Abstract
Neurobiological mechanisms underlying comorbid posttraumatic stress disorder (PTSD) and cocaine use disorder (CUD) are unknown. We aimed to develop an animal model of PTSD + CUD to examine the neurobiology underlying cocaine-seeking in the presence of PTSD comorbidity. Rats were exposed to cat urine once for 10-minutes and tested for anxiety-like behaviors one week later. Subsequently, rats underwent long-access (LgA) cocaine self-administration and extinction training. Rats were re-exposed to the trauma context and then immediately tested for cue-primed reinstatement of cocaine-seeking. Plasma and brains were collected afterwards for corticosterone assays and real-time qPCR analysis. Urine-exposed (UE; n = 23) and controls not exposed to urine (Ctrl; n = 11) did not differ in elevated plus maze behavior, but UE rats displayed significantly reduced habituation of the acoustic startle response (ASR) relative to Ctrl rats. A median split of ASR habituation scores was used to classify stress-responsive rats. UE rats (n = 10) self-administered more cocaine on Day 1 of LgA than control rats (Ctrl + Coc; n = 8). Re-exposure to the trauma context prevented cocaine reinstatement only in stress-responsive rats. Ctrl + Coc rats had lower plasma corticosterone concentrations than Ctrls, and decreased gene expression of corticotropin releasing hormone (CRH) and Glcci1 in the hippocampus. Rats that self-administered cocaine displayed greater CRH expression in the amygdala that was independent of urine exposure. While we did not find that cat urine exposure induced a PTSD-like phenotype in our rats, the present study underscores the need to separate stressed rats into cohorts based on anxiety-like behavior in order to study individual vulnerability to PTSD + CUD.
Introduction
Post-traumatic stress disorder (PTSD) develops in a subset of people exposed to a traumatic event (American Psychiatric Association, Citation2013). Cocaine use disorder (CUD) is highly comorbid with PTSD, with 43% of individuals with CUD meeting criteria for lifetime PTSD (Back et al., Citation2000) and 34% of trauma-exposed individuals meeting criteria for lifetime CUD (Khoury et al., Citation2010). Comorbid PTSD and CUD (PTSD + CUD) is associated with heightened severity of PTSD and CUD symptoms and worse treatment outcomes relative to individuals without comorbidity for these disorders (Najavits et al., Citation2007).
The neurobiological mechanisms underlying PTSD + CUD are unknown, and animal models that recapitulate PTSD + CUD are essential for assessing these changes. While many studies have examined stress and cocaine interactions, these studies include the entire trauma-exposed group as the study population. For example, Eagle et al. (Citation2015) exposed rats to a single-prolonged-stressor (SPS) followed by cocaine self-administration, extinction, and reinstatement and found that SPS exposure does not influence cocaine-seeking. This strategy is limited in that it does not separate stressor-exposed rats into cohorts based on anxiety-like behaviors following exposure. This separation is crucial to the study of PTSD, as only 15–25% of trauma-exposed individuals develop PTSD (Breslau et al., Citation2003).
Here, we aimed to develop a rodent model of PTSD + CUD by combining the “CBC model” of PTSD with the “extinction-reinstatement model” of addiction in order to assess PTSD-induced changes in cocaine-related behaviors. The CBC model is a well-characterized animal model of PTSD that separates rats exposed to a single brief predator stressor into “PTSD-like” and “Resilient” cohorts based on “cut-off-behavioral criteria” (CBC) derived from elevated plus maze (EPM) and acoustic startle response (ASR) tests 7 days post-exposure (Cohen et al., Citation2012). Using this model, approximately 25% of rats show “PTSD-like” behaviors (Cohen & Zohar, Citation2004; Cohen et al., Citation2007), a rate similar to PTSD in trauma-exposed humans. Moreover, similar to humans, “PTSD-like” rats show long-lasting symptoms of anxiety and hyper-arousal outside the stress context (Cohen & Zohar, Citation2004). In the extinction-reinstatement model of addiction, following operant drug self-administration, animals undergo extinction training such that the response that previously yielded drug no longer does so. Extinction training is followed by reinstatement testing, in which stimuli (stress, drug or drug-paired cues) known to induce relapse in human addicts also induce a reinstatement of the operant response (Epstein et al., Citation2006). We hypothesized that “PTSD-like” rats would exhibit increased cocaine intake, more active lever presses during extinction training, and exaggerated stress + cue-primed reinstatement.
Glucocorticoids are implicated in the etiology of both PTSD and CUD. Cortisol is attenuated immediately after trauma exposure in humans that develop PTSD (Ehring et al., Citation2008) and rats that self-administer cocaine display reduced basal corticosterone levels (Mantsch & Goeders, Citation2000). The amygdala, medial prefrontal cortex, dorsal hippocampus, and nucleus accumbens are part of the neurocircuitry disrupted by both PTSD and CUD. Our overarching hypothesis tested here is that the subset of stress-responsive rats would display changes in the expression of stress-related genes (FKBP5, corticotropin releasing hormone (CRH), Glcci1) in these brain regions relative to rats that were not responsive to stress. Furthermore, we predicted that cocaine self-administration would potentiate these effects.
Methods
Animals
Out-bred adult male Sprague–Dawley rats (275–300 g; Charles River) were individually housed in a temperature (22 °C) and humidity controlled vivarium on a reverse 12-h light–dark cycle (lights on at 19:00 h, lights off at 07:00 h), with all behavioral procedures and urine exposure initiated within 3–4 hours of the onset of the dark cycle. Rats were given free access to rat chow and water and a 6- to 10-day acclimation period before testing procedures began. All testing procedures were approved and regulated by the Institutional Animal Care and Use Committee at the University of Florida (UF) in Gainesville, FL. A total of 55 rats were used for this experiment.
Materials and equipment
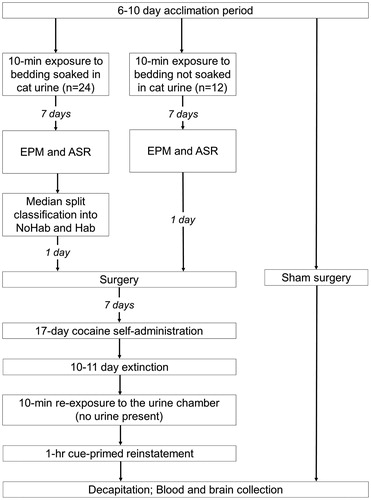
The urine-exposure chamber was a transparent plexiglas cylinder (diameter =60 cm) with a removable bedding tray and mesh floor (Wonder Bubble Modular Biobubble from BioBubble Pets, Boca Raton, FL). A standard EPM apparatus (Med Associates, St. Albans, VT) was used in the current study. The plus-shaped maze (56 × 56 cm) was made of black plexiglass and elevated 50 cm above the floor. To assess the ASR, we used ventilated sound-proof acoustic chambers (51 cm ×55 cm ×31 cm; San Diego Instruments, San Diego, CA). They were equipped with a plexiglass restraint tube, two speakers, and a transducer system through which the startle responses were recorded. The background white noise for the chamber was set to 68 dB. The cocaine self-administration chambers used were standard two-lever operant chambers (Med Associates, St. Albans, VT). Presses on the active lever resulted in the delivery of 0.2 mg/infusion of cocaine in 0.05 mL; infusions were paired with illumination of the stimulus light and a tone (2900 Hz). Cocaine hydrochloride [(-) cocaine-HCl] was kindly provided by the National Institute on Drug Abuse (NIDA) and dissolved in 0.9% sterile saline. All testing procedures were conducted under bright white light, with the exception of the EPM, which was conducted under conditions of dim white light. A timeline of all testing procedures is shown in .
Figure 1. Timeline of testing procedures. Twenty-four rats were exposed to bedding soaked in cat urine and 12 rats were exposed to unsoaked bedding. All 36 rats were then tested in the elevated plus maze and acoustic startle test. Urine-exposed rats were then classified as stress-responsive (NoHab) or stress-resilient (Hab) based on a median split of acoustic startle responses. Sixteen urine-exposed and eight unexposed rats were then anesthetized and surgically implanted with a jugular vein catheter. Six urine-exposed rats experienced catheter failure or illness during the self-administration-extinction component of the study, hence their brains and blood samples were not collected. An additional group of rats that were not exposed to urine or cocaine, but were given anesthesia and underwent sham surgery, served as control rats for brain (n = 8) and plasma corticosterone analyses (n = 11). NoHab = urine-exposed rats that did not habituate the acoustic startle response; Hab = urine-exposed rats that habituated the acoustic startle response. EPM = elevated plus maze; ASR = acoustic startle response.
Procedure
Induction of PTSD-like behaviors
Exposure to cat urine
Bedding soaked with cat urine was obtained from a UF cat colony that was composed of both male and female cats with intact gonads. The bedding was placed in the removable tray of the urine-exposure chamber. Urine exposed rats (UE; n = 24) were individually placed in the chamber once for ten minutes. Rats were never in direct contact with the cat urine. Control rats (n = 12) were placed in the chamber with unscented bedding.
