body weight (Mlynarik et al., Citation2007). The general locomotor activity of AVP-deficient rats was not influenced if measured in the open-field, wire suspension or stationary beam test making these rats suitable for behavioral testing.
Experimental manipulations and behavioral tests
Before providing some previously unpublished data, we state that all manipulations were performed in accordance with regulations set by the European Communities Council Directive (2010/63/EU) and were approved by our Institutional Animal Care and Use Committee. For anesthesia we used a mixture of ketamine (50 mg/kg; Produlab Pharma B.V., SJ Raamsdonksveer, the Netherlands), xylasine (10 mg/kg; Produlab Pharma B.V., SJ Raamsdonksveer, the Netherlands) and pipolphen (5 mg/kg; Egis Pharmaceuticals PLC, Budapest, Hungary) throughout. If not stated otherwise, blood samples were collected from awake, freely moving rats via chronically implanted jugular venous catheters and treated as described in detail elsewhere (Zelena et al., Citation2009).
Anxiety-like behavior
To measure innate anxiety, three different paradigms were used:
(1) The elevated plus-maze test (EPM test) was introduced as an apparatus for testing innate anxiety in laboratory rodents (Stemmelin et al., Citation2005). We performed the 5 min test using a metal maze painted dark gray (elevated 70 cm above the floor, arm length: 50 cm; arm width: 15 cm; central platform: 15 cm ×15 cm; height of closed arm walls: 40 cm) (Balazsfi et al., Citation2015). On the EPM the time spent on the open arms provides a typical measure for low (relatively long time on the open arms) or high (relatively little time on the open arms) anxiety. Closed arm entries are considered as a parameter for the locomotor activity of the animals, while the ratio of open arm/total entries (frequency) is taken as a measure for locomotion-independent anxiety.
(2) The defensive withdrawal test (Takahashi et al., Citation1989) employs a modified setup of the open-field activity box (100 cm ×100 cm with 50 cm side walls) (Balazsfi et al., Citation2015). This open-field contains a dark tube (diameter 10 cm ×21 cm; closed on one end) that divides the total area into two compartments: an illuminated, unprotected area with walls and a dark, protective tube. When starting the 5 min experimental session, the experimental subjects are placed inside the tube and the tube is put into the open-field. We analyzed the latency to leave the tube and the percentages of time spent in tube and in the open area (separately for center and periphery). An increased latency to leave the tube and a reduced time spent in the open area is usually interpreted as indicating anxiety-like behavior (Engelmann et al., Citation1996).
(3) Marble burying tests another facet of innate fear, which is the aversive impact of novel objects (Balazsfi et al., Citation2015). The rats are placed individually in an unfamiliar box (41.3 cm × 26 cm × 29.8 cm high, Ferplast, Geo Maxi, Italy) containing a 5 cm deep layer of bedding holding 15 glass marbles (25 mm diameter) on its surface in a given pattern. The number of marbles that the rat buries is recorded during a 30 min observation period and a low number reflects a less-anxious phenotype.
Depressive-like behavior
To assess depressive-like behavior in our experimental subjects we used the FS test (Balazsfi et al., Citation2015). For this test the experimental animal is placed in a glass cylinder (45 cm tall and 14 cm in diameter) filled with water (at 20 ± 1 °C) to a height of 30 cm and allowed to swim freely for 10 min. At the beginning, animals usually try to escape by struggling but, after a while, they will give up and will spend most of the time in an immobile posture, floating. Typically, the percentage of time spent struggling (intense movements of forepaws usually directed against the walls when the animal continuously breaks the water surface), swimming (making active swimming motions, more than those necessary to merely keep the head above water) and floating (activity is minimized to occasional and small movements of legs or tail necessary to keep the head above the water) are measured as critical parameters. Struggling is an escape behavior and together with swimming represents active coping in the face of stressful situations (“non-depressive”), while enhancement in time spent floating is considered to reflect “depressive”-like behavior.
We focused in our experiments on adult male rats but in some cases females, including lactating rats, were also used. We compared AVP-deficient (di/di) rats with their heterozygous littermates (di/+), or closely related normal (+/+) Brattleboro rats, as the Long Evans strain cannot be a proper control because of separation from the Brattleboro line more than 50 years ago (Bohus & de Wied, Citation1998). summarizes the different studies cited in the text, including information about the sex and genotype of the controls (di/+ or +/+) used to compare the results for di/di rats.
Table 1. Summary of the data obtained in defined behavioral tests with different sex/reproductive states for vasopressin (AVP)-deficient Brattleboro rats (di/di) under different treatment conditions.
Indications for an anxiety- and depressive-like behavioral profile
The congenital lack of AVP leads to attenuated depression-like behavior in the FS and sucrose preference tests, while behavior on the EPM remains unaffected (Mlynarik et al., Citation2007) (, first row). We observed significantly enhanced open arm exploration (a typical sign for low innate anxiety) in di/di rats compared to controls if the EPM test was preceded by other experimental manipulations (including behavioral testing and control injections). These observations indicated that a genotype difference in anxiety-related behavior in di/di rats may exist, but is subtle and context-dependent. These findings are in good agreement with the effect of the V1b antagonist SSR149415, which produced clear-cut anxiolytic-like activity in models involving traumatic stress exposure, but not in classical tests of anxiety (Griebel et al., Citation2002). In Brattleboro rats we further found reduced self-grooming activity in di/di rats. Other authors reported that the duration of self-grooming was associated with a decrease in open arm exploration on the EPM, and both behaviors could be reduced by anxiolytic treatment in Sprague–Dawley rats (Bagdy et al., Citation2001). This suggests that the reduced self-grooming in our di/di rats is a sign of attenuated anxiety.
