Abstract
Studies in rodents have shown that early life trauma leads to anxiety, increased stress responses to threatening situations, and modifies food intake in a new environment. However, these associations are still to be tested in humans. This study aimed to verify complex interactions among anxiety diagnosis, maternal care, and baseline cortisol on food intake in a new environment in humans. A community sample of 32 adolescents and young adults was evaluated for: psychiatric diagnosis using standardized interviews, maternal care using the Parental Bonding Inventory (PBI), caloric consumption in a new environment (meal choice at a snack bar), and salivary cortisol. They also performed a brain fMRI task including the visualization of palatable foods vs. neutral items. The study found a three-way interaction between anxiety diagnosis, maternal care, and baseline cortisol levels on the total calories consumed (snacks) in a new environment. This interaction means that for those with high maternal care, there were no significant associations between cortisol levels and food intake in a new environment. However, for those with low maternal care and who have an anxiety disorder (affected), cortisol was associated with higher food intake; whereas for those with low maternal care and who did not have an anxiety disorder (resilient), cortisol was negatively associated with lower food intake. In addition, higher anxiety symptoms were associated with decreased activation in the superior and middle frontal gyrus when visualizing palatable vs. neutral items in those reporting high maternal care. These results in humans mimic experimental research findings and demonstrate that a combination of anxiety diagnosis and maternal care moderate the relationship between the HPA axis functioning, anxiety, and feeding behavior in adolescents and young adults.
Introduction
Prenatal life, infancy, and adolescence are critical periods characterized by an increased vulnerability to stress (Kassi et al., Citation2000). Therefore, insults during such periods can lead to persistent changes, such as neuronal damage (McClelland et al., Citation2010), that continue into adult life (Macmillan et al., Citation2001) and increase the individual’s vulnerability to many disorders, including psychiatric conditions (Cattaneo et al., Citation2015). For instance, poor quality of maternal care and physical/sexual abuse can lead to persistent hyperactivation of the hypothalamus-pituitary-adrenal (HPA) axis responsivity (Mcgowan et al., Citation2009), to anxiety, and to depression in adult life (Parker, Citation1979; Parker et al., Citation1999).
Human studies have demonstrated that psychiatric disorders and HPA axis dysfunction have also been associated with metabolic syndrome and obesity (Raikkonen et al., Citation2007; Skilton et al., Citation2008), as well as with feeding behaviors (Hearon et al., Citation2014; Miller-Matero et al., Citation2014). Women with high levels of chronic stress show attenuated neuroendocrine responses to an acute stressor, and an increased caloric ingestion of highly palatable, sugar/fat enriched “comfort-foods” (Tryon et al., Citation2013). In addition, woman who classify themselves as chronically stressed show increased food intake, resulting in an increased waist circumference and body mass index, in addition to decreased cortisol levels when compared to controls (Tomiyama et al., Citation2011). Evidence suggests that glucocorticoid excess is involved in the development of obesity and abdominal fat deposition via increased food intake (Adam & Epel, Citation2007).
In rats, despite the vast literature demonstrating that chronic stress in adult life leads to anxiety and increased intake of palatable foods (Dallman et al., Citation2005; Ely et al., Citation1997), little is known about the effect of early life stress on the intake of palatable foods. It has been shown that early life handling increases palatable food intake in adulthood (Silveira et al., Citation2006, Citation2008, Citation2010), but these adult rats are well known for their enhanced capacity to deal with stress (Levine, Citation1957; Silveira et al., Citation2011). Anxiety and depressive-like symptoms provoked by early life stress can be reduced by chronic consumption of a palatable cafeteria diet (Maniam & Morris, Citation2010). Another rodent model that is associated with impaired maternal care is based on the reduction of the nesting material offered to dams during the first few days of postnatal life (Dalle Molle et al., Citation2012; Ivy et al., Citation2008). This early life stress model is associated with increased anxiety-like behaviors in adult rats, such as less time spent in the open arms of the Elevated Plus Maze Task (Dalle Molle et al., Citation2012) and decreased food intake in the Novelty Suppressed Feeding Task, accompanied by altered HPA axis responsivity to stress (Machado et al., Citation2013).