Elevated plus maze
One week after urine exposure, rats were assessed in the EPM. Rats were placed onto the center of the EPM, facing an open arm, and permitted to explore the maze for five minutes. Each EPM session was recorded by a high-resolution CCD camera connected to a computer. The number of open and closed arm entries and the time spent in open and closed arms were quantified using Ethovision XT 7.0 (Noldus Information Technology, Leesburg, VA).
Acoustic startle response
Immediately following the EPM test, rats were individually placed in the ASR chambers. Rats underwent 30 startle trials during which 110 dB noise was presented for 40 ms followed by a 30 to 45 s inter-trial interval. The percent initial ASR score was computed as follows: [(average startle response of the last six trials)/(average startle response of the first six trials)] × 100, with higher scores indicating less habituation. As outlined by the CBC model (Cohen et al., Citation2012), we compared EPM and ASR responses of urine-exposed (UE) rats to those of control (Ctrl) rats in order to verify that urine-exposure had a significant effect on behavior. We also aimed to classify UE rats into cohorts of “PTSD-like” and “Resilient” rats based on anxious behaviors in the EPM and ASR using the CBC model (Cohen et al., Citation2012).
Cocaine self-administration, extinction and reinstatement
One day following EPM and ASR testing, a subset of urine exposed (n = 16) and Ctrl (n = 8) rats were anesthetized with ketamine HCl (87.5 mg/kg in 1 mL/kg, intramuscular, i.m.) and xylazine (5 mg/kg in 1 mL/kg, i.m.) for catheter implantation. One end of the catheter (silastic tubing, 12.5 cm long) was inserted into the jugular vein and secured in place by suture while the other end passed subdermally to exit through an incision on the back where it was attached to a stainless steel guide cannula (Plastics One, Inc., Roanoke, VA) affixed to an elastomer harness (Instech, Plymouth Meeting, PA). Catheter patency was maintained by daily injections of 0.1 mL heparin (100 IU/mL) prepared in 0.9% physiological saline. The antibiotic Timentin (60 mg/kg in 1 mL/kg; GlaxoSmithKline, Research Triangle Park, NC) was administered intravenously for one week during a post-surgery recovery period. Rats were then individually placed in an operant chamber where they were given the opportunity to self-administer cocaine using a fixed ratio-1 (FR-1) schedule of reinforcement. Self-administration took place during daily 1-hr sessions (short-access; ShA) for 7 days followed by daily 6-hr sessions (long-access; LgA) for 10 days. The use of an initial ShA component prior to LgA cocaine self-administration can result in an escalation of drug intake during the LgA component while the omission of the ShA component results in a decrease in intake over the course of LgA self-administration (Knackstedt & Kalivas, Citation2007). This escalation of cocaine intake in the LgA paradigm is considered to recapitulate the increased intake in humans as they progress from social to compulsive use (Ahmed & Koob Citation1998). Lever presses on the active lever resulted in delivery of intravenous cocaine paired with a light + tone complex (drug-paired cues) and were followed by a 20-s timeout. Inactive lever presses had no consequences but were recorded. Following self-administration, rats underwent daily 2-hr extinction sessions for 10–11 days during which cocaine and drug-paired cues were no longer delivered upon lever-pressing. Rats were re-exposed to the urine-exposure chamber (no urine present) once for ten minutes and, immediately afterwards, underwent a 1-hr reinstatement test wherein drug-paired cues were again delivered upon lever pressing (stress + cue reinstatement). Immediately upon completion of reinstatement testing, rats were killed via rapid decapitation without anesthesia. This time corresponded to approximately 4.5 hours after the onset of the dark cycle. At that time, trunk blood was collected for a corticosterone assay.
Plasma corticosterone analysis
An additional group of rats that were not exposed to urine or cocaine served as Ctrl rats for brain (n = 8) and corticosterone analyses (n = 11). These rats were administered ketamine HCl and xylazine as described above and underwent sham surgery in order to control for the effect of anesthesia and surgery. After a 1-month period of daily handling and no exposure to operant chambers, blood samples used for plasma corticosterone analyses were collected immediately after decapitation, approximately 4.5 hours after the onset of the dark cycle. Blood samples were collected into chilled tubes lined with 15% K3 EDTA solution (Thermo Fisher Scientific, Wilmington, DE). The tubes were then centrifuged at 3975g for 15 min. Plasma was collected and then stored at −80 °C until analysis via radioimmunoassay (RIA). The plasma corticosterone concentration was determined using a 125I kit from MP Biomedicals (Santa Ana, CA) as done previously (Krause et al., Citation2011). According to the manufacturer, the limit of detection for this kit is 7.7 ng/ml. The intra- and inter-assay coefficients of variation were 8.6% and 13.6%, respectively.
Gene expression analysis by real time quantitative RT-PCR
Brains were rapidly extracted and snap-frozen in isopentane cooled to –30 °C on dry ice. Brains were stored at –80 °C until processed. Frozen brains were sliced into 2-mm-thick coronal brain sections using a rat brain matrix (ASI Instruments, Warren, MI). Tissues of the medial prefrontal cortex (mPFC), dorsal hippocampus (dHipp), amygdala (AMY), and nucleus accumbens (NAc) were micropunched (2 mm diameter) according to a rat brain atlas (Paxinos & Watson Citation2007; ). Frozen brain tissue was then submerged in 1 mL TRI-Reagent (Applied Biosystems/Ambion, Austin, TX) and homogenized with Tissuelyser LT (Qiagen, Valencia, CA). Immediately post homogenization, 1-bromo-3-chloro-propane was added and samples were centrifuged for 10 min at 10,000g. Ethanol (70%) was then added to the supernatant and the ethanol/supernatant mixture was immediately transferred to RNeasy spin columns. RNA extraction and DNase incubation were completed using an RNeasy Mini Kit following the manufacturer’s protocol (Qiagen, Valencia, CA). RNA quantity was then assessed with a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). Reverse transcription of RNA was then completed using the iScript cDNA Synthesis Kit following the manufacturer’s protocol (Bio-Rad, Hercules, CA). Real time quantitative polymerase chain reaction (qPCR) was conducted in duplicate using the 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) using TaqMan Gene Expression Master Mix and validated by TaqMan Gene Expression assays (Thermo Fisher Scientific, Wilmington, DE). RPL32 was chosen as our housekeeping gene because it is stable under conditions similar to those of the present study (Zhang et al., Citation2009). We used the threshold cycle results for the samples to calculate relative changes in gene expression in Excel using the Delta Delta CT method (Livak & Schmittgen, Citation2001). Based on concurrent, unpublished work in our laboratory, we chose to assay CRH in all brain regions, Glcci1 in only the amygdala and dHipp, and FKBP5 in the amygdala. As we failed to find significant changes in CRH mRNA in the NAc and mPFC, this also informed our decision to omit the examination of FKBP5 and Glcci1 mRNAs in these brain regions.
Statistical analysis
Following Cohen and colleagues’ protocol (Cohen et al., Citation2012), we first examined data by comparing study groups (urine-exposed rats versus control rats not exposed to urine). Data were then analyzed according to the study phenotype, which was based on the rats’ anxiety-like behaviors. As such, independent samples t-tests and one-way ANOVAs were used to assess group and phenotypic differences in anxiety-like behaviors, cocaine-related behaviors, plasma corticosterone, and gene expression. Two-way Repeated Measures (RM) ANOVA tests were used to examine main and interactive effects of Group and Time in cocaine self-administration and extinction. Independent of Group or Time main effects detected with ANOVAs; paired samples t-tests were used to test for the reinstatement of cocaine-seeking, which is defined as a significant increase in responding during the test relative to the average of the last two days of extinction training. Bivariate (Pearson’s) correlations were used to assess the association between percent initial ASR, cocaine-related behaviors, corticosterone concentrations, and gene expression. Significant main effects were followed by Least Significant Differences (LSD) post-hoc tests. Data two standard deviations above or below the mean were identified as outliers, which resulted in one UE rat and one Ctrl rat being excluded from all analyses and individual samples being removed from gene expression analyses. All statistical analyses were performed using SPSS 17.0 and 23.0 (IBM, Aramonk, NY).
