Abstract
Glucocorticoids (GCs) are used to treat numerous diseases, but their use in limited by adverse side effects. One such effect is occasional increased anxiety. Since the intensity of hepatic microsomal oxidation has been shown to alter responses to GC, we examined the possibility that rats with lower rates of hepatic GC metabolism would have increased anxiety. We hypothesized that the resulting, excessive GC would stimulate brain monoamine oxidase A (MAO-A), which would reduce brain serotonin, and thereby increase anxiety. Hepatic microsomal oxidative intensity was evaluated by the hexobarbital sleep time (HST) test. Results showed that rats with lower rates of hepatic GC metabolism had elevated brain MAO-A activity, reduced brain serotonin, and more anxiety than rats with higher rates of hepatic GC metabolism. We suggest that the HST test, as an integrative test of microsomal oxidation status, should be useful for predicting individual sensitivity to GC and to other drugs metabolized by the hepatic microsomal oxidation system.
Introduction
Physiological concentrations of endogenous glucocorticoids (GCs) can exert anxiolytic effects (File et al., Citation1979). Recently, the GCs have been used to treat anxiogenic stress disorders, including posttraumatic stress disorder (PTSD), a syndrome characterized by deficiency of endogenous GCs (Yehuda & Seckl, Citation2011). However, widespread use of GC therapy for PTSD is limited by its side effects (Fardet et al., Citation2012; Judd et al., Citation2014). Although exact mechanisms of the GC neuropsychiatric effect are not completely clear, activated expression of monoamine oxidase (MAO) can be regarded as a participant in the neuropsychiatric side effects of GCs. This hypothesis is supported by data of Johnson et al. (Citation2010) demonstrating the ability of M30, a new MAO-A and MAO-B inhibitor, to restrict dexamethasone-induced neuronal apoptosis. This increased MAO activity stimulates free radical oxidation and eventually leads to apoptotic death of neurons (Naoi et al., Citation2012). Another side effect is an occasional inverse response to GC that is evident as increased anxiety (Judd et al., Citation2014). Since GC effectiveness for asthma treatment was shown to be inversely related to the intensity of hepatic microsomal oxidation (Moore et al., Citation2013), it is logical to suggest that GC-induced anxiety could be due to depressed hepatic metabolism of GC. Excessive GC stimulates brain MAO-A, which reduces brain serotonin (Nagatsu, Citation2004), and this elevates anxiety (Ravindran & Stein, Citation2010). Considering the potential side effects of GC, it is not surprising that patient responses to GC therapy vary.
To address the issue of GC-induced anxiety, we performed a pilot study to relate brain MAO-A activity, brain serotonin, and anxiety with the rate of hepatic GC metabolism in rats. In vivo hepatic microsomal oxidative intensity was evaluated by the hexobarbital sleep time (HST) test (Baird et al., Citation1975). In addition, we measured the total activity of CYP3A, cytochrome P450 isoforms from the 3A family, which metabolize GCs including triamcinolone acetonide (TA) (Moore et al., Citation2013).
Results showed that rats with lower rates of hepatic GC metabolism, as reflected by longer HST, had elevated MAO-A activity, reduced brain serotonin, and more anxiety than rats with higher rates of hepatic GC metabolism.
Methods
Animals
The study was performed on 42 adult Sprague-Dawley male rats, acquired from the breeding facility Pushchino (Pushchino, Russia). The rats were housed in standard rat cages and fed rat chow and tap water ad libitum. The temperature in the housing facility was controlled at 21 °C, and lights were set to a 12:12 h light–dark cycle, with lights on at 7:00 am. Three weeks were allowed for the rats to acclimate before the initiation of the study protocol. During this period, the rats were handled and weighed once per day. This investigation conformed to the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health, and the investigation was approved by the South Ural State Medical University Ethics Review Board.
Hexobarbital sleep time test
An index of the overall intensity of hepatic microsomal oxidation in rats is the duration of sleep induced by the barbiturate derivative, hexobarbital (Baird et al., Citation1975). Depending on their HST, the rats were divided into groups with high intensity of microsomal oxidation (HST ≤15 min, fast metabolizers of TA [FM]) or with low intensity of microsomal oxidation (HST >15 min, slow metabolizers of TA [SM]) (Kozochkin et al., Citation2014). It is logical that FM and SM might respond to drugs differently in ways other than sleep time. Specifically, the HST might predict individual responses to halogenated GC.