While the relationship between childhood trauma (Alvarez et al., Citation2007; Mamun et al., Citation2007; Noll et al., Citation2007), poor/stressful conditions (Giskes et al., Citation2008; Kestila et al., Citation2009), and the development of adulthood obesity has been established, little is known about how variations in maternal care influence long-term feeding behavior in humans, especially when considering anxiety symptoms. Resiliency and vulnerability in relation to the effects of the neonatal environment on anxiety are practically unknown although some studies exist in rodents (Silveira et al., Citation2011). Moreover, the neurobiological response to palatable foods associated with the perceived maternal care in infancy has not yet been explored.
Studies involving functional magnetic resonance imaging (fMRI) and childhood trauma have demonstrated that trauma experienced in early life is associated with acceleration in the development of pituitary gland volume (Ganella et al., Citation2015), and no differences in response to viewing food images in the fMRI (Elsey et al., Citation2015) in adolescent girls was found. An increase in amygdala activity when visualizing sad faces has been reported in children (Suzuki et al., Citation2014). In addition, both self-reported stress-induced anxiety and cue-induced favorite food cravings are linked to hypoactive striatal circuits in adolescents (Hommer et al., Citation2013).
The aim of the present study was to investigate the interaction between perceived maternal care, anxiety symptoms in adolescence, baseline cortisol, and the neurobehavioral response to palatable foods. Thus, the study evaluated the relationship between baseline cortisol levels and food intake in a new environment in adolescents reporting high and low maternal care scores. In addition, the study evaluated the brain responses to the visualization of palatable foods vs. neutral items in a brain fMRI task, in those reporting high or low maternal care according to their score of anxiety symptoms. Our hypothesis was that there would be an interaction between perceived maternal care and anxiety symptoms in the brain response to the visualization of palatable food images.
Methods
Seventy-five participants took an anthropometric and feeding behavior assessment at the HCPA Clinical Research Center (CPC-HCPA), and 43 underwent fMRI at the PUCRS Institute of Brain (InsCer – PUCRS). The participants filled out the Parental Bonding Inventory (PBI). After anthropometric measures were performed during the fasting, participants received a voucher (with equal value for all) to purchase a snack of their choice in the cafeteria (feeding behavior in a new environment). If the participant did not use the full value of the voucher, the change was returned to the researcher. In a subsequent visit, structural and functional images were viewed to evaluate the brain responses to the visualization of palatable foods vs. neutral items. The saliva was collected using a Salivit Labvitrus® to assess cortisol. A psychiatric evaluation was carried out with a clinical evaluation which included a structured clinical interview [Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL)] (Kaufman et al., Citation1997) held with the adolescents; and the Brazilian version of the MINI instrument (International Neuropsychiatric Interview) applied to individuals over −18.
The adolescents and young adults selected for this study originated from a community sample selected from six schools that belonged to the service area of the Santa Cecilia Health Unit, located in the city center of Porto Alegre, Rio Grande do Sul, Brazil. In 2008, the children and adolescents were invited to participate in the multidimensional evaluation and treatment of anxiety in children and adolescents, which included a psychiatric and nutritional assessment (Salum et al., Citation2011). Two hundred and forty-two participants completed the assessment in 2008, out of which 229 were eligible for the current study (six were excluded due to mental retardation; seven, due to kinship with another participant). The study was designed to carry out a detailed reevaluation in approximately 30% of the eligible sample. The present study is the fourth wave of a long-term follow-up study, as suggested by Barbieri et al. (Citation2006). The reevaluation, carried out in 2013/2014, included: (1) psychiatric assessment; (2) anthropometric and feeding behavior assessment; (3) blood and saliva collection for DNA extraction and biochemical evaluation; and (4) task-based fMRI. Seventy-five participants attended the data collection in the Clinical Research Center of the Hospital de Clínicas de Porto Alegre (CPC-HCPA) and 43 underwent fMRI at the Brain Institute of Rio Grande do Sul (InsCer). Of the 43 participants that completed the brain fMRI, baseline cortisol was detected in saliva in only 32 of them.