Results
PTSD Classification
As stated above, one UE rat and one Ctrl rat were excluded from EPM and ASR analyses due to ASR responses two standard deviations above the mean. The remaining UE rats (n = 23) and Ctrl rats (n = 11) did not differ in time spent in the open arms of the EPM (; t (32) = 0.65, n.s.) or the number of entries into the open arms (; t (32) = 0.40, n.s.), with UE rats displaying a range of 2–8 open arm entries (mean = 4.61; SEM =0.31) and Ctrl rats displaying a range of 2–7 open arm entries (mean = 4.82; SEM =0.40). UE rats displayed a range of 6–16 total arm entries (mean = 9.61; SEM =0.61) and Ctrl rats displayed a range of 4–15 total arm entries (mean = 9.64; SEM =0.83). An independent samples t-test revealed that UE rats habituated significantly less than Ctrl rats in the ASR (; t (32) = 2.14, p < 0.05). In the CBC model, “PTSD-like” behaviors are evidenced by (1) zero open arm entries and five minutes spent in the closed arms of the EPM and (2) mean startle amplitude >800 units and no habituation in the ASR. “Resilient” behaviors are evidenced by (1) 8 + open arm entries and 0–1 minutes spent in the closed arms of the EPM and (2) mean startle amplitude ≤700 units and habituation in the ASR (Cohen et al., Citation2012). As such, although we aimed to use EPM and ASR CBC for “PTSD-like” classification, here we classified rats based on percent initial ASR scores alone. To do so, we computed the median percent initial ASR among UE rats and conducted a median split (median= 87.45). UE rats with percent initial ASR scores above the median were classified as “no habituation” (NoHab; n = 11; 47.83%) and UE rats with percent initial ASR scores below the median were classified as “habituation” (Hab; n = 12; 52.17%). Only 3 (27.27%) of the Ctrl rats had ASR scores above the median, whereas 8 (72.73%) of the Ctrl rats had ASR scores below the median. These rates indicate that the NoHab phenotype occurred as a result of urine-exposure and was not a pre-existing factor. Importantly, in order to recapitulate the DSM-5 PTSD criteria of trauma exposure, Ctrl rats were not subdivided into “NoHab” or “Hab” rats for the remainder of the analyses.
Figure 2. Cat urine exposure did not influence elevated plus maze (EPM) behaviors but resulted in attenuated habituation of the acoustic startle response (ASR). Panels A and B: Time spent in the open arms of the EPM did not differ as a result of urine exposure (A) or phenotype (B). Panels C and D: Urine exposure did not alter the number of EPM open arm entries (C), nor did phenotype (D). Panel E: UE rats displayed less habituation of the ASR relative to controls (Ctrls) [t-test, p < 0.05]. Panel F: A one-way ANOVA revealed phenotype effects for percent initial ASR [p < 0.001], with LSD post-hoc tests revealing that NoHab rats habituated the ASR less than both the Hab rats and Ctrl rats. Ctrl = control rats not exposed to urine (n = 11); Urine = urine-exposed rats (n = 23); NoHab = urine-exposed rats that did not habituate the acoustic startle response (n = 11); Hab = urine-exposed rats that habituated the acoustic startle response (n = 12); Habituation assessment: percent initial ASR = [(average startle response of the last six ASR trials)/(average startle response of the first six ASR trials)] × 100, with higher percent initial ASR scores indicating lower habituation of the ASR. * = p < 0.05 compared to Ctrl; # = p < 0.05 compared to Hab, t-test (E) or LSD post-hoc test (F). Data are presented as mean ± SEM.
![Figure 2. Cat urine exposure did not influence elevated plus maze (EPM) behaviors but resulted in attenuated habituation of the acoustic startle response (ASR). Panels A and B: Time spent in the open arms of the EPM did not differ as a result of urine exposure (A) or phenotype (B). Panels C and D: Urine exposure did not alter the number of EPM open arm entries (C), nor did phenotype (D). Panel E: UE rats displayed less habituation of the ASR relative to controls (Ctrls) [t-test, p < 0.05]. Panel F: A one-way ANOVA revealed phenotype effects for percent initial ASR [p < 0.001], with LSD post-hoc tests revealing that NoHab rats habituated the ASR less than both the Hab rats and Ctrl rats. Ctrl = control rats not exposed to urine (n = 11); Urine = urine-exposed rats (n = 23); NoHab = urine-exposed rats that did not habituate the acoustic startle response (n = 11); Hab = urine-exposed rats that habituated the acoustic startle response (n = 12); Habituation assessment: percent initial ASR = [(average startle response of the last six ASR trials)/(average startle response of the first six ASR trials)] × 100, with higher percent initial ASR scores indicating lower habituation of the ASR. * = p < 0.05 compared to Ctrl; # = p < 0.05 compared to Hab, t-test (E) or LSD post-hoc test (F). Data are presented as mean ± SEM.](/cms/asset/7bf60606-9755-497c-a91d-571b95c3c212/ists_a_1189898_f0002_b.jpg)
One-way ANOVA tests revealed that NoHab, Hab, and Ctrl rats did not differ in time spent in the open arms of the EPM (; F (2,31) = 0.52, n.s.) or the number of entries into the open arms (; F (2,31) = 0.86, n.s.), with NoHab rats displaying a range of 3–7 open arm entries (mean = 5.00; SEM =0.38), Hab rats displaying a range of 2–8 open arm entries (mean =4.25; SEM =0.48), and Ctrl rats displaying a range of 2–7 open arm entries (mean =4.82; SEM =0.40). NoHab rats displayed a range of 6–15 total arm entries (mean = 10.18; SEM =0.80), Hab rats displayed a range of 6–16 total arm entries (mean =9.08; SEM =0.92) and Ctrl rats displayed a range of 4–15 total arm entries (mean =9.64; SEM =0.83). In order to support our use of a median split to distinguish the NoHab and Hab phenotypes, we used a one-way ANOVA to test for phenotypic differences in percent initial ASR scores. The ANOVA found significant group differences in percent initial ASR (F (2,31) = 26.327, p < 0.001). As shown in , post-hoc analyzes revealed that NoHab rats habituated significantly less than the Hab rats (p < 0.001) and Ctrl rats (p < 0.001), whereas Hab rats and Ctrl rats did not differ in percent initial ASR.
Cocaine self-administration, extinction and reinstatement
A total of 16 UE rats and 8 Ctrl rats were included in the self-administration portion of the study. The UE rats were selected based on their percent initial ASR scores (highest and lowest) and the Ctrl rats were selected at random. We have 24 operant chambers and were limited to 24 rats for this component of the study. Six UE rats (four NoHab and two Hab rats) experienced catheter failure or illness during the self-administration-extinction component of the study, and thus their cocaine intake data were only included in , and only if they completed the entire component (e.g. completed all 7 days of ShA).
Figure 3. Cat urine-exposed + cocaine (Urine + Coc) rats self-administered more cocaine infusions on Day 1 of Long Access (LgA) relative to control + cocaine (Ctrl + Coc) rats. Panel A: Number of cocaine infusions over the course of Short Access (ShA) and on Day 1 of ShA did not differ as a result of urine exposure. Panel B: The number of cocaine infusions over the course of LgA self-administration did not differ as a result of urine exposure, but Urine + Coc rats self-administered more cocaine infusions on the first day of LgA relative to Ctrl + Coc rats [t-test, p < 0.05]. Panel C: The number of active lever presses over the course of extinction sessions did not differ as a result of urine exposure. Panel D: Paired samples t-tests revealed that both Urine + Coc [t-test, p < 0.01] and Ctrl + Coc [t-test, p < 0.01] displayed significantly greater lever pressing during the stress + cue-primed reinstatement test compared to the average of the last two days of extinction. Ctrl + Coc = control rats not exposed to urine that self-administered cocaine (n = 8); Urine + Coc = urine-exposed rats that self-administered cocaine (n = 10). * = p < 0.05 compared to Ctrl; ^ = p < 0.05 compared to extinction. Data are presented as mean ± SEM.