Regarding the HPA axis parameters, mild genotype-dependent differences in plasma ACTH concentrations, and an unchanged corticosterone release in response to both EPM and FS exposure were observed. Interestingly, dexamethasone (a synthetic glucocorticoid) more profoundly suppressed the stressor-induced corticosterone increase in di/di rats compared to their controls, a typical characteristic of “non-depressed” patients (Carroll, Citation1982; Rush et al., Citation1996).
Replacement of peripheral AVP signaling by DDAVP administration
Behavioral changes.
In subsequent experiments we investigated whether the behavioral changes observed in Brattleboro rats are due to the lack of AVP signaling in the brain and/or in the blood (Balazsfi et al., Citation2015) (, fourth row). The latter situation would suggest that the behavioral changes are secondary to, for instance, the lack of antidiuretic control and, therefore could be explained by a pronounced motivation of di/di rats to search for water. We chronically treated our Brattleboro rats with the V2-receptor agonist desmopressin (1-deamino-8-d-arginine vasopressin, DDAVP) to compensate for the missing antidiuretic action of endogenous AVP (Zelena et al., Citation2006). The behavioral consequences of this treatment were analyzed using a battery of tests primarily aimed at investigating emotional behavior and learning and memory that had previously not been applied in such depth in studies testing the effects of DDAVP in AVP deficient rats (Feifel et al., Citation2007; Zelena et al., Citation2006). In this experiment, our results confirmed previous findings suggesting an anxiolytic-like and a reduced depressive-like behavioral profile in AVP-deficient rats. We found that di/di rats spent more time on the open arms of the EPM, spent less time in the periphery of the defensive withdrawal apparatus and showed less burying behavior in the marble burying test, implying a reduced innate anxiety state. Furthermore, di/di rats showed less floating behavior during the FS test suggesting a “non-depressive” phenotype. As expected, DDAVP treatment compensated the peripheral effects of AVP-deficiency, but failed to have a significant impact on the rats’ behavior. Thus, we can conclude that lack of peripheral AVP does not explain the behavioral changes in di/di rats.
c-Fos immunopositivity.
We not only focused on behavior in the FS test, but also investigated the impact of exposure to the defined stressor on c-Fos synthesis in the lateral septum and amygdala, as endogenous AVP signaling in both septum and medial amygdala has been reported to modulate the behavioral response during FS (Ebner et al., Citation2002; Stemmelin et al., Citation2005). This technique is used to map cellular activity as c-Fos protein is among the first to be expressed in response to a wide range of stimuli and initiates the synthesis of other proteins.
The number of FS-induced c-Fos-positive cells was similar in the septum of all three studied groups (control, di/di and di/di with DDAVP treatment). In contrast, c-Fos immunoreactivity in the medial amygdala was higher in di/di compared to control rats, with almost identical levels in di/di and DDAVP-treated di/di rats. This was in agreement with previous data showing that in the septum AVP administration of a V1 antagonist promoted enhanced floating, indicating a more “depressive-like” phenotype (Ebner et al., Citation1999), while in the amygdala the same treatment produced an “anti-depressive”-like behavioral profile (Ebner et al., Citation2002). Our results provided the first hint for a more prominent role of AVP signaling in the medial amygdala than in the septum to mediate anxiety- and depression-like behavior.
Mimicking local, intracerebral release of AVP
Based on previous findings, it was reasonable to hypothesize that during stressor exposure AVP released into the PVN may reach critically high levels and travel via the extracellular fluid in behaviorally relevant concentrations to the septum (Engelmann et al., Citation2004) (. Landgraf and colleagues suggested that the septum might be a key brain area in AVP signaling for anxiety-like behavior from a study that showed reduced anxiety behavior after local V1 receptor antisense oligonucleotide administration (Landgraf et al., Citation1995). Hence, we concentrated on the PVN and septum.
To mimic central AVP release in our di/di rats we first used retrodialysis to deliver AVP into the PVN (for experimental details, see Zelena et al. (Citation2009); control rats were intact) or lateral septum (for experimental details see: (Ebner et al., Citation1999), control rats received vehicle Ringer lactate solution) (). The rats received synthetic AVP via the microdialysis probe (dialysis rate: 200 μl/h; gross dosage: 10 μg AVP/ml), which was started 1 h before behavioral testing (Zelena et al., Citation2009). In control rats a maximal AVP concentration of ca. 6 pg/100 μl was detected in the microdialysates collected during FS and basal levels were just above undetectable (Zelena et al., Citation2009). Considering a recovery of ca. 2% (e.g. restricted by passage through the microdialysis membrane (Landgraf & Ludwig, Citation1991)), we estimated that this reflects an extracellular concentration of ca. 3 μg AVP/ml in the intact rats. We decided to administer synthetic AVP to our di/di rats in a dose that was estimated to cause a significantly lower extracellular AVP concentration (ca. 50 pg AVP/ml considering a delivery of 0.5% for nonapeptide retrodialysis (Engelmann et al., Citation1992)) to avoid undesired local pharmacological effects, including blood-vessel constriction (Smock et al., Citation1987) and considering a higher sensitivity of central vasopressin receptors in di/di rats (Burnard et al., Citation1985).