Parental care was measured using the PBI and diagnosis of anxiety using the SCARED-C instrument. The study evaluated brain responses to a task in which participants had to evaluate palatable foods; the task also included the evaluation of the frequency of “neutral items” (such as tools and tableware). The fMRI task was carried out with participants who reported high or low maternal care, according to their score of anxiety symptoms. Socioeconomic classification was based on the ABEP criteria (Brazilian Association of Research Companies), which ranks socioeconomic status according to the possession of certain items and the educational level of the head of the family: Class A – 35 to 46; Class B – 23 to 34; Class C – 14 to 22; Class D – 8 to 13; and Class E – 0 to 7. The study was approved by the Institutional Ethics Committee of the HCPA (GPPG/HCPA, project number 12-0254) and followed national and international guiding principles for research involving humans, including Resolution 196/96 of the National Health Council. Ethics committee approval and a subsequent written informed consent (either from the subjects or their guardians) were obtained from all participants prior to the study. Confidentiality, with respect to identity, privacy, and confidentiality of data, was ensured.
Nutritional evaluation
The anthropometric assessment was performed at the HCPA Clinical Research Center (CPC-HCPA) throughout the morning by trained researchers. All participants were fasting when the measurements were taken. Weight, height, and waist circumference were measured using accurate and calibrated equipment (Filizola®, Harpenden®). Each measure was collected twice; the average of the two assessments was used.
Psychiatric evaluation
The psychiatric evaluation was carried out with a clinical evaluation followed by (1) a structured clinical interview – Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., Citation1997), held with the adolesents and (2) the Brazilian version of the MINI instrument (International Neuropsychiatric Interview) (Amorim, Citation2000), applied to the individuals over −18. These instruments were applied by medical and psychology students trained for such activities and supervised by a child/adolescent psychiatrist. Anxiety was measured in two forms: categorical presence of any anxiety diagnosis using the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) in children and using the Mini-International Neuropsychiatric Interview (M.I.N.I.) in adults; and a continuous score of the SCARED scale (Isolan et al., Citation2011) was used in the fMRI data.
Feeding behavior in a new environment
A collection of the anthropometric measures were performed while participants were fasting for 4 h. They then received a voucher to purchase a snack of their choice in the cafeteria of the HCPA Clinical Research Center (CPC-HCPA). Participants were free to choose any snack as long as it did not cost more than what the voucher allowed; their food choices were photographed. Subsequently, food choices were entered into an Excel® data spreadsheet (Microsoft). For each participant, the nutritional composition of the selected snack was calculated with the aid of a household measures chart (Pinheiro et al., Citation2005) and the USDA National Nutrient Database (USDA, Citation2013).The objective of testing consumption in a new environment was an attempt to translate the novelty-suppressed feeding test paradigm to humans (Machado et al., Citation2013). Classically, the inhibition of food intake induced by the exposure to novelty is interpreted as a measure of anxiety (Gross et al., Citation2002; Zhang et al., Citation2010).
Maternal care
Perceived maternal care was assessed by the Brazilian version of the PBI (Parker, Citation1979). This questionnaire measures perceived care and the overprotection that individuals may have received from their mother or father during the first 16 years of life. Maternal care scores were calculated following the author’s guidelines, and using the Kendler version of the PBI (Kendler, Citation1996). The cutoff point for PBI scales was ≥18 for maternal warmth (MW), used in this study to categorize the individuals into high and low maternal care groups.
Salivary cortisol measurements
Saliva was collected using a Salivit Labvitrus® kit, frozen, and subsequently transported to the HCPA Clinical Laboratory for quantitation of cortisol by chemiluminescence method. Baseline cortisol was used for the main analysis. The analysis was repeated using the area under the curve of three points (AUC_ground, pré-fMRI, immediately after fMRI, and 30 min after the fMRI), as suggested by Pruessner et al. (Citation2003). For this analysis, only a smaller sample size was available (n = 24) because some of the individuals had lost samples (undetectable measurements) at one of the three points.
Functional fMRI acquisition and data processing
All participants who attended the evaluation at the CPC-HCPA were invited to participate in the brain neuroimaging stage of the study. The exclusion criteria for the fMRI exam was assessed via telephone contact (presence of metals in the body, brackets, a recent tattoo, and pregnancy), after which point the exam was scheduled at the Brain Institute. Participants were asked to keep fasting for at least 4 h. About 30 min before the exam, they received a standard snack [cereal bar + box of juice = 174 kcal, 39 g carbohydrate (90% of total calories), 0.9 g protein (2% of total calories), and 1.6 g of lipids (8% of total calories)].