![Figure 3. Cat urine-exposed + cocaine (Urine + Coc) rats self-administered more cocaine infusions on Day 1 of Long Access (LgA) relative to control + cocaine (Ctrl + Coc) rats. Panel A: Number of cocaine infusions over the course of Short Access (ShA) and on Day 1 of ShA did not differ as a result of urine exposure. Panel B: The number of cocaine infusions over the course of LgA self-administration did not differ as a result of urine exposure, but Urine + Coc rats self-administered more cocaine infusions on the first day of LgA relative to Ctrl + Coc rats [t-test, p < 0.05]. Panel C: The number of active lever presses over the course of extinction sessions did not differ as a result of urine exposure. Panel D: Paired samples t-tests revealed that both Urine + Coc [t-test, p < 0.01] and Ctrl + Coc [t-test, p < 0.01] displayed significantly greater lever pressing during the stress + cue-primed reinstatement test compared to the average of the last two days of extinction. Ctrl + Coc = control rats not exposed to urine that self-administered cocaine (n = 8); Urine + Coc = urine-exposed rats that self-administered cocaine (n = 10). * = p < 0.05 compared to Ctrl; ^ = p < 0.05 compared to extinction. Data are presented as mean ± SEM.](/cms/asset/b3b181fe-eafa-4f04-a40e-ed7591d85405/ists_a_1189898_f0003_b.jpg)
The effects of urine exposure on cocaine self-administration
Short-access
UE and Ctrl rats that self-administered cocaine (Urine + Coc; Ctrl + Coc; respectively) did not differ in the number of cocaine infusions over the course of ShA (), as a two-way RM ANOVA did not find a significant main effect of Group (F (1,20) = 1.12, n.s.) or a Group × Time interaction (F (6,120) = 1.65, n.s.). However, there was a significant effect of Time (F (6,120) = 4.27, p < 0.05). A two-way RM ANOVA did not find a significant main effect of Group (F (1,20) = 0.02, n.s.) or Time (F (6,120) = 1.55, n.s.), or a Group × Time interaction (F (6,120) = 1.24, n.s.) for inactive lever presses over the course of ShA (data not shown). Urine + Coc and Ctrl + Coc rats did not differ in the total number of cocaine infusions during ShA (t (16) = 1.14, n.s.), as an independent samples t-test was not significant (data not shown). Because cocaine infusions on the first day of ShA was positively correlated with percent initial ASR (see below), we examined group differences in cocaine intake and inactive lever presses on the first day of ShA. Urine + Coc and Ctrl + Coc rats did not differ in the number of cocaine infusions (; t (21) = 1.90, n.s.) or inactive lever presses (data not shown; t (21) = 0.97, n.s.) on the first day of ShA.
Long-access
Urine + Coc and Ctrl + Coc rats did not differ in the number of infusions over the course of LgA sessions (), as a two-way RM ANOVA did not find a significant main effect of Group (F (1,17) = 1.54, n.s.) or a Group × Time interaction (F (9,153) = 0.73, n.s.). There was a significant effect of Time (F (9,153) = 6.86, p < 0.001), as there was an increase in self-administration of cocaine infusions during LgA. A two-way RM ANOVA did not find a significant main effect of Group (F (1,17) = 0.73, n.s.) or Time (F (9,153) = 0.69, n.s.), or a Group × Time interaction (F (9,153) = 0.85, n.s.) for inactive lever presses over the course of LgA (data not shown). Urine + Coc and Ctrl + Coc rats did not differ in the total number of cocaine infusions during LgA (t (16) = 1.15, n.s.), as an independent samples t-test was not significant (data not shown). Urine + Coc rats self-administered significantly more cocaine infusions on the first day of LgA relative to Ctrl + Coc rats (; t (20) = 2.31, p < 0.05), as indicated by a significant independent samples t-test, conducted upon finding that cocaine infusions on the first day of ShA were positively correlated with percent initial ASR (see below). There were no Group differences in the number of inactive lever presses on the first day of LgA (t (20) = 1.84, n.s.), as an independent samples t-test was not significant (data not shown).
The effects of urine exposure on extinction
Urine + Coc and Ctrl + Coc rats did not differ in the number of active lever presses over the course of extinction sessions (), as a two-way RM ANOVA did not find a significant main effects of Group (F (1,16) = 1.04, n.s.) or Group × Time interaction (F (9,144) = 0.90, n.s.). There was a significant effect of Time (F (9,144) = 15.85, p < 0.001), as there was a decrease in active lever presses during extinction. A two-way RM ANOVA did not find a significant main effect of Group (F (1,16) = 0.16, n.s.) for inactive lever presses over the course of extinction sessions. However, there was a significant effect of Time (F (9, 144) = 5.49, p < 0.001) and a significant Group × Time interaction (F (9,144) = 2.58, p < 0.01) ().
The effects of urine exposure on stress + cue reinstatement
Urine + Coc and Ctrl + Coc rats did not differ in the number of active lever presses during extinction versus reinstatement, as a two-way RM ANOVA did not find a significant main effect of Group (F (1,16) = 3.03, n.s.) or a Group × Time interaction (F (1,16) = 2.40, n.s.). There was a significant effect of Time (F (1,16) = 32.55, p < 0.001), as there was an increase in active lever presses during the test compared to reinstatement. Paired samples t-tests comparing active lever presses during reinstatement to extinction revealed that both Urine + Coc and Ctrl + Coc rats significantly reinstated cocaine-seeking (; t (9) = 5.51, p < 0.01; t (7) = 4.31, p < 0.01; respectively). Urine + Coc and Ctrl + Coc rats did not differ in the number of inactive lever presses during extinction versus reinstatement (data not shown), as a two-way RM ANOVA did not find a significant main effect of Group (F (1,16) = 0.09, n.s.) or a Group × Time interaction (F (1,16) = 2.11, n.s.). There was no effect of Time (F (1,16) = 1.89, n.s.), indicating that the increase in responding during the reinstatement test was specific to the lever that previously delivered cocaine. Paired samples t-tests revealed that there were no differences in inactive lever presses during the last two days of extinction compared to inactive lever presses during reinstatement testing for both Urine + Coc and Ctrl + Coc rats (data not shown; t (9) = 0.06, n.s.; t (7) = 1.85, n.s.; respectively).
The effects of study phenotype on cocaineself-administration
Short-access
Ctrl + Coc rats, NoHab rats that self-administered cocaine (NoHab + Coc), and Hab rats that self-administered cocaine (Hab + Coc) did not differ in the number of cocaine infusions over the course of ShA (), as a two-way RM ANOVA did not find a significant main effects of Group (F (2,19) = 0.67, n.s.) or Group × Time interaction (F (12,114) = 1.37, n.s.). However, there was a significant effect of Time (F (6,114) = 3.49, p < 0.01). A two-way RM ANOVA did not find a significant main effect of Group (F (2,19) = 0.01, n.s.) or Time (F (6,114) = 2.09, n.s.), or a Group × Time interaction (F (12,114) = 0.64, n.s.) for inactive lever presses over the course of ShA (data not shown). Ctrl + Coc, NoHab + Coc, and Hab + Coc did not differ in the total number of infusions during ShA (data not shown; F (2,15) = 0.62, n.s.), as a one-way ANOVA test was not significant. Percent initial ASR was significantly and positively correlated with the number of infusions on the first day of ShA (; r= 0.53, p < 0.01, n = 23). Given that higher percent initial ASR scores indicate less ASR habituation, these results indicate that reduced habituation of the ASR is associated with greater cocaine infusions on the first day of ShA. Conducted upon finding this result, one-way ANOVA tests did not find phenotypic differences in the number of cocaine infusions (; F (2,20) = 3.13, n.s.) or inactive lever presses (data not shown; F (2,20) = 0.50, n.s) on the first day of ShA. Percent initial ASR was not correlated with the total number of ShA infusions (data not shown; r= 0.11, n.s., n = 18).
Figure 4. Exposure to the trauma context prevented cocaine reinstatement in cat urine- exposed rats not habituating (NoHab + Coc) the acoustic startle response (ASR), but not in the control + cocaine (Ctrl + Coc) and habituating + cocaine (Hab + Coc) rats. Panel A: The number of cocaine infusions over the course of Short Access (ShA) self-administration did not differ as a result of phenotype. Panel B: Percent initial ASR was positively correlated with the number of cocaine infusions on the first day of ShA self-administration (p < 0.01, n = 23). Panel C: The number of cocaine infusions over the course of Long Access (LgA) self-administration did not differ by phenotype. Panel D: The number of active lever presses over the course of extinction did not differ by phenotype. Panel E: Ctrl + Coc and Hab + Coc rats reinstated cocaine-seeking [paired t-tests, p < 0.01; p < 0.05, respectively], whereas NoHab + Coc rats did not. Ctrl + Coc = control rats not exposed to urine that self-administered cocaine (n = 8); NoHab + Coc = NoHab rats that self-administered cocaine (n = 5); Hab + Coc = Hab rats that self-administered cocaine (n = 5); Habituation assessment: percent initial ASR = [(average startle response of the last six ASR trials)/(average startle response of the first six ASR trials)] × 100, with higher percent initial ASR scores indicating lower habituation of the ASR. ^ = p < 0.05 compared to extinction. Data are presented as mean ± SEM.