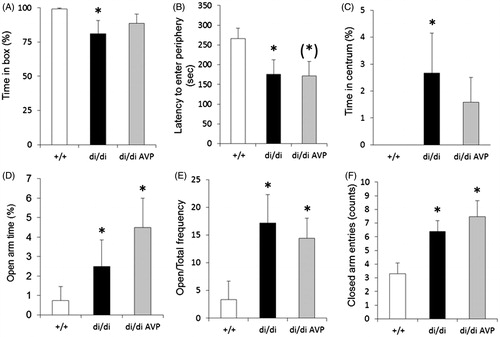
Behaviors after mimicking intra-PVN release of AVP by microdialysis
We found that untreated di/di rats showed a less “anxious” phenotype both in defensive withdrawal and EPM tests when compared with their controls (). AVP retrodialysis into the PVN failed to alter the behavioral profile of di/di rats in both cases. However, in a separate study it was shown that retrodialysing AVP into the PVN facilitated the return of plasma corticosterone (and oxytocin) concentrations to basal levels at a late timepoint (105 min) after onset of a 10 min acute stressor exposure (FS) in di/di rats (Zelena et al., Citation2009). The findings together suggest that the primary physiological function of intra-PVN released AVP is linked to the control of the activity of the central nervous part of the HPA axis under acute stress conditions rather than to behavioral modulation.
Figure 2. Vasopressin (AVP) microdialysis into the PVN and behavior tests. Mimicking intracerebral release of vasopressin (AVP) by retrodialysis of AVP into the paraventricular nucleus of the hypothalamus (PVN) in AVP-deficient Brattleboro rats (di/di, n = 10). Selected behavioral parameters were measured in the defensive withdrawal (A–C) and elevated plus maze (D–F) tests. Data are means ± SEM. +/+: non-AVP-deficient controls (n = 10); di/di AVP: AVP-deficient (Brattleboro) rats receiving AVP retrodialysis (n = 13). Statistical analysis was conducted using the nonparametric module of the StatSoft 12.0 program (Tulsa, OK) with Mann–Whitney pair-wise comparisons. *p < 0.05 vs +/+; (*): p = 0.08 vs +/+.
Behaviors after mimicking intra-septal release of AVP by microdialysis
AVP signaling in the septum may originate not only from the PVN, but also from other areas including the bed nucleus of the stria terminalis (Compaan et al., Citation1993) and the medial amygdala (Caffe et al., Citation1987). Thus, in additional experiments we investigated whether retrodialysis of AVP into the septum may have behavioral consequences for our Brattleboro rats. In this experimental series, we detected only a tendency towards an “anxiolytic phenotype” of di/di rats in the defensive withdrawal and EPM tests; none of the differences reached statistical significance (). However, it is possible that the manipulations associated with the microdialysis procedure per se may have masked a possible anxiolytic-like effect of AVP-deficiency.
Figure 3. Vasopressin (AVP) microdialysis into the septum and behavior tests. Effects of AVP retrodialysis into the septum of AVP-deficient Brattleboro rats (di/di, n = 11) on selected behavioral parameters measured in the defensive withdrawal (A–C), elevated plus maze (D–F) and forced swim (G–I) tests. Data are means ± SEM. +/+: non-AVP-deficient controls (n = 10); di/di AVP: AVP-deficient (Brattleboro) rats receiving AVP retrodialysis (n = 11). Statistical analysis was conducted using the nonparametric module of the StatSoft 12.0 program with Mann–Whitney pair-wise comparisons. **p < 0.01 vs +/+; (*): p = 0.065 vs +/+; (#): p = 0.056 vs di/di.
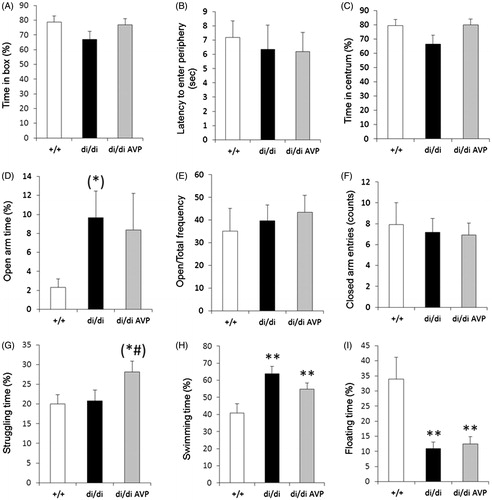
A more robust and statistically significant effect was found in the FS test. Namely, untreated di/di rats spent less time floating and more time swimming (). However, in the FS test retrodialysis of AVP into the septum failed to significantly affect the behavior of di/di rats.
Rescue of AVP synthesis in the PVN by viral vector
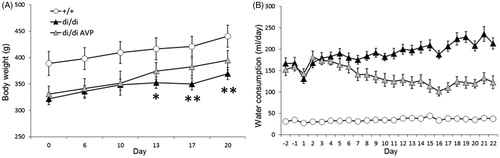
A possible explanation for the lack of significant behavioral results following the AVP-retrodialysis in di/di rats may be due to the artificial timing and dynamics of the experiment. An experimental approach to overcome this limitation may be provided by local rescue of AVP synthesis in the PVN of di/di rats by using an adeno-associated virus (AAV) vector construct containing the intact AVP nucleotide sequence (). The AAV is widely used in gene therapy, because of its lack of pathogenicity and its ability to stably integrate into the host cell genome. Thus, a previously successfully tested AVP-AAV vector (vAVP; Type 2, 7 × 109 genome copies/μl, 2 μl/injection site) (Ideno et al., Citation2003) was stereotaxically and bilaterally injected into the PVN of di/di rats. Controls received AAV containing a β-galactosidase nucleotide sequence (7.36 × 109 genome copies/μl, 2 μl/injection site). To confirm the functional rescue of local AVP synthesis we measured water consumption and body weight changes (). Our results demonstrated that restitution of AVP synthesis in the PVN significantly diminished water consumption and increased body weight. After two weeks, water consumption reached a stable level, thereby suggesting that subsequent behavioral testing would be in the context of effective and normally regulated AVP synthesis in the PVN.