Acquisition of fMRI images
Image acquisition was performed using a 3.0 Tesla magnetic resonance imaging scanner (GE Healthcare Signa HDxt) with an eight-channel head coil for signal reception. T1 structural images were acquired with voxels in anisotropic spatial resolution of 1 mm3 for 170 contiguous slices with a matrix size of 256 × 256 (frequency and phase). Inversion-recovery imaging consisting of a TE of 2.18 ms and TR of 6.1 ms was used. Structural acquisition was followed by the acquisition of functional images.
The acquisition of fMRI was performed by acquiring ecoplanar T2 × weighted images (EPI BOLD) with the following parameters: 26 interleaved axial slices, slice thickness of 4.0 mm and gap of 0.4 mm, FOV 240 mm ×240 mm, matrix size of 80 × 64, TE = 30 ms, TR= 2000 ms, and a 90° flip angle. The task was divided into three sessions; each session lasted 7 min and 46s and resulted in the acquisition of 233 volumes. During the brain scan, the participants performed the task described below.
fMRI paradigm – palatable foods/neutral foods/neutral items
The paradigm used in the fMRI study was adapted from Page et al. (Citation2011) and was designed to assess the brain responses of individuals during the presentation of highly palatable foods, neutral foods, and neutral items (non-edible objects, such as tools). Figures were selected from a database of images from the UFRGS School of Psychology (Deluchi, Citation2014) and the International Affective Picture System (IAPS) (Lang et al., Citation1997). A pilot study that evaluated adolescents at the same age range as our sample was performed to determine which images would be classified as highly palatable and neutral.
The paradigm was presented using the E-Prime software (Version 2, Psychological Software Tools Inc.). The presentation was divided into three sessions that lasted approximately 7 min each. Each experimental session included 21 randomized images (seven highly palatable foods, seven neutral foods, and seven neutral items), for a total 63 images across the experiment. Each image represented a trial which consisted of (1) image presentation (4s); (2) presentation of a 4-point scale, following the question: “How much do you like this food?” (5s); (3) presentation of another 4-point scale, following the question: “How much do you want to eat this food right now?” (5s). Individuals had to respond to the scales in (2) and (3) by pressing buttons; the scale ranged from 1 (not at all) to 4 (very much). A 6-s rest was inserted between trials (this rest was not explicitly modeled in the experiment). A 28-s baseline (rest) was also inserted in each of the three sessions; this 28-s baseline was modeled in the experiment (baseline for activation maps). The following questions were asked for the neutral items: “How much is this object relevant to you?” and “How much do you use this object?” Both questions were followed by a 4-point scale that resembled the scales in the food trials. Before the imaging session, participants practiced the task outside of the scanner; the practice trials followed the same structure as the actual experiment, but using different images.
More details about the fMRI preprocessing and the distribution of activation methods are available in the Supplementary Material.
Statistical analyses
Data were entered and analyzed using the Statistical Package for Social Sciences (SPSS), version 22.0 (SPSS Inc., IBM Company, Chicago, IL). Descriptive statistics were performed comparing characteristics of the individuals categorized as High (equal to or above 18) or Low (below 18) on the Maternal Warmth domain score of the PBI. The chi-square test was used for categorical variables and student’s t-test for continuous variables. The primary analysis was based on the model proposed by Holmbeck (Citation2002), consisting of the Generalized Linear Model (GLM) using the amount of calories eaten in a new environment as a dependent variable, and the three predictors: perceived maternal care (categorical), anxiety diagnosis (categorical), and baseline salivary cortisol (continuous). This was followed by a post-hoc analysis, as proposed by Holmbeck (Citation2002). In all analyses, we considered a significance level of 5%.
Results
Seventy-five healthy youths participated in the anthropometric and feeding behavior assessments performed in 2013. The subjects were not significantly different, in terms of age, sex, ethnicity, maternal schooling, birth weight, BMI, and socioeconomic status, from the remaining participants of the initial population sample (data not shown).
shows the participants’ characteristics, according to the perceived quality of maternal care received in infancy. The two groups did not show significant differences in relation to sex, skin color, maternal education, socioeconomic status, and BMI. However, as expected, adolescents reporting poor maternal care have increased scores of anxiety symptoms, as measured by the SCARED-C (p = 0.038).
Table 1. Subject’s characteristics, categorized as High (equal to or above 18) or Low (below 18) on the Maternal Warmth domain score of the Parental Bonding Inventory.