![Figure 4. Exposure to the trauma context prevented cocaine reinstatement in cat urine- exposed rats not habituating (NoHab + Coc) the acoustic startle response (ASR), but not in the control + cocaine (Ctrl + Coc) and habituating + cocaine (Hab + Coc) rats. Panel A: The number of cocaine infusions over the course of Short Access (ShA) self-administration did not differ as a result of phenotype. Panel B: Percent initial ASR was positively correlated with the number of cocaine infusions on the first day of ShA self-administration (p < 0.01, n = 23). Panel C: The number of cocaine infusions over the course of Long Access (LgA) self-administration did not differ by phenotype. Panel D: The number of active lever presses over the course of extinction did not differ by phenotype. Panel E: Ctrl + Coc and Hab + Coc rats reinstated cocaine-seeking [paired t-tests, p < 0.01; p < 0.05, respectively], whereas NoHab + Coc rats did not. Ctrl + Coc = control rats not exposed to urine that self-administered cocaine (n = 8); NoHab + Coc = NoHab rats that self-administered cocaine (n = 5); Hab + Coc = Hab rats that self-administered cocaine (n = 5); Habituation assessment: percent initial ASR = [(average startle response of the last six ASR trials)/(average startle response of the first six ASR trials)] × 100, with higher percent initial ASR scores indicating lower habituation of the ASR. ^ = p < 0.05 compared to extinction. Data are presented as mean ± SEM.](/cms/asset/ea678537-679a-42a2-8695-d87b66f46272/ists_a_1189898_f0004_b.jpg)
Long-access
Ctrl + Coc, NoHab + Coc, and Hab + Coc did not differ in the number of cocaine infusions over the course of LgA sessions (), as a two-way RM ANOVA did not find significant main effects of Group (F (2,16) = 0.86, n.s.) or Group × Time interaction (F (18,144) = 0.75, n.s.). There was a significant effect of Time (F (9,144) = 6.31, p < 0.01), as rats increased cocaine self-administration during LgA. A two-way RM ANOVA did not find a significant main effect of Group (F (2,16) = 1.00, n.s.) or Time (F (9,144) = 1.07, n.s.), or a Group × Time interaction (F (18,144) = 1.16, n.s.) for inactive lever presses over the course of ShA (data not shown). There were no Group differences in the number of cocaine infusions on the first day of LgA (; F (2,19) = 2.72, n.s.), the number of inactive lever presses on the first day of LgA (data not shown; F (2,19) = 1.63, n.s.), or the total number of infusions during LgA (data not shown; F (2,15) = 0.78, n.s.), as a one-way ANOVA test was not significant. Percent initial ASR was not significantly correlated with the number of infusions on the first day of LgA (data not shown; r= 0.36, n.s., n = 22) or the total number of LgA infusions (data not shown; r= 0.01, n.s., n = 18).
The effects of study phenotype on extinction of the cocaine-seeking response
Ctrl + Coc, NoHab + Coc, and Hab + Coc did not differ in the number of active lever presses over the course of extinction sessions (), as a two-way RM ANOVA did not find a significant main effects of Group (F (2,15) = 1.09, n.s.) or Group × Time interaction (F (18,135) = 0.86, n.s.). There was a significant effect of Time (F (9,135) = 13.32, p < 0.01), as there was a decrease in active lever presses during extinction. A two-way RM ANOVA did not find a significant main effect of Group (F (2,15) = 0.08, n.s.) or a Group × Time interaction (F (18,135) = 1.47, n.s.) for inactive lever presses over the course of extinction sessions (). However, there was a significant effect of Time (F (9,135) = 6.04, p < 0.001).
The effects of study phenotype on stress + cue reinstatement
Ctrl + Coc, NoHab + Coc, and Hab + Coc rats did not differ in the number of active lever presses during extinction or reinstatement, as a two-way RM ANOVA did not find a significant main effect of Group (F (2,15) = 2.48, n.s.) or Group × Time interaction (F (2,15) = 1.93, n.s.). There was a significant effect of Time (F(1,15) = 26.61, p < 0.001). Paired samples t-tests found that Ctrl + Coc rats and Hab + Coc rats significantly reinstated cocaine-seeking (; t (7) = 4.31, p < 0.01; t (4) = 2.79, p < 0.05; respectively), whereas NoHab + Coc rats did not (; t (4) = 2.76, n.s.). Ctrl + Coc, NoHab + Coc, and Hab + Coc rats did not differ in the number of inactive lever presses during extinction versus reinstatement, as a two-way RM ANOVA did not find a significant main effect of Group (F (2,15) = 0.06, n.s.) or Group × Time interaction (F (2,15) = 2.30, n.s.). There was no effect of Time (F (1,15) = 0.83, n.s.), indicating that the increase in responding during the reinstatement test was specific to the lever that previously delivered cocaine. Paired samples t-tests found that there were no differences in inactive lever presses during the last two days of extinction compared to inactive lever presses during reinstatement testing for Ctrl + Coc, NoHab + Coc, and Hab + Coc rats (data not shown; t (7) = 1.85, n.s.; t (4) = 1.27, n.s.; t (4) = 1.07, n.s.; respectively). Percent initial ASR did not correlate with active lever presses on the first day of extinction (data not shown; r= -0.13, n.s., n = 18) or active lever presses during reinstatement (data not shown; r= -0.12, n.s., n = 18).
Plasma corticosterone concentrations
Blood samples for corticosterone analyses were collected immediately after completion of 1-hour cue-primed reinstatement testing. Plasma corticosterone concentrations of Ctrl rats, Ctrl + Coc rats, and Urine + Coc rats was similar, as a one-way ANOVA found no significant difference in group mean scores (; F (2,26) = 3.05, n.s.). The one-way ANOVA comparing plasma corticosterone concentrations of Ctrl rats, Ctrl + Coc rats, NoHab + Coc rats, and Hab + Coc rats was not significant (; F (3,25) = 2.14, n.s.). However, because the literature supports the a priori hypothesis that we would observe corticosterone differences between Ctrl and Ctrl + Coc rats (Mantsch & Goeders, Citation2000), we compared Ctrl and Ctrl + Coc rats and determined that Ctrl + Coc rats had significantly lower plasma corticosterone relative to Ctrl rats (; t (17) = 2.87, p < 0.05). Plasma corticosterone concentration was not correlated with percent initial ASR, cocaine-related behaviors, or gene expression.
Figure 5. Lower plasma corticosterone concentrations in control + cocaine (Ctrl + Coc) rats than in controls (Ctrls). Corticosterone concentrations did not differ across study groups (Panel A) or study phenotypes (Panel B). Independent sample t-tests indicated significant differences between Ctrl + Coc and Ctrl rats [Panels A,B; p < 0.05]. Ctrl = control rats not exposed to urine or cocaine (n = 11); Ctrl + Coc = control rats not exposed to urine and self-administered cocaine (n = 8); Urine + Coc = urine-exposed rats that self-administered cocaine (n = 10); NoHab + Coc = NoHab rats that self-administered cocaine (n = 5); Hab + Coc = Hab rats that self-administered cocaine (n = 5). * = p < 0.05 compared to Ctrl. Data are presented as mean ± SEM.
![Figure 5. Lower plasma corticosterone concentrations in control + cocaine (Ctrl + Coc) rats than in controls (Ctrls). Corticosterone concentrations did not differ across study groups (Panel A) or study phenotypes (Panel B). Independent sample t-tests indicated significant differences between Ctrl + Coc and Ctrl rats [Panels A,B; p < 0.05]. Ctrl = control rats not exposed to urine or cocaine (n = 11); Ctrl + Coc = control rats not exposed to urine and self-administered cocaine (n = 8); Urine + Coc = urine-exposed rats that self-administered cocaine (n = 10); NoHab + Coc = NoHab rats that self-administered cocaine (n = 5); Hab + Coc = Hab rats that self-administered cocaine (n = 5). * = p < 0.05 compared to Ctrl. Data are presented as mean ± SEM.](/cms/asset/adc869b1-46f0-4d1d-acc4-d3b62aa79280/ists_a_1189898_f0005_b.jpg)
Gene expression
CRH expression
Ctrl rats, Ctrl + Coc rats, NoHab + Coc rats, and Hab + Coc rats did not differ in CRH expression in the mPFC or NAc, as one-way ANOVA tests did not reveal significant group differences (; F (3, 22) = 1.29, n.s.; F (3, 21) = 0.48 n.s.; respectively). Significant group differences were found for the expression of CRH in the dHipp (; F (3, 20) = 17.90, p < 0.001). LSD post-hoc comparisons revealed that the expression of dHipp CRH in the Ctrl + Coc rats was significantly lower than the Ctrl rats (p < 0.001), NoHab + Coc rats (p < 0.01), and Hab + Coc rats (p <0.05). NoHab + Coc rats and Hab + Coc rats did not differ from Ctrl rats. In addition, there were significant group differences in the expression of CRH in the amygdala (; F (3, 20) = 15.12, p < 0.001). LSD post-hoc comparisons revealed that the expression in amygdala of CRH in the Ctrl + Coc rats, NoHab + Coc rats, and Hab + Coc rats were all significantly higher than the Ctrl rats (p < 0.001; p < 0.01; p < 0.01; respectively). CRH expression was not correlated with percent initial ASR, cocaine-related behaviors, or corticosterone.