Hexobarbital solution was administered, 60 mg/kg, i.p. When the rat showed signs of awakening, it was placed on its back for observation of its righting reflex. HST was designated as the time between injection of hexobarbital and successful recovery of the righting reflex, which was defined as the ability of the animal to turn over onto its paws within 15 s after being placed on its back. For each rat, HST was measured three times, and the results were averaged.
GC treatment
On the 14th day after HST was determined, 13 FM and 9 SM were injected with a halogenated GC, TA (VEB Berlin-Chemie, Berlin, Germany), 2 mg/kg, i.p. Thirteen FM and 8 SM were injected with a similar volume of normal saline to serve as untreated control rats.
Behavioral test for anxiety
On the 1th and 4th day after the TA injection, anxiety was evaluated using a 10-min, elevated X-Maze behavioral test. The time the rat stayed in the enclosed arms of the maze was taken as an index of its level of anxiety.
Collection of brain tissue and measurements of MAO-A activity and serotonin concentrations
Following the behavior test, the rats were killed by diethyl ether inhalation anesthesia (2.75 ml/L air), and brain cortex, midbrain, medulla with pons were collected (Glowinski & Iversen, Citation1966). Brain homogenate was prepared in 0.067 M sodium phosphate buffer (1/10 w/v; рН = 7.2) and centrifuged for isolation of mitochondria (Satav & Katyare, Citation2004). MAO-A activity was measured in the presence of selegeline, a selective MAO-B inhibitor (Tipton et al., Citation2006) at 250 nm with 4 mM 5-hydroxytriptamine creatinine sulfate as a substrate. Brain serotonin was assayed using an ELISA kit (Biosource, Nivelle, Belgium) following the procedure recommended by the manufacturer. For this assay, the lowest level of sensitivity is 0.5 ng/ml.
Collection of liver tissue and measurements of cytochrome P450 from the 3A family (CYP3A) activities in hepatic microsomes
Livers were homogenized in 1.15% KCl. The homogenates were centrifuged at 9000 × g for 20 min, and the supernatant was then centrifuged at 100,000 × g for 60 min. Microsomal pellets were resuspended in 0.1 M Tris-HCl buffer (pH 7.4) containing 0.5 mM dithiothreitol, 0.1 mM EDTA, and 20% glycerol. Microsomal protein concentrations were determined by the Bradford protein assay method, using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA) and bovine serum albumin (BSA; Sigma-Aldrich Inc., St. Louis, MO) as the standard, according to the protocol provided by the manufacturer.
The total activity of CYP3A was determined by measuring the amount of formaldehyde formed in the reaction of CYP3A-dependent N-demethylation of erythromycin (Werringloer, Citation1978). The reaction system contained 50 mM potassium phosphate buffer (pH = 7.4), 3 mM MgCl2 (Fluka, Buches, Switzerland) 12.5 mmol/L erythromycin (Sigma-Aldrich, St-Louis, MO), and 0.5–1 mg microsomal protein. The reaction was initiated by adding 0.25 mmol NADPН (Merck, Darmstadt, Germany) and stopped by placing the samples on ice. Then 200 μl of 15% trichloracetic acid was added, and the samples were centrifuged. Formaldehyde concentration was measured in the supernatant spectrophotometrically (405 nm) using the Nash’s reagent containing 2M ammonium acetate, 0.05 M glacial acetic acid, and 0.02 M acetyl acetone.
Statistical analyses
All measurements were done in duplicate or triplicate, and mean values were computed for each rat. Values are presented as means ± SD. The non-parametric Wilcoxon–Mann–Whitney U test was used to determine significance of intergroup differences for groups with different HST. This statistical analysis was performed using Statistica version 8 for Windows.