Figure 4. Body weight (A) and water consumption (B) after injection into the PVN of adeno-associated virus (AAV) containing the intact AVP nucleotide sequence (vAVP). Time-course of changes in body weight (A) and water consumption (B) in vasopressin (AVP)-deficient (di/di) Brattleboro rats after bilateral injection of adeno-associated virus (AAV) containing AVP-nucleotide into the paraventricular nucleus of the hypothalamus (PVN): di/di vAVP group; controls were either di/di rats given AAV containing galactosidase nucleotide sequence, or untreated +/+ non-AVP-deficient controls; n = 10 per group. Data are means ± SEM. Statistical analysis was conducted using the repeated measure ANOVA module of the StatSoft 12.0 program with Newman–Keuls post hoc comparisons. (A) *p < 0.05; **p < 0.01 vs +/+; (B) the main effects of group, time as well as their interaction were statistically significant (p < 0.01); for clarity (B) does not contain statistical symbols, the main effects of group, time as well as their interaction were significant (p < 0.01).
Behavior
In line with previous behavioral results there was a tendency towards anxiolysis in di/di rats in both the defensive withdrawal and EPM tests. This tendency was abolished by rescuing AVP synthesis in the PVN with the vector treatment (). Moreover, in the FS test the di/di rats showed initially a significantly more active behavior (struggling), which was partly diminished after rescue of AVP synthesis in the PVN (). Although these results suggest that AVP of PVN origin may indeed influence the anxiety- and depressive-like behavior in rats, none of the behavioral results reached the level of statistical significance; in addition the treatment had no impact on behavior in the marble burying test ().
Figure 5. Behavior tests after rescue of vasopressin (AVP) synthesis by bilateral injection of adeno-associated virus (AAV) containing the intact AVP nucleotide sequence (vAVP) into the PVN of AVP-deficient Brattleboro rats (di/di) rats. Data are shown for selected parameters measured in the tests for: defensive withdrawal (A–C, n = 4–5 per group), elevated plus maze exposure (D–F, n = 9 per group) and forced swimming (G–I, n = 9 per group). Data are means ± SEM. +/+: AVP-non-deficient controls; di/di vAVP: AVP-deficient rats with adeno-associated virus vector treatment (). Statistical analysis was conducted using the nonparametric module of the StatSoft 12.0 program with Mann–Whitney pair-wise comparisons. *p < 0.05 vs +/+; (*): p = 0.06 vs +/+; (#): p = 0.06 vs di/di.
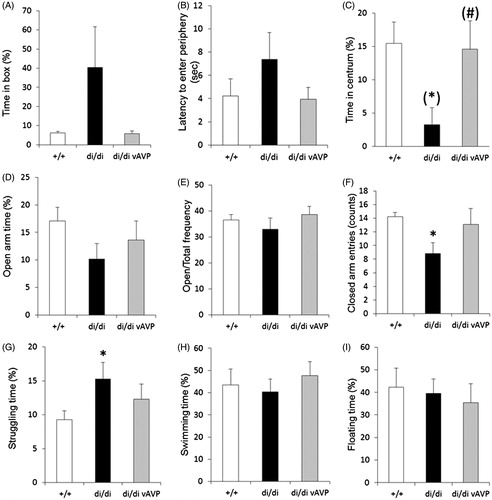
Figure 6. Marble burying (MB) test behavior (A) and ACTH secretory response to an acute stressor (B) after bilateral injection of adeno-associated virus (AAV) containing the intact AVP nucleotide sequence (vAVP) into the PVN of AVP-deficient Brattleboro rats (di/di) rats. (A) Selected parameters measured in the marble burying test (MB, A, n = 9 rats per group); (B) adrenocorticotropin (ACTH) responses (n = 10 per group). In B rats were urethane anesthetized and challenged by an intraperitoneal injection of hypertonic saline; blood samples were collected after 15 min for ACTH RIA. Data are means ± SEM. +/+ =AVP-non-deficient controls; di/di vAVP = AVP-deficient rats receiving vAVP treatment (see ). Statistical analysis was conducted using the repeated measure (A) or one way (B) ANOVA module of the StatSoft 12.0 program with Newman Keuls post hoc comparisons. (A) The main effects of time and group × time interactions were statistically significant (p < 0.01); for clarity (A) does not contain statistical symbols; (B) **p < 0.01 vs +/+; #p < 0.05 vs di/di.
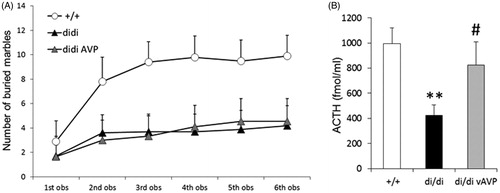
HPA stress axis
When we measured activation of the HPA axis after intraperitoneal hypertonic saline injection (a strong stressor) in urethane anesthetized rats, we found that the blunted ACTH response in di/di rats was normalized after AVP synthesis rescue in the PVN (). Thus, it seems that the impact of AVP synthesized and released in the PVN is primarily on the regulation of the HPA axis to stress, rather than on the regulation of behavioral responses.