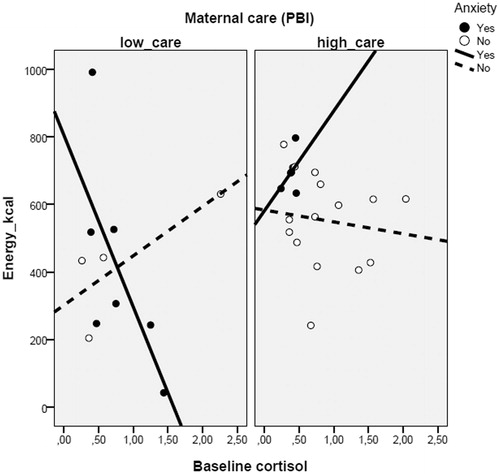
The study found a significant three-way interaction between anxiety diagnosis, perceived maternal care, and salivary cortisol on food intake levels (Wald = 15.635; df = 3; p = 0.001), as well as the main effects of the anxiety diagnosis (Wald = 8.022; df = 1; p = 0.005) and the salivary cortisol levels (Wald = 4. 141; df = 1; p = 0.042) (). Post-hoc analysis of the interaction, according to Holmbeck (Citation2002), allowed us to demonstrate that among those reporting high maternal care, there is no relationship between cortisol levels and energy intake in the new environment independent of the presence (p = 0.264) or absence (p = 0.590) of anxiety symptoms. On the other hand, there is a dual response in those reporting low maternal care. In those with low maternal care and with a positive anxiety diagnosis (affected), cortisol was negatively correlated with energy intake in a new environment (B = −385.65; p = 0.001), whereas in those without anxiety (resilient), there is a positive correlation between cortisol and energy intake (B = 215.85; p = 0.014). The analysis was repeated using the AUC and remained unchanged (data not shown).
Figure 1. GLM showing the interaction between cortisol, anxiety, and maternal care (PBI scores) (p = 0.001) on the total caloric intake in a new environment in adolescents. Post-hoc analysis shows that in those reporting low maternal care, the intake varies according to the presence of anxiety, decreasing as cortisol increases in the anxious individuals, and positively correlating with cortisol in those without anxiety.
The fMRI analysis revealed brain regions that responded to the images of palatable foods compared to neutral items and were highly correlated to anxiety scores and perceived maternal care. In the group of adolescents who reported high maternal care, we found a negative correlation between anxiety scores and brain activation in the superior frontal gyrus (X = −16, Y = 46, Z = 34; FWE-corrected p value = 0.010) and middle frontal gyrus (X = −28, Y = 20, Z = 46, FWE-corrected p value = 0.040). On the other hand, only visual areas were active for adolescents who reported low maternal care scores (, ); there were no significant correlation clusters in other areas of the brain. Finally, independent of the anxiety scores, adolescents who reported high maternal care showed a negative correlation in the precuneus (X = 40, Y = −72, Z = 36, FWE-corrected p value <0.05) ().
Figure 2. Brain areas of negative correlation in the high maternal care group as anxiety levels increase (superior and middle frontal gyrus). The same group has a negative correlation in the precuneus, independent of anxiety scores. For a detailed description, see Contrast of palatable foods > neutral items. Student’s t-test, FWE was corrected for multiple comparisons, p < 0.05.
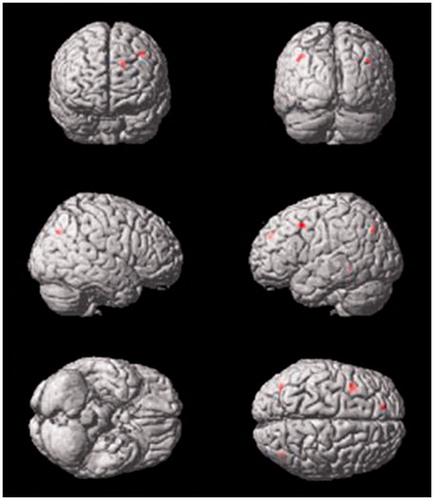
Table 2. Brain fMRI results in response to palatable foods in contrast to neutral items.