Results
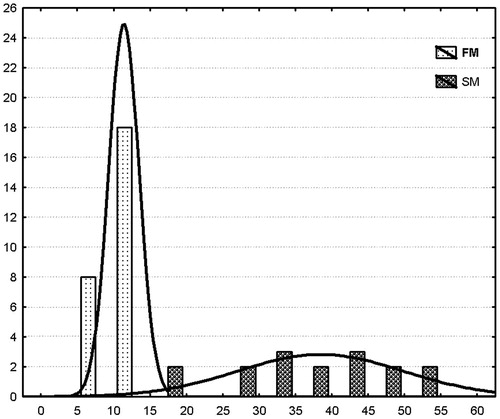
The HST test identified rats with short HST duration (HST = 11.34 ± 2.01 min, FM, n = 26) and long HST duration (38.25 ± 11.07 min, SM, n = 16). The histogram in shows the bimodal distribution of FM and SM rats plotted as a function of their hexobarbital sleep duration.
Figure 1. A frequency histogram showing distributions of the number of fast and slow metabolizing rats as a function of hexobarbital sleep duration. FM, fast metabolizers of triamcinolone acetonide; SM, slow metabolizers of triamcinolone acetonide. Abscissa, sleep duration, min; ordinate, number of rats.
By 24 h following administration of TA in a high dose, all rats exhibited evidence of increased anxiety-like behavior. This was evident as increased time of stay in the closed arm of the X-Maze, which was 18% longer for TA-treated FM than for untreated control rats (525 ± 43 for TA treatment, n = 13; 440 ± 40 s for control, n = 13; p < 0.05). Similarly, time of stay in the closed arms of the X-Maze was 22% longer for TA-treated SM rats than for untreated control rats (524 ± 26 s for TA treatment, n = 9; 429 ± 42 s for control, n = 7; p < 0.05).
In 96 h after TA administration, time spent in the enclosed arms of the X-Maze was significantly longer (509 ± 19 s, n = 9) than for SM control rats (450 ± 16 s, n = 8; p < 0.05), and those with TA treatment spent a correspondingly shorter time in the open arms of the maize (p < 0.05). For FM, time spent in the enclosed arms did not differ significantly (control 432 ± 48 s, n = 13; TA treatment 461 ± 38 s, n = 13; p > 0.05). Thus, increased anxiety-like behavior was manifested in SM but not in FM.
shows that in SM, TA injection reduced the serotonin level by 56% in medulla oblongata (p < 0.05), by 37% in midbrain (p < 0.05), and by 30% in cortex (p < 0.05). These reduced serotonin levels were associated with significant increases in MAO-A activity in medulla oblongata (+100%, p < 0.01), in midbrain (+50%, p < 0.01), and in cerebral cortex (+30%, p < 0.05). In FM, statistically significant changes in brain serotonin were absent, and no significant changes in MAO-A activity were observed in these brain structures. Thus, in SM but not in FM, TA injection resulted in higher MAO-A activity, lower brain serotonin concentrations, and more severe anxiety.
Table 1. Effect of triamcinolone acetonide (TA) on serotonin concentration and MAO-A activity in brain structures of fast and slow metabolizers of TA.
Differences in indices of total activities of GC-responsive isoforms of cytochrome P450 observed after the TA treatment were consistent with the observed differences in behavioral activity of FM and SM, in serotonin concentrations (), and in MAO-A activities () in brain structures. The TA treatment was associated with a 350% increase in CYP3A activity in FM rats (0.52 ± 0.062 nmol/min/g protein for control and 1.82 ± 0.98 nmol/min/g protein for TA treatment, p < 0.0001) and a 51% increase in SM rats (0.45 ± 0.12 for control and 0.68 ± 0.1 for TA treatment, p < 0.005).
Discussion
Among rats of similar sex, age, and strain, their HST varied significantly. Thus, the HST test permitted us to segregate rats according to their hepatic microsomal oxidase activity (Baird et al., Citation1975), i.e., FM or SM ().
Notably, we used a high dose of TA, and as might be expected, at 24 h after injection, both FM and SM rats showed increased anxiety-like behavior. At 96 h after TA administration, increased anxiety-like behavior was observed only in SM rats. In addition, CYP3A activity was considerably lower than in FM than SM rats. Although the reaction of erythromycin N-demethylation is not related with GC metabolism, the reaction intensity specifically reflects activity of “GC-responsive” cytochrome P450 isoforms. This is supported by the data of Wrighton et al. (Citation1985) who showed that monoclonal antibodies to rat isofoms of cytochrome P450 CYP3A inhibit metabolism of erythromycin-related macrolide antibiotics.