Focus on female Brattleboro rats as experimental subjects – virgins
Behavior.
Although most of the experimental studies have been done in males (Bosch & Neumann, Citation2012; Fodor et al., Citation2012), the prevalence of anxiety and depression is higher in females (Bitew, Citation2014; Silva et al., Citation2014). Hence, we also examined the anxiety- and depressive-like behavior in female Brattleboro rats (, second row) (Fodor et al., Citation2016). As the locomotor activity was not different between the genotypes, the females of this strain are also suitable for being tested in behavioral paradigms. In some anxiety- (EPM) and all depression-like measures (FS, sucrose preference/anhedonia) di/di females showed an anxiolytic- and non-depressive-like behavioral profile similar to males. However, it is important to emphasize that we employed a wide range of anxiety-related behavioral tests (open-field, marble burying, novelty-induced hypophagia, sampling phase of the novel object recognition test) in which we failed to confirm the results obtained on the EPM in female di/di rats. Thus, congruent with the literature (Griebel et al., Citation2002, Citation2012; Salome et al., Citation2006), both male and female data suggest that central AVP signaling might be more important in the generation of depression than that in anxiety.
HPA stress axis and c-Fos.
Regarding regulation of the HPA axis activity in AVP deficiency, we found no differences among the females of all genotypes in their basal HPA hormone levels, or in levels in response to open-field testing and FS. However, the release of ACTH and corticosterone induced by EPM-exposure was blunted in di/di virgins. In most of the studied brain areas, c-Fos levels were higher in di/di rats under resting conditions. Interestingly, the increased number of c-Fos-positive cells induced by stressor-exposure (FS) was lower in di/di females than in the controls. Thus, behavioral alterations in di/di Brattleboro virgins could not be fully explained by changes in stress-hormones but, in contrast to males (Balazsfi et al., Citation2015; Sterrenburg et al., Citation2011), reduced neuronal activation in several brain areas after stress (including PVN, septum and medial amygdala) may contribute to the altered behavior. However, whereas in our hands the neuronal activation in both the septum and medial amygdala of di/di virgins was approximately half of that found in controls (Fodor et al., Citation2016), a study on Wistar rats showed that higher female anxiety correlated with enhanced V1a receptor expression in only the amygdala, and not in the septum (Poirier et al., Citation2013).
Focus on female Brattleboro rats as experimental subjects – lactation
Behavior.
We also examined the affective-like behavior in lactating Brattleboro rats (Fodor et al., Citation2012) (, third row). On the EPM there was only a tendency towards an increased anxiolytic-like phenotype in di/di mothers. The data obtained in the FS and the sucrose preference tests suggested that di/di mothers displayed a “non-depressive” behavioral profile.
HPA stress axis and c-Fos.
Previous studies have shown that AVP-deficiency diminishes HPA-axis changes related to lactation equivalent to those in a state of chronic stress, and also the acute stressor-induced ACTH and corticosterone release, suggesting that endogenous AVP of hypothalamic origin might contribute to stress adaptation not only in males but also in lactating rats (Fodor et al., Citation2013). Under basal conditions, we counted more c-Fos positive cells in the PVN and amygdala (but not the septum) of di/di mothers. In these areas the FS-induced increase in the number of c-Fos positive cells was smaller in di/di mothers than in controls (by ca. 50%), while in the septum it was even higher (ca. two-fold).
Conclusions from lactating Brattleboro rats.
At first, AVP-deficiency could be interpreted as being protective against developing depression during pregnancy and/or lactation. This may be linked to the blunted HPA-axis activity and is paralleled by an altered cellular activity in defined limbic brain structures. However, di/di females also show a higher prevalence of maternal neglect (Fodor et al., Citation2012), demonstrating that the behavioral consequences of central AVP deficiency do not simply produce a beneficial phenotype for the survival of the offspring. Further studies are needed to investigate in more detail sex specific consequences of AVP action on behavior.
Overall conclusions from Brattleboro rat studies
Although there is evidence that AVP synthesized in extra-hypothalamic brain areas, and signaling in the septum and medial amygdala as a neurotransmitter may play an important role for the generation of emotions (Compaan et al., Citation1993), our focus was on AVP synthesized within the hypothalamus and released into the hypothalamic extracellular space. We hypothesized that AVP signaling, originating from such intra-hypothalamic release, may be important not only for the control of the endocrine HPA axis stress response but also for emotional behavior. Using the Brattleboro rat as a model for congenital absence of central AVP synthesis we confirmed findings from previous studies that this nonapeptide acting at the brain level may play an important role for the development of anxiety- and depressive-like behavior not only in males, but also in (evidently more vulnerable) females and lactating mothers. Our data suggest that the signaling function of AVP within the brain might be more important in depression than in anxiety. This fits well with the reported enhanced effectiveness of the V1b antagonist SSR149415 in FS but not EPM tests in rodents (Griebel et al., Citation2002) as well as in major depression compared to generalized anxiety disorder in patients (Griebel et al., Citation2012).