Figure 6. Cocaine- and stress-induced differences in the expression of stress- and plasticity-related genes in the brain. Panel A: CRH mRNA. Expression in dorsal hippocampus (dHipp) was lower in the Ctrl + Coc rats than in all other groups of rats [ANOVA, LSD, p < 0.001]; expression in the amygdala (Amy) was greater in the Ctrl + Coc, NoHab + Coc, and Hab + Coc rats than in the Ctrl rats [ANOVA, LSD,p < 0.001)]. Panel B: Glcci1 mRNA. Expression in dHipp was significantly lower in the Ctrl + Coc rats than in all other groups [ANOVA, LSD, p < 0.001]. Panel C: FKBP5 mRNA. Expression in the amygdala (Amy) did not differ among study phenotypes. Ctrl = control rats not exposed to urine or cocaine (n = 8); Ctrl + Coc = control rats not exposed to urine that self-administered cocaine (n = 8); NoHab + Coc = rats not habituating acoustuc startle response and self-administered cocaine (n = 5); Hab + Coc = rats habituating acoustuc startle response and self-administered cocaine (n = 5). * = p < 0.05 compared to Ctrl; + = p < 0.05 compared to NoHab + Coc; # = p < 0.05 compared to Hab + Coc, all using the LSD post-hoc test. Data are presented as mean ± SEM. Panel D: Outlines of coronal rat brain sections according to the Rat Brain Atlas (Paxinos & Watson Citation2007) that guided tissue dissections of the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), dorsal hippocampus (dHipp), and amygdala (Amy).
![Figure 6. Cocaine- and stress-induced differences in the expression of stress- and plasticity-related genes in the brain. Panel A: CRH mRNA. Expression in dorsal hippocampus (dHipp) was lower in the Ctrl + Coc rats than in all other groups of rats [ANOVA, LSD, p < 0.001]; expression in the amygdala (Amy) was greater in the Ctrl + Coc, NoHab + Coc, and Hab + Coc rats than in the Ctrl rats [ANOVA, LSD,p < 0.001)]. Panel B: Glcci1 mRNA. Expression in dHipp was significantly lower in the Ctrl + Coc rats than in all other groups [ANOVA, LSD, p < 0.001]. Panel C: FKBP5 mRNA. Expression in the amygdala (Amy) did not differ among study phenotypes. Ctrl = control rats not exposed to urine or cocaine (n = 8); Ctrl + Coc = control rats not exposed to urine that self-administered cocaine (n = 8); NoHab + Coc = rats not habituating acoustuc startle response and self-administered cocaine (n = 5); Hab + Coc = rats habituating acoustuc startle response and self-administered cocaine (n = 5). * = p < 0.05 compared to Ctrl; + = p < 0.05 compared to NoHab + Coc; # = p < 0.05 compared to Hab + Coc, all using the LSD post-hoc test. Data are presented as mean ± SEM. Panel D: Outlines of coronal rat brain sections according to the Rat Brain Atlas (Paxinos & Watson Citation2007) that guided tissue dissections of the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), dorsal hippocampus (dHipp), and amygdala (Amy).](/cms/asset/3688dd27-1b91-48ec-8102-ff537d548b58/ists_a_1189898_f0006_b.jpg)
Glcci1 expression
Significant group differences were found for the expression of Glcci1 in the dHipp (; F (3, 21) = 10.75, p <0.001). LSD post-hoc comparisons revealed that the mean relative expression of dHipp Glcci1 in the Ctrl + Coc rats was significantly lower than the Ctrl rats, NoHab + Coc rats, and Hab + Coc rats (p < 0.001; p < 0.05; p < 0.001; respectively). However, there were no significant group differences in the expression of Glcci1 in the amygdala (; F (3, 21) = 2.02, n.s.). Glcci1 expression was not correlated with percent initial ASR, cocaine-related behaviors, or plasma corticosterone concentration.
FKBP5 expression
Ctrl rats, Ctrl + Coc rats, NoHab + Coc rats, and Hab + Coc rats did not differ in the expression of FKBP5 in the amygdala, as a one-way ANOVA test did not reveal significant group differences (; F (3, 22) = 0.11, n.s.). FKBP5 expression was not correlated with percent initial ASR, cocaine-related behaviors, or plasma corticosterone concentration.
Discussion
In the present study, a single exposure to cat urine failed to produce a PTSD-like phenotype when using identical criteria to those in the CBC model (Cohen et al., Citation2012). Our failure to produce a PTSD-like phenotype using the CBC model was likely due to our use of cat urine from a domestic colony, given that a PTSD-like phenotype in the CBC model is produced by exposing rats to feral cat urine (Hagit Cohen, personal communication). This is supported by the evidence of different urine scent profiles and urine-marking behaviors between domestic cats and their feral counterparts (Turner & Bateson, Citation2000).
While having no effect on EPM behavior, exposure to cat urine attenuated the habituation of the ASR relative to controls not exposed to urine. This particular rodent behavior has special translational relevance, as deficits in habituation to auditory startle are seen in individuals with PTSD (Shalev, Citation2001). Rats with an anxiety-like phenotype show increased acoustic startle responses and no habituation relative to rats that do not show this phenotype (Plappert et al., Citation1993), and the anxiolytic drug alprazolam reduces the acoustic startle response in healthy humans (Rodriguez-Fornells et al., Citation1999). Due to the presence of an effect of urine exposure on the ASR, we classified rats as stress-responsive and stress-resilient using a median split of percent initial ASR scores. Upon the classification of a subset of urine-exposed rats that were more responsive to the predator stressor, we examined both group effects (i.e. Ctrl versus UE), and phenotypic effects (i.e. NoHab + Coc, versus Hab + Coc) on our dependent variables of cocaine-seeking, corticosterone concentrations, and gene expression.
Cocaine self-administration, extinction and reinstatement
We found that cocaine self-administration remained low during short-access, but progressively increased during long-access. This “escalation” in cocaine consumption during long-access is considered to recapitulate the transition from regulated drug use to uncontrollable use in humans (Ahmed & Koob, Citation1998). Although Urine + Coc and Ctrl + Coc rats did not differ in cocaine self-administration during short-access, percent initial ASR was significantly correlated with the number of infusions on the first day of short-access in that less habituation of the ASR (i.e. more anxiety) was associated with a greater number of cocaine infusions on Day 1 of short-access (). Urine + Coc rats also self-administered significantly more cocaine infusions on the first day of long-access relative to Ctrl + Coc rats (). These results indicate that exposure to a predator stressor and/or the subsequent increase in anxiety does not produce a long-lasting increase in cocaine self-administration but can enhance intake during critical transition periods of drug intake.
All groups of rats decreased active lever pressing throughout extinction in a similar manner ( and ). This was unexpected given that individuals with PTSD + CUD have worse substance-related treatment outcomes relative to CUD-individuals without PTSD (Najavits et al., Citation2007) which could be manifested in the current model of cocaine addiction as increased responding under extinction conditions. Furthermore, PTSD patients typically exhibit a deficit in the ability to extinguish both trauma-related (Wessa & Flor, Citation2007) and trauma-unrelated memories (Orr et al., Citation2000). The lack of a relationship between anxiety phenotype and extinction learning here may be due to our inability to recapitulate a PTSD phenotype in our rats.
One prominent result of the present study was that cue-primed reinstatement of cocaine-seeking immediately following re-exposure to the trauma context (i.e. stress + cue-primed reinstatement) was attenuated only in the urine-exposed rats that did not habituate the ASR, whereas control rats and urine-exposed rats that habituated the ASR significantly reinstated cocaine-seeking (). Highlighting the importance of separating rats into cohorts based on anxiety-like responses to trauma-exposure, we did not observe this effect when including the entire trauma-exposed group as the study population. Specifically, both the urine-exposed group and the control group showed significant cue-primed reinstatement of cocaine-seeking following re-exposure to the trauma context (). Based on a large body of literature suggesting that stress incites the reinstatement of cocaine-seeking (Epstein et al., Citation2006), we had initially hypothesized that re-exposure to the trauma context would potentiate reinstatement and not prevent it. However, studies finding a facilitatory effect of stress on the reinstatement of cocaine-seeking do not use conditioned stressors. That is, the first exposure to the stressor occurs immediately prior to the reinstatement test. In contrast, a conditioned cue that predicts foot-shock presentation does not induce the reinstatement of cocaine-seeking (Shaham et al., Citation2000). Rats display freezing behaviors following predator stress (Asok et al., Citation2013) and urine-exposed rats display significantly more freezing behaviors after exposure to a trauma-cue compared to controls (Cohen et al., Citation2008). Thus, it is possible that the urine-exposed rats that did not habituate the ASR in the present study responded to the trauma context re-exposure with freezing behavior (and hence less lever presses) during the reinstatement test that immediately followed it. However, this hypothesis could not be tested directly, as we do not have a video record of rats’ behavior during reinstatement testing. Future research that examines freezing behaviors during cue-primed reinstatement following trauma context re-exposure is warranted.