Discussion
These findings mimic our previous reports in rodents showing that a combination of disruptions in the quality of maternal care and a positive diagnosis of an anxiety disorder, modify the relationship between neuroendocrine responses to stress on food intake in a new environment (Machado et al., Citation2013). Interestingly, no associations between cortisol levels and food intake were found for those with high maternal care, independent of the anxiety status. In the fMRI study, we saw that high maternal care is associated with a negative correlation in the precuneus facing the palatable foods, independent of the anxiety levels, as well as a negative correlation in the superior and middle frontal gyrus with crescent anxiety scores. These findings were not seen in the low maternal care group.
Our results are in accordance with Turner et al. (Citation2015) suggesting that positive parent–child relationships may protect overweight adolescents from developing clinical eating disorders and from psychological distress later in life. Among overweight youth, higher levels of reported family connectedness were associated with lower levels of unhealthy behaviors and psychosocial distress (Mellin et al., Citation2009). The results showed that in children with high maternal care, food intake was unrelated to changes in the HPA axis. Therefore, our findings might shed light on some underlying mechanisms involving the relationship between HPA functioning and food intake. Our results correspond with D’Argenio et al. (Citation2009), who suggested that not only sexual abuse (Alvarez et al., Citation2007; Mamun et al., Citation2007; Noll et al., Citation2007) or poverty (Giskes et al., Citation2008; Kestila et al., Citation2009), but also less severe forms of early life stress are linked to the risk of developing obesity later in life (D’Argenio et al., Citation2009). Our findings also correspond with other studies showing that emotional abuse and neglect, particularly during infancy, are linked to obesity in adult men and women (Gunstad et al., Citation2006; Pederson & Wilson, Citation2009). Finally, Pompili et al. (Citation2014) showed that a history of moderate-to-severe childhood maltreatment is associated with distinct depressive constructs such as alcohol and/or drug abuse, as well as with suicidal behaviors.
It has been suggested that the perceived quality of maternal care persistently modifies the structure and function of frontal regions including the superior and middle frontal gyri (Kim et al., Citation2010). The association between the perception of maternal care and function may be associated with the differential brain responses to food images in the maternal care group. The frontal gyrus is related to cognition (Casey et al., Citation2011; Metcalfe & Mischel, Citation1999) and is involved in decision-making and impulse control (Aron et al., Citation2004; Casey et al., Citation2002). One would expect that as anxiety increases, the activation of these areas would decrease (Hartley & Phelps, Citation2012), such as in the results for the high maternal care group. The absence of frontal-lobe activation differences in relation to anxiety for the low maternal care group also calls for further investigation.
The precuneus is involved in consciousness and mental self-representation, having its activity decreased when there is engagement in objective tasks, not self-oriented tasks (Cavanna & Trimble, Citation2006). Interestingly, thinking about the costs and benefits of eating palatable foods was associated with decreased activity in this area (Yokum & Stice, Citation2013). It is possible that the negative correlation in this area seen in the high maternal care group is related to the better engagement of these individuals in the task during brain imaging.
This study is limited by the sample size and by using a retrospective assessment of the parental bonding, although this instrument has been widely used in the related literature. In addition, this is a cross-sectional evaluation of a longitudinal study, therefore only associations can be described and no causality can be inferred. Finally, the food task may have been affected by the decision to allow participants to purchase whatever they wanted from the cafeteria. However, forcing the individuals to eat a standard meal that they might not enjoy could prompt stress/anxiety and therefore affect our measurements. Moreover, this approach seemed to be more naturalistic and closer to what might be eaten on a regular basis.
Conclusions
Neurobehavioral responses to palatable food in adolescents vary according to the perceived maternal care during infancy, to the current presence of anxiety symptoms, and cortisol levels. Awareness about these influences may be important to prevent obesity in vulnerable populations.
Funding information
Financial support was received from: Brazilian National Council for Technological and Scientific Development, Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE/HCPA).
Disclosure statement
The authors declare no conflict of interest.
References
- Adam TC, Epel ES. (2007). Stress, eating and the reward system. Physiol Behav 91:449–58.
- Alvarez J, Pavao J, Baumrind N, Kimerling R. (2007). The relationship between child abuse and adult obesity among California women. Am J Prev Med 33:28–33.
- Amorim P. (2000). Mini International Neuropsychiatric Interview (MINI): validação de entrevista breve para diagnóstico de transtornos mentais. Rev Bras Psiquiatr 22:106–15.
- Aron AR, Robbins TW, Poldrack RA. (2004). Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–7.