Previous publications have suggested that AVP of magnocellular PVN origin may reach the septum to link the endocrine stress response regulation with behavior (Engelmann et al., Citation2000, Citation2004) (. The results presented here do not easily support this hypothesis. In contrast, the function of centrally released AVP originating within the PVN in controlling HPA-axis activity and, to a limited extent, also for body fluid homeostasis is further supported by our experiments performed with di/di rats.
Interestingly, in response to defined stressors AVP is released not only from the PVN, but also from the SON, originating from the somata of local magnocellular neurons and/or their dendrites (Wotjak et al., Citation1998; Zelena et al., Citation2013). Further studies may focus on the role central AVP plays when originating from the SON neurons and released to signal in adjacent brain areas, including the medial amygdala, which are known to contribute to the generation of emotional behavior.
Acknowledgements
We would like to thank K. Demeter for his help with the 3D figures.
Disclosure statement
We disclose any possible conflict of interest in the conduct and reporting of research.
This study was supported by OTKA grants F48783, IN67249 and NN71629, Hungary and DFG grants EN366/6-1 and EN366/8-1, Germany. The agencies had no further role in study design, in the collection, analysis or interpretation of the data.
References
- Aguilera G. (1994). Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 15:321–50.
- Albers HE. (2015). Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol 36:49–71.
- Altemus M, Cizza G, Gold PW. (1992). Chronic fluoxetine treatment reduces hypothalamic vasopressin secretion in vitro. Brain Res 593:311–13.
- Appenrodt E, Schnabel R, Schwarzberg H. (1998). Vasopressin administration modulates anxiety-related behavior in rats. Physiol Behav 64:543–7.
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. (2001). Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol 4:399–408.
- Balazsfi D, Pinter O, Klausz B, Kovacs KB, Fodor A, Torok B, Engelmann M, Zelena D. (2015). Restoration of peripheral V2 receptor vasopressin signaling fails to correct behavioral changes in Brattleboro rats. Psychoneuroendocrinology 51:11–23.
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. (2005). The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47:503–13.
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. (2004). Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29:483–93.
- Bitew T. (2014). Prevalence and risk factors of depression in Ethiopia: a review. Ethiop J Health Sci 24:161–9.
- Bohus B, de Wied D. (1998). The vasopressin deficient Brattleboro rats: a natural knockout model used in the search for CNS effects of vasopressin. Prog Brain Res 119:555–73.
- Bosch OJ, Neumann ID. (2012). Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61:293–303.
- Breuer ME, van Gaalen MM, Wernet W, Claessens SE, Oosting RS, Behl B, Korte SM, et al. (2009). SSR149415, a non-peptide vasopressin V1b receptor antagonist, has long-lasting antidepressant effects in the olfactory bulbectomy-induced hyperactivity depression model. Naunyn Schmiedebergs Arch Pharmacol 379:101–6.
- Burnard DM, Pittman QJ, Veale WL. (1985). Brattleboro rats display increased sensitivity to arginine vasopressin-induced motor disturbances. Brain Res 342:316–22.
- Caffe AR, van Leeuwen FW, Luiten PG. (1987). Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol 261:237–52.
- Carroll BJ. (1982). Clinical applications of the dexamethasone suppression test for endogenous depression. Pharmacopsychiatria 15:19–25.
- Compaan JC, Buijs RM, Pool CW, De Ruiter AJ, Koolhaas JM. (1993). Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res Bull 30:1–6.
- Cryan JF, Slattery DA. (2007). Animal models of mood disorders: recent developments. Curr Opin Psychiatry 20:1–7.
- Dallman MF. (1993). Stress update adaptation of the hypothalamic–pituitary–adrenal axis to chronic stress. Trends Endocrinol Metab 4:62–9.
- De Bellis MD, Gold PW, Geracioti TD, Jr., Listwak SJ, Kling MA. (1993). Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 150:656–7.
- de Wied D. (1978). Vasopressin in affective illness. Lancet 2:273.
- Dinan TG, Scott LV. (2005). Anatomy of melancholia: focus on hypothalamic-pituitary-adrenal axis overactivity and the role of vasopressin. J Anat 207:259–64.
- Douglas AJ, Johnstone LE, Neumann I, Leng G, Russell JA. (1994). Oxytocin neurones in the supraoptic nucleus (SON) are inhibited by endogenous opioids in late pregnant rats. Gene Ther 1:S84.
- Ebner K, Wotjak CT, Holsboer F, Landgraf R, Engelmann M. (1999). Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats. Eur J Neurosci 11:997–1002.
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. (2002). Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci 15:384–8.
- Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, et al. (2007). Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res 178:123–7.
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. (1999). Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol 11:867–72.
- Engelmann M, Landgraf R, Wotjak CT. (2004). The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol 25:132–49.
- Engelmann M, Ludwig M, Landgraf R. (1992). Microdialysis administration of vasopressin and vasopressin antagonists into the septum during pole-jumping behavior in rats. Behav Neural Biol 58:51–7.
- Engelmann M, Thrivikraman KV, Su Y, Nemeroff CB, Montkowski A, Landgraf R, Holsboer F, Plotsky PM. (1996). Endocrine and behavioral effects of airpuff-startle in rats. Psychoneuroendocrinology 21:391–400.
- Engelmann M, Wotjak CT, Ebner K, Landgraf R. (2000). Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol 85:125S–30S.
- Feifel D, Melendez G, Priebe K, Shilling PD. (2007). The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res 181:278–86.
- Fodor A, Klausz B, Pinter O, Daviu N, Rabasa C, Rotllant D, Balazsfi D, et al. (2012). Maternal neglect with reduced depressive-like behavior and blunted c-fos activation in Brattleboro mothers, the role of central vasopressin. Horm Behav 62:539–51.