Akin to our finding that urine exposure alone did not influence reinstatement, studies utilizing the single-prolonged-stressor (SPS) model in combination with cocaine self-administration have found that when considering the entire stress-exposed group as the study population, SPS does not influence the reinstatement of cocaine-seeking (Eagle et al., Citation2015). When examining the entire cohort of rats exposed to a SPS, a decrease in cocaine intake during long-access self-administration was found relative to un-stressed controls (Enman et al., Citation2015). Assessing anxiety prior to initiation of cocaine self-administration allowed us to perform correlational analyzes between these measures and cocaine intake () and to detect a relationship between anxiety and cocaine intake. Thus, by categorizing rats based on anxiety measures following a stressor, we were able to detect differences in both cocaine intake and stress + cue-primed reinstatement.
Plasma corticosterone concentrations
We found that unexposed control rats that self-administered cocaine had significantly lower corticosterone concentrations compared to unexposed control rats that did not self-administer cocaine. While we did not control for all procedures used on the cocaine self-administering rats (e.g. infusions, operant learning), we consider that our results are due to cocaine exposure. Rats that self-administer cocaine have been shown to have reduced basal corticosterone following 24 hours of withdrawal (Mantsch & Goeders, Citation2000). The present results indicate that this reduction persists into 2 weeks of withdrawal and is present following drug-paired cues.
Studies using the CBC model have found that “PTSD-like” Sprague-Dawley rats have significantly higher plasma corticosterone concentrations 7-days post-urine exposure relative to “Resilient” rats and controls (Cohen et al., Citation2007). In the present study, corticosterone was assessed 39–42 days after urine-exposure, during which time rats underwent cocaine self-administration, extinction training, trauma context re-exposure, and a single reinstatement test. We found that corticosterone concentrations of urine-exposed rats (regardless of study phenotype) did not differ from unexposed control rats that either did or did not self-administer cocaine. We speculate that these differences in corticosterone concentrations were not detected because the addition of cocaine dampens these effects.
Gene expression
We examined stress-related gene expression in the amygdala, nucleus accumbens, medial prefrontal cortex, and dorsal hippocampus because these regions are associated with both anxiety and cocaine addiction. Trauma reminders (such as context) increase activity in the basolateral amygdala in rats (Ritov et al., Citation2014), which is a region implicated in cue- and context-primed reinstatement of cocaine-seeking (Fuchs et al., Citation2005; Meil & See, Citation1997). Both the nucleus accumbens and the medial prefrontal cortex are necessary for cue- and stress-primed reinstatement of cocaine-seeking (Kalivas & McFarland, Citation2003; Mantsch et al., Citation2014). Moreover, disrupted medial prefrontal cortex activity is evident in both PTSD and CUD (Kalivas & O’Brien, Citation2008; Taghva et al., Citation2013). Finally, the hippocampus is critical for contextual fear (Maren & Fanselow, Citation1997) and context-primed reinstatement of cocaine-seeking following extinction (Fuchs et al., Citation2005). We assessed the expression of the CRH gene in the amygdala, nucleus accumbens, medial prefrontal cortex, and dorsal hippocampus, Glcci1 in the dorsal hippocampus and amygdala, and FKBP5 in the amygdala. Brain tissue was collected immediately following the reinstatement test and one hour following the re-exposure to the “trauma” context. As we only examined gene expression at this one point in time, limited conclusions can be made about which of the preceding experimental procedures directly altered gene expression.
The CRH gene encodes for the peptide corticotropin-releasing hormone (CRH), which is synthesized and released by the parvocellular cells of the hypothalamic paraventricular nucleus upon activation of the HPA axis. CRH neurons are also present outside of the hypothalamus, including in limbic areas such as the central nucleus of the amygdala and the hippocampus (Palkovits et al., Citation1985). CRH projections in the extended amygdala, including the accumbens shell, are involved in stress-primed reinstatement (Kalivas & McFarland, Citation2003). In the present study, we found that CRH gene expression in the amygdala was increased in all groups that self-administered cocaine regardless of ASR habituation (). The increase in expression is consistent with the finding that CRH antagonism prevents the ability of acute stress to induce the reinstatement of cocaine-seeking (Shaham et al., Citation1998). Given that the ability of non-contingent cocaine administration to increase CRH mRNA expression in the amygdala dissipates as early as 3 days after withdrawal (Erb et al., Citation2004), our results highlight a difference in CRH expression in the amygdala between contingent- and non-contingent cocaine self-administration. Central amygdala CRH gene expression is increased 3 h following exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) (Asok et al., Citation2013), but amygdala CRH mRNA levels return to baseline levels 48-hours after restraint stress (Kalin et al., Citation1994). This is in agreement with our findings that urine-exposure did not alter CRH mRNA expression in amygdala beyond those changes produced by cocaine.
We found that CRH gene expression in the dorsal hippocampus was reduced by cocaine but increased by urine-exposure (). Although there are evidently no studies of hippocampal CRH expression following stress- and/or cue-primed cocaine reinstatement, “PTSD-like” rats (but not in “Resilient” rats) have increased CRH mRNA expression in the hippocampus CA1 and CA3 regions 7-days post-exposure to soiled cat litter (Kozlovsky et al., Citation2012). As we found that rats that received urine exposure in combination with cocaine self-administration displayed greater dorsal hippocampus CRH mRNA expression than those that received only cocaine, our results are consistent with the idea that predator stress can upregulate CRH gene expression in this brain region.
We did not find stress- or cocaine-induced changes in CRH gene expression in the nucleus accumbens or medial prefrontal cortex. These results are in agreement with another study that found no difference in nucleus accumbens CRH gene expression between stress-responsive and stress-resilient rats (Koya et al., Citation2005). Taken together, our results suggest that CRH gene expression in the nucleus accumbens and medial prefrontal cortex are unaffected by cat urine-exposure or protracted withdrawal from cocaine self-administration.
Glcci1 expression is considered to be an indicator of glucocorticoid receptor activity. Dexamethasone, a glucocorticoid receptor agonist, significantly increases Glcci1 expression in various non-neuronal cell lines (Tantisira et al., Citation2011), suggesting that glucocorticoid binding to glucocorticoid receptors induces Glcci1 gene expression. In the present study, cocaine reduced Glcci1 gene expression in the dorsal hippocampus (but not the amygdala) and this effect was not seen after urine-exposure (). Thus, we speculate that glucocorticoid receptor activity in the dorsal hippocampus, but not the amygdala, is decreased in trauma-unexposed rats that self-administer cocaine, thus leading to decreased dorsal hippocampus Glcci1 expression. Notably, these results are consistent with our findings that unexposed control rats that self-administered cocaine had significantly lower corticosterone concentrations relative to unexposed control rats that did not self-administer cocaine (). Given the possible role of Glcci1 in glucocorticoid sensitivity (Quax et al., Citation2015) and support for increased glucocorticoid receptor sensitivity in PTSD (Yehuda, Citation2009), we expected Glcci1 gene expression to differ between urine-exposed rats that did not habituate the ASR and urine-exposed rats that habituated the ASR. It is possible that Glcci1 expression in other brain regions differs between these two groups, or that we did not find differences due to our failure to produce a PTSD-like phenotype. The latter is a likely conclusion as we did not observe phenotype-related plasma corticosterone concentration differences here.
Exposure to inescapable foot-shock increases hippocampal GR binding capacity 14 days post-exposure (van Dijken et al., Citation1993). While measures of GR expression are not conclusive indexes of GR activity, increased hippocampal GR expression (Eagle et al., Citation2013; Liberzon et al., Citation1999) and concomitant enhanced negative feedback in the HPA axis (Liberzon et al., Citation1999) are seen in rats 7 and 14 days after SPS-exposure. Notably, enhanced negative feedback in the HPA axis is evident among individuals with PTSD (Yehuda et al., Citation1993). GR expression in the amygdala remains unchanged following SPS-exposure (Eagle et al., Citation2013). This is in agreement with our results of no change in Glcci1 gene expression in the amygdala, and increased gene expression of hippocampal Glcci1 in both stressed (urine-exposed) groups relative to rats not exposed to urine but also taking cocaine ().
FKBP5, a co-chaperone of heat shock protein 90, inhibits glucocorticoid receptor translocation to the nucleus and reduces glucocorticoid receptor sensitivity. Given that peripheral FKBP5 gene expression is low in individuals with PTSD compared to trauma-exposed individuals without PTSD (Levy-Gigi et al., Citation2013), we expected reduced amygdala FKBP5 expression in the urine-exposed rats that did not habituate the ASR relative to the urine-exposed rats that habituated the ASR. However, we did not detect group differences in FKBP5 gene expression in the amygdala (). This is likely because FKBP5 gene expression in blood and amygdala do not fully overlap in rats exposed to the CBC model (Daskalakis et al., Citation2014). Our results are in agreement with studies that suggest no link between FKBP5 and CUD (Levran et al., Citation2014).