Relationships between intensity of hepatic microsomal oxidation and the presence or absence of GC-induced anxiety are illustrated in . Similar relationships between GC, MAO activity, and anxiety were observed in rats subjected to repeated restraint stress (Tseilikman et al., Citation2015; Volchegorskii et al., Citation2004). In these studies, post-stress anxiety developed in parallel with lipid peroxidation (LP) in brain. Inhibition of MAO demonstrated that the stress-induced increase in LP was MAO-dependent (Tseilikman et al., Citation2009). Importantly, this mode of restraint stress is associated with depressed CYP3A catalytic activity (Tseilikman et al., Citation2016). Thus, the increased anxiety observed in the SM of the current study is consistent with depressed GC metabolism and the increased anxiety-like behavior observed after restraint stress.
Figure 2. Regulation of MAO-dependent responses to glucocorticoids by intensity of hepatic microsomal oxidation in fast and slow metabolizers of triamcinolone acetonide. GC: glucocorticoid; MAO: monoamine oxidase; PTSD: posttraumatic stress disorder.
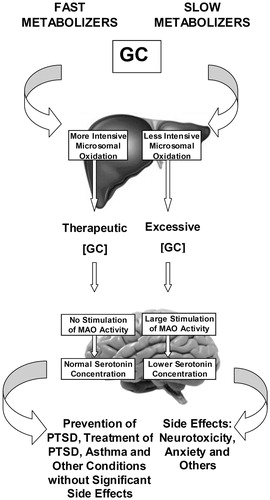
It should be noted that the behavioral test for anxiety and the analyses of serotonin were made 4 days after TA treatment. This time was sufficient to detect significant differences between SM and FM, but we do not know for how long the prolonged effects of TA treatment would have been detectable. On the other hand, we would expect differences between SM and FM to have been less at times closer to the TA treatment.
SM are expected to have a longer effect of TA due to their lower rate of GC metabolism. Apparently, the development of anxiety in SM is related with reduced serotonin levels in cortex, midbrain, and medulla oblongata, which contain the majority of serotonergic neurons (in the raphe nuclei). Our data showed that in these brain areas, MAO-A activity was increased as well. Since MAO-A oxidatively deaminates serotonin (Nagatsu, Citation2004), it is specifically this MAO-A activation that would have led to decreased serotonin concentrations in these brain structures. The increased MAO-A activity, in turn, most likely resulted from prolonged action of GC in SM.
GCs, including TA, are used for correction of delayed, post-stress behavioral disorders. In our earlier studies (Volchegorskii et al., Citation2004), we found that TA significantly decreased the extent of anxiogenic behavioral abnormalities in rats by preventing significant post-stress MAO activation in brain. However, in 29% of the stressed rats, TA treatment failed to correct the behavioral abnormalities. Although the rats in the current study were not subjected to stress, the current findings of a dependence of TA effectiveness on variable baseline intensity of microsomal oxidation could explain why TA treatment was not effective in some stressed rats of our earlier study. Moreover, the current findings are consistent with data of Moore et al. (Citation2013) showing that GC effectiveness for treatment of asthma varied with the activity of the hepatic CYP3A enzyme family. Evaluation of the overall intensity of hepatic microsomal oxidation, as performed in the current study, was appropriate since other enzymes, such as 11β hydroxysteroid dehydrogenase type 2 (11β HSD-2), are also involved in GC metabolism (Yehuda & Seckl, Citation2011).
In summary, the hexobarbital sleep test allowed us to identify, beforehand, animals that would respond abnormally to the halogenated GC, TA, with a side effect of increased anxiety-like behavior. Thus, this integrative test of microsomal oxidation status should be useful for predicting individual responses to GC and to other drugs metabolized by the hepatic microsomal oxidation system. This test should be a particularly useful component in experimental drug screening.
Funding information
This study was supported by the Ministry of Education and Science of the RF as a part of base State Program (Project code 1696) and by grant 14-04-01381 from the Russian Foundation for Basic Research.
Disclosure statement
No organization had any role in the writing or the decision to submit the article for publication. The authors report no conflicts of interest.