- Fodor A, Kovacs KB, Balazsfi D, Klausz B, Pinter O, Demeter K, Daviu N, et al. (2016). Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiol Behav 158:100–11.
- Fodor A, Pinter O, Domokos A, Langnaese K, Barna I, Engelmann M, Zelena D. (2013). Blunted HPA axis response in lactating, vasopressin-deficient Brattleboro rats. J Endocrinol 219:89–100.
- Friedmann AS, Memoli VA, Yu XM, North WG. (1993). Biosynthesis of vasopressin by gastrointestinal cells of Brattleboro and Long-Evans rats. Peptides 14:607–12.
- Garcia FD, Coquerel Q, Kiive E, Dechelotte P, Harro J, Fetissov SO. (2011). Autoantibodies reacting with vasopressin and oxytocin in relation to cortisol secretion in mild and moderate depression. Prog Neuropsychopharmacol Biol Psychiatry 35:118–25.
- Gillespie CF, Nemeroff CB. (2005). Hypercortisolemia and depression. Psychosom Med 67:S26–S8.
- Goekoop J, de Winter R, Wolterbeek R, Wiegant V. (2011). Support for two increased vasopressinergic activities in depression at large and the differential effect of antidepressant treatment. J Psychopharmacol (Oxford) 25:1304–12.
- Goekoop JG, de Winter RF, Wolterbeek R, Spinhoven P, Zitman FG, Wiegant VM. (2009). Reduced cooperativeness and reward-dependence in depression with above-normal plasma vasopressin concentration. J Psychopharmacol (Oxford) 23:891–7.
- Gold PW, Goodwin FK, Reus VI. (1978). Vasopressin in affective illness. Lancet 1:1233–6.
- Griebel G, Beeske S, Stahl SM. (2012). The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry 73:1403–11.
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. (2002). Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA 99:6370–5.
- Griebel G, Simiand J, Stemmelin J, Gal CS, Steinberg R. (2003). The vasopressin V1b receptor as a therapeutic target in stress-related disorders. Curr Drug Targets CNS Neurol Disord 2:191–200.
- Hodgson RA, Mullins D, Lu SX, Guzzi M, Zhang X, Bleickardt CJ, Scott JD, et al. (2014). Characterization of a novel vasopressin V1b receptor antagonist, V1B-30N, in animal models of anxiety-like and depression-like behavior. Eur J Pharmacol 730:157–63.
- Ideno J, Mizukami H, Honda K, Okada T, Hanazono Y, Kume A, Saito T, et al. (2003). Persistent phenotypic correction of central diabetes insipidus using adeno-associated virus vector expressing arginine-vasopressin in Brattleboro rats. Mol Ther 8:895–902.
- Inder WJ, Donald RA, Prickett TC, Frampton CM, Sullivan PF, Mulder RT, Joyce PR. (1997). Arginine vasopressin is associated with hypercortisolemia and suicide attempts in depression. Biol Psychiatry 42:744–7.
- Ivell R, Schmale H, Krisch B, Nahke P, Richter D. (1986). Expression of a mutant vasopressin gene: differential polyadenylation and read-through of the mRNA 3′ end in a frame-shift mutant. EMBO J 5:971–7.
- Keck ME, Welt T, Muller MB, Uhr M, Ohl F, Wigger A, Toschi N, et al. (2003). Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology 28:235–43.
- Kokras N, Sotiropoulos I, Pitychoutis PM, Almeida OF, Papadopoulou-Daifoti Z. (2011). Citalopram-mediated anxiolysis and differing neurobiological responses in both sexes of a genetic model of depression. Neuroscience 194:62–71.
- Krahn DD, Meller WH, Shafer RB, Morley JE. (1985). Cortisol response to vasopressin in depression. Biol Psychiatry 20:918–21.
- Landgraf R. (2006). The involvement of the vasopressin system in stress-related disorders. CNS Neurol Disord Drug Targets 5:167–79.
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. (1995). V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci 15:4250–8.
- Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, Nussbaumer M, et al. (2007). Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev 31:89–102.
- Landgraf R, Ludwig M. (1991). Vasopressin release within the supraoptic and paraventricular nuclei of the rat brain: osmotic stimulation via microdialysis. Brain Res 558:191–6.
- Landgraf R, Neumann ID. (2004). Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 25:150–76.
- Liebsch G, Wotjak CT, Landgraf R, Engelmann M. (1996). Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci Lett 217:101–4.
- Ludwig M, Bull PM, Tobin VA, Sabatier N, Landgraf R, Dayanithi G, Leng G. (2005). Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J Physiol (Lond) 564:515–22.
- Ludwig M, Leng G. (1998). Intrahypothalamic vasopressin release. An inhibitor of systemic vasopressin secretion? Adv Exp Med Biol 449:163–73.
- Ludwig M, Stern J. (2015). Multiple signalling modalities mediated by dendritic exocytosis of oxytocin and vasopressin. Philos Trans R Soc Lond B Biol Sci 370. doi: 10.1098/rstb.2014.0182.
- Meller WH, Kathol RC, Jaeckle RS, Lopez JF. (1987). Stimulation of the pituitary-adrenal axis with arginine vasopressin in patients with depression. J Psychiatr Res 21:269–77.
- Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, et al. (2006). Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry 59:594–602.
- Mlynarik M, Zelena D, Bagdy G, Makara GB, Jezova D. (2007). Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm Behav 51:395–405.