Conclusions
In conclusion, exposing rats to urine from non-feral cats did not induce a PTSD-like phenotype in the present experiment. Thus, we were not successful in developing an animal model of PTSD + CUD. However, we were able to distinguish a difference in stress responsivity one week after urine exposure that was manifested as failure to habituate the acoustic startle response. The results of the present study indicate that stress responsiveness influences cocaine-seeking. Our results indicate that corticosterone concentrations, as well as CRH and Glcci1 gene expression in the dorsal hippocampus, were increased by cat urine exposure but decreased by cocaine. Moreover, CRH gene expression in the amygdala was increased by cocaine but not further upregulated by urine-exposure. The use of a median split analysis on only one behavioral index of anxiety is not a strong criterion for the classification of a PTSD-like phenotype. Our future studies will examine the ability of other stressors to induce anxiety using multiple measures as employed in the CBC model. Although we did not generate a PTSD-like phenotype here, the results of the present study highlight the importance of separating trauma-exposed rats into cohorts of stress-responsive and stress-resilient groups.
Funding information
This work was supported by Institute for Translational Neuroscience Subcontract 8738sc awarded to LK. Award Number: W81XWH-12-2-0048. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702-5014 is the awarding and administering acquisition office.
Disclosure statement
The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. The authors declare no conflicts of interest.
References
- Ahmed SH, Koob GF. (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. 5th ed.) Washington, DC: American Psychiatric Association.
- Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. (2013). Immediate early gene and neuropeptide expression following exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT). Behav Brain Res 248:85–93.
- Back S, Dansky BS, Coffey SF, Saladin ME, Sonne S, Brady KT. (2000). Cocaine dependence with and without post-traumatic stress disorder: a comparison of substance use, trauma history and psychiatric comorbidity. Am J Addict 9:51–62.
- Breslau N, Davis GC, Schultz LR. (2003). Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry 60:289–94.
- Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z. (2012). Animal model for PTSD: from clinical concept to translational research. Neuropharmacology 62:715–24.
- Cohen H, Maayan R, Touati-Werner D, Kaplan Z, M AM, Loewenthal U, Kozlovsky N, Weizman R. (2007). Decreased circulatory levels of neuroactive steroids in behaviourally more extremely affected rats subsequent to exposure to a potentially traumatic experience. Int J Neuropsychopharmacol 10:203–9.
- Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. (2008). Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry 64:708–17.
- Cohen H, Zohar J. (2004). An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann N Y Acad Sci 1032:167–78.
- Daskalakis NP, Cohen H, Cai G, Buxbaum JD, Yehuda R. (2014). Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc Natl Acad Sci USA 111:13529–34.
- Eagle AL, Knox D, Roberts MM, Mulo K, Liberzon I, Galloway MP, Perrine SA. (2013). Single prolonged stress enhances hippocampal glucocorticoid receptor and phosphorylated protein kinase B levels. Neurosci Res 75:130–7.
- Eagle AL, Singh R, Kohler RJ, Friedman AL, Liebowitz CP, Galloway MP, Perrine SA. (2015). Single prolonged stress effects on sensitization to cocaine and cocaine self-administration in rats. Behav Brain Res 284:218–24.
- Ehring T, Ehlers A, Cleare AJ, Glucksman E. (2008). Do acute psychological and psychobiological responses to trauma predict subsequent symptom severities of PTSD and depression? Psychiatry Res 161:67–75.
- Enman NM, Arthur K, Ward SJ, Perrine SA, Unterwald EM. (2015). Anhedonia, Reduced Cocaine Reward, and Dopamine Dysfunction in a Rat Model of Posttraumatic Stress Disorder. Biol Psychiatry 78:871–9.
- Epstein DH, Preston KL, Stewart J, Shaham Y. (2006). Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16.
- Erb S, Funk D, Borkowski S, Watson SJ, Akil H. (2004). Effects of chronic cocaine exposure on corticotropin-releasing hormone binding protein in the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroscience 123:1003–9.
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309.
- Kalin NH, Takahashi LK, Chen FL. (1994). Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res 656:182–6.
- Kalivas PW, McFarland K. (2003). Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56.
- Kalivas PW, O’Brien C. (2008). Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–80.
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. (2010). Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress Anxiety 27:1077–86.
- Knackstedt LA, Kalivas PW. (2007). Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322:1103–9.
- Koya E, Spijker S, Homberg JR, Voorn P, Schoffelmeer AN, De Vries TJ, Smit AB. (2005). Molecular reactivity of mesocorticolimbic brain areas of high and low grooming rats after elevated plus maze exposure. Brain Res Mol Brain Res 137:184–92.
- Kozlovsky N, Zohar J, Kaplan Z, Cohen H. (2012). Microinfusion of a corticotrophin-releasing hormone receptor 1 antisense oligodeoxynucleotide into the dorsal hippocampus attenuates stress responses at specific times after stress exposure. J Neuroendocrinol 24:489–503.
- Krause EG, de Kloet AD, Flak JN, Smeltzer MD, Solomon MB, Evanson NK, Woods SC, et al. (2011). Hydration state controls stress responsiveness and social behavior. J Neurosci 31:5470–6.
- Levran O, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, Kreek MJ. (2014). Drug addiction and stress-response genetic variability: association study in African Americans. Ann Hum Genet 78:290–8.
- Levy-Gigi E, Szabo C, Kelemen O, Keri S. (2013). Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol Psychiatry 74:793–800.
- Liberzon I, Lopez J. F, Flagel S. B, Vazquez D. M, Young E. A. (1999). Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol 11:11–17.
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–8.
- Mantsch JR, Goeders NE. (2000). Effects of cocaine self-administration on plasma corticosterone in rats: relationship to hippocampal type II glucocorticoid receptors. Prog Neuropsychopharmacol Biol Psychiatry 24:633–46.
- Mantsch JR, Vranjkovic O, Twining RC, Gasser PJ, McReynolds JR, Blacktop JM. (2014). Neurobiological mechanisms that contribute to stress-related cocaine use. Neuropharmacology 76:383–94.
- Maren S, Fanselow MS. (1997). Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem 67:142–9.
- Meil WM, See RE. (1997). Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87:139–48.
- Najavits LM, Harned MS, Gallop RJ, Butler SF, Barber JP, Thase ME, Crits-Christoph P. (2007). Six-month treatment outcomes of cocaine-dependent patients with and without PTSD in a multisite national trial. J Stud Alcohol Drugs 68:353–61.
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. (2000). De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 109:290–8.
- Palkovits M, Brownstein MJ, Vale W. (1985). Distribution of corticotropin-releasing factor in rat brain. Fed Proc 44:215–19.
- Paxinos G, Watson C. (2007). The rat brain in sterotaxic coordinates. San Diego: Academic Press.
- Plappert CF, Pilz PK, Schnitzler HU. (1993). Acoustic startle response and habituation in freezing and nonfreezing rats. Behav Neurosci 107:981–7.
- Quax RA, Koper JW, Huisman AM, Weel A, Hazes JM, Lamberts SW, Feelders RA. (2015). Polymorphisms in the glucocorticoid receptor gene and in the glucocorticoid-induced transcript 1 gene are associated with disease activity and response to glucocorticoid bridging therapy in rheumatoid arthritis. Rheumatol Int 35:1325–33.
- Ritov G, Ardi Z, Richter-Levin G. (2014). Differential activation of amygdala, dorsal and ventral hippocampus following an exposure to a reminder of underwater trauma. Front Behav Neurosci 8:18.
- Rodriguez-Fornells A, Riba J, Gironell A, Kulisevsky J, Barbanoj MJ. (1999). Effects of alprazolam on the acoustic startle response in humans. Psychopharmacology (Berl) 143:280–5.
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. (1998). CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 137:184–90.
- Shaham Y, Erb S, Stewart J. (2000). Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33:13–33.
- Shalev AY. (2001). What is posttraumatic stress disorder? J Clin Psychiatry 62 (Suppl):4–10.
- Taghva A, Oluigbo C, Corrigan J, Rezai AR. (2013). Posttraumatic stress disorder: neurocircuitry and implications for potential deep brain stimulation. Stereotact Funct Neurosurg 91:207–19.
- Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, Lange C, et al. (2011). Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med 365:1173–83.
- Turner DC, Bateson PPG. (2000). The domestic cat: the biology of its behaviour. Cambridge, UK: Cambridge University Press.
- van Dijken HH, de Goeij DC, Sutanto W, Mos J, de Kloet ER, Tilders FJ. (1993). Short inescapable stress produces long-lasting changes in the brain-pituitary-adrenal axis of adult male rats. Neuroendocrinology 58:57–64.
- Wessa M, Flor H. (2007). Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry 164:1684–92.
- Yehuda R. (2009). Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci 1179:56–69.
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. (1993). Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 150:83–6.
- Zhang R, Packard BA, Tauchi M, D'Alessio DA, Herman JP. (2009). Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci USA106:5913–18.