References
- Baird MB, Nicolosi RJ, Massie HR, Samis HV. (1975). Microsomal mixed-function oxidase activity and senescence-I. Hexobarbital sleep time and induction of components of the hepatic microsomal enzyme system in rats of different ages. Exp Gerontol 10:89–99.
- Fardet L, Petersen I, Nazareth I. (2012). Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry 169:491–7.
- File SE, Vellucci SV, Wendlandt S. (1979). Corticosterone – an anxiogenic or an anxiolytic agent? J Pharm Pharmacol 31:300–5.
- Glowinski J, Iversen LL. (1966). Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 13:655–9.
- Johnson S, Tazik S, Lu D, Johnson C, Youdim MB, Wang J, Rajkowska G, Ou XM. (2010). The new inhibitor of monoamine oxidase, M30, has a neuroprotective effect against dexamethasone-induced brain cell apoptosis. Front Neurosci 4:180.
- Judd LL, Schettler PJ, Brown ES, Wolkowitz OM, Sternberg EM, Bender BG, Bulloch K, et al. (2014). Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry 10:1045–51.
- Kozochkin DA, Bolotov AA, Tseilikman VE, Tishevskaya NV, Abramovskikh OS, Nikitina AA, Komelkova MV, Misharina ME. (2014). Calculation of discriminant function characteristics for dividing animals into phenotypic groups by the time of hexobarbital sleep. Sovremennye Problemy Nauki i Obrazovaniya 1:27–32 (in Russian).
- Moore CD, Roberts JK, Orton CR, Murai T, Fidler TP, Reilly CA, Ward RM, Yost GS. (2013). Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos 41:379–89.
- Nagatsu T. (2004). Progress in monoamine oxidase (MAO) research in relation to genetic engineering. Neurotoxicology 25:11–20.
- Naoi M, Maruyama W, Inaba-Hasegawa K. (2012). Type A and B monoamine oxidase in age-related neurodegenerative disorders: their distinct roles in neuronal death and survival. Curr Top Med Chem 12:2177–88.
- Ravindran LN, Stein MB. (2010). The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry 71:839–54.
- Satav JG, Katyare SS. (2004). Effect of streptozotocin-induced diabetes on oxidative energy metabolism in rat liver mitochondria – a comparative study of early and late effects. Indian J Clin Biochem 19:23–31.
- Tipton KF, Davey G, Motherway M. (2006). Monoamine oxidase assays. Curr Protoc Toxicol Chapter 4:Unit 4.21.
- Tseilikman VE, Kozochkin DA, Sinitskii AI, Tseilikman OB, Lapshin MS, Kuzina OV, Komelkova MV, Telesheva IB. (2016). Influence of repeated episodes 1-hour immobilization stress on glucoсorticoids metabolizing enzymes activities in liver. Bull Exp Biol Med, 160:614–16.
- Tseilikman VE, Sinitsky AI, Poyarkov KA, Vojdaev EV. (2009). Effect of deprenyl on free radical oxidation in rat brain during immobilization stress. Bull Exp Biol Med 148:856–8.
- Tseilikman VE, Sinitsky AI, Tseilikman OB, Deev RV, Lapshin MS, Kozochkin DA. (2015). Glucocorticoid-related regulation of LPO in brain cortex during anxiogenic stress Glucocorticoid-dependent regulation of lipid peroxidation in the cerebral cortex under anxiogenic stress. Bull Exp Biol Med 159:729–31.
- Volchegorskii IA, Tseilikman VE, Smirnov DS, Ship SA, Borisenkov AV. (2004). Decreases in glucocorticoid sensitivity as a factor of stress-producing changes in the activity of monoamine oxidase, lipid peroxidation, and behavior in rats. Neurosci Behav Physiol 4:697–701.
- Werringloer J. (1978). Assay of formaldehyde generated during microsomal oxidation reactions. Meth Enzymol 52:297–302.
- Wrighton SA, Maurel P, Schuetz EG, Watkins PB, Young B, Guzelian PS. (1985). Identification of the cytochrome P-450 induced by macrolide antibiotics in rat liver as the glucocorticoid responsive cytochrome P-450p. Biochemistry 24:2171–8.
- Yehuda R, Seckl J. (2011). Minireview: stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology 152:4496–503.