- Muller MB, Landgraf R, Keck ME. (2000). Vasopressin, major depression, and hypothalamic-pituitary-adrenocortical desensitization. Biol Psychiatry 48:330–3.
- Murgatroyd C, Spengler D. (2011). Epigenetic programming of the HPA axis: early life decides. Stress 14:581–9.
- Murgatroyd C, Wu Y, Bockmuhl Y, Spengler D. (2010). Genes learn from stress: how infantile trauma programs us for depression. Epigenetics 5:194–9.
- Nussey SS, Ang VT, Jenkins JS, Chowdrey HS, Bisset GW. (1984). Brattleboro rat adrenal contains vasopressin. Nature 310:64–6.
- Poirier GL, Cordero MI, Sandi C. (2013). Female vulnerability to the development of depression-like behavior in a rat model of intimate partner violence is related to anxious temperament, coping responses, and amygdala vasopressin receptor 1a expression. Front Behav Neurosci 7:35.
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. (1996). Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry 53:137–43.
- Rotzinger S, Lovejoy DA, Tan LA. (2010). Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 31:736–56.
- Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR, Jr., Weissenburger JE, Crowley GT, et al. (1996). The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatry 57:470–84.
- Salome N, Stemmelin J, Cohen C, Griebel G. (2006). Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 187:237–44.
- Sawyer WH, Valtin H, Sokol HW. (1964). Neurohypophysial principles in rats with familial hypothalamic diabetes insipidus (Brattleboro strain). Endocrinology 74:153–5.
- Schmale H, Richter D. (1984). Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature 308:705–9.
- Scott LV, Dinan TG. (1998). Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci 62:1985.
- Silva MT, Galvao TF, Martins SS, Pereira MG. (2014). Prevalence of depression morbidity among Brazilian adults: a systematic review and meta-analysis. Rev Bras Psiquiatr 36:262–70.
- Smock T, Cach R, Topple A. (1987). Action of vasopressin on neurons and microvessels in the rat hippocampal slice. Exp Brain Res 66:401–8.
- Sokol W, Valtin H. (1982). The Brattleboro rat. Ann NY Acad Sci 394:1–802.
- Somova L, Ivanova E, Zaharieva S, Machuganska A. (1986). Changes of adrenal vasopressin during hemorrhagic shock in rats with hereditary diabetes insipidus (Brattleboro strain). Acta Physiol Pharmacol Bulg 12:70–5.
- Stemmelin J, Lukovic L, Salome N, Griebel G. (2005). Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology 30:35–42.
- Sterrenburg L, Borch A, Peeters BW, Pinter O, Zelena D, Roubos EW, Kozicz T. (2011). Acute ether stress differentially affects corticotropin-releasing factor and urocortin 1 in the Brattleboro rat. Brain Res 1398:21–9.
- Stewart LQ, Roper JA, Scott Young W, 3rd, O'Carroll AM, Lolait SJ. (2008). The role of the arginine vasopressin Avp1b receptor in the acute neuroendocrine action of antidepressants. Psychoneuroendocrinology 33:405–15.
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE (1989). Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci 103:648–54.
- Valtin H, Schroeder HA. (1964). Familial hypothalamic diabetes insipidus in rats (Brattleboro strain). Am J Physiol 206:425–30.
- van Londen L, Goekoop JG, Kerkhof GA, Zwinderman KH, Wiegant VM, De Wied D. (2001). Weak 24-h periodicity of body temperature and increased plasma vasopressin in melancholic depression. Eur Neuropsychopharmacol 11:7–14.
- van Londen L, Kerkhof GA, van den Berg F, Goekoop JG, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, de Wied D. (1998). Plasma arginine vasopressin and motor activity in major depression. Biol Psychiatry 43:196–204.
- van West D, Del-Favero J, Aulchenko Y, Oswald P, Souery D, Forsgren T, Sluijs S, et al. (2004). A major SNP haplotype of the arginine vasopressin 1B receptor protects against recurrent major depression. Mol Psychiatry 9:287–92.
- Veenema AH. (2009). Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol 30:497–518.
- Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young WS. 3rd. (2002). Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry 7:975–84.
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, et al. (2004). Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology 29:1–14.
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. (1998). Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience 85:1209–22.
- Wotjak CT, Landgraf R, Engelmann M. (2008). Listening to neuropeptides by microdialysis: echoes and new sounds? Pharmacol Biochem Behav 90:125–34.
- Zelena D. (2012). Vasopressin in health and disease with a focus on affective disorders. Cent Nerv Syst Agents Med Chem 12:286–303.
- Zelena D, Langnaese K, Domokos A, Pinter O, Landgraf R, Makara GB, Engelmann M. (2009). Vasopressin administration into the paraventricular nucleus normalizes plasma oxytocin and corticosterone levels in Brattleboro rats. Endocrinology 150:2791–8.
- Zelena D, Mergl Z, Makara GB. (2006). The role of vasopressin in diabetes mellitus-induced hypothalamo-pituitary-adrenal axis activation: studies in Brattleboro rats. Brain Res Bull 69:48–56.
- Zelena D, Pinter O, Langnaese K, Richter K, Landgraf R, Makara GB, Engelmann M. (2013). Oxytocin in Brattleboro rats: increased synthesis is contrasted by blunted intrahypothalamic release from supraoptic nucleus neurones. J Neuroendocrinol 25:711–18.
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. (2010). Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci 30:7017–22.