- Barbieri MA, Bettiol H, Silva AA, Cardoso VC, Simoes VM, Gutierrez MR, Castro JA, et al. (2006). Health in early adulthood: the contribution of the 1978/79 Ribeirão Preto birth cohort. Braz J Med Biol Res 39:1041–55.
- Cattaneo A, Macchi F, Plazzotta G, Veronica B, Bocchio-Chiavetto L, Riva MA, Pariante CM. (2015). Inflammation and neuronal plasticity: a link between childhood trauma and depression pathogenesis. Front Cell Neurosci 9:40–52.
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Jonides J, et al. (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci USA 108:14998–5003.
- Casey BJ, Tottenham N, Fossella J. (2002). Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Dev Psychobiol 40:237–54.
- Cavanna AE, Trimble MR. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain J Neurol 129:564–83.
- Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Salum GA, Manfro GG, Silveira PP. (2012). Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry 2:e195.
- Dallman MF, Pecoraro NC, la Fleur SE. (2005). Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immunity 19:275–80.
- D’Argenio A, Mazzi C, Pecchioli L, Di Lorenzo G, Siracusano A. (2009). Troisi A. Early trauma and adult obesity: is psychological dysfunction the mediating mechanism? Physiol Behav 98:543–6.
- Deluchi M. (2014). Viés atencional para pistas associadas a alimentos em adultos obesos com e sem o Transtorno da Compulsão Alimentar Periódica: Universidade Federal do Rio Grande do Sul.
- Elsey J, Coates A, Lacadie CM, McCrory EJ, Sinha R, Mayes LC, Potenza MN. (2015). Childhood trauma and neural responses to personalized stress, favorite-food and neutral-relaxing cues in adolescents. Neuropsychopharmacology 40:1580–9.
- Ely DR, Dapper V, Marasca J, Correa JB, Gamaro GD, Xavier MH, Michalowski MB, et al. (1997). Effect of restraint stress on feeding behavior of rats. Physiol Behav 61:395–8.
- Ganella DE, Allen NB, Simmons JG, Schwartz O, Kim JH, Sheeber L, Whittle S. (2015). Early life stress alters pituitary growth during adolescence – a longitudinal study. Psychoneuroendocrinology 53:185–94.
- Giskes K, van Lenthe FJ, Turrell G, Kamphuis CB, Brug J, Mackenbach JP. (2008). Socioeconomic position at different stages of the life course and its influence on body weight and weight gain in adulthood: a longitudinal study with 13-year follow-up. Obesity (Silver Spring) 16:1377–81.
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, et al. (2002). Serotonin 1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416:396–400.
- Gunstad J, Paul RH, Spitznagel MB, Cohen RA, Williams LM, Kohn M, Gordon E. (2006). Exposure to early life trauma is associated with adult obesity. Psychiatry Res 142:31–7.
- Hartley CA, Phelps EA. (2012). (2012). Anxiety and decision-making. Biol Psychiatry 72:113–18.
- Hearon BA, Quatromoni PA, Mascoop JL, Otto MW. (2014). The role of anxiety sensitivity in daily physical activity and eating behavior. Eat Behav 15:255–8.
- Holmbeck GN. (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol 27:87–96.
- Hommer RE, Seo D, Lacadie CM, Chaplin TM, Mayes LC, Sinha R, Potenza MN. (2013). Neural correlates of stress and favorite-food cue exposure in adolescents: a functional magnetic resonance imaging study. Hum Brain Mapp 34:2561–73.
- Isolan L, Salum GA, Osowski AT, Amaro E, Manfro GG. (2011). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED) in Brazilian children and adolescents. J Anxiety Disord 25:741–8.
- Ivy AS, Brunson KL, Sandman C, Baram TZ. (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154:1132–42.
- Kassi E, Kyrou I, Tsigos C, Chrousos G. (2000). Stress, Endocrine Physiology and Pathophysiology [Updated 2012 Jun 1]. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al., editors. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc., available at http://www.ncbi.nlm.nih.gov/books/NBK278995/
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–8.
- Kendler KS. (1996). Parenting: a genetic-epidemiologic perspective. Am J Psychiatry 153:11–20.
- Kestila L, Rahkonen O, Martelin T, Lahti-Koski M, Koskinen S. (2009). Do childhood social circumstances affect overweight and obesity in early adulthood? Scand J Public Health 37:206–19.
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, Swain JE. (2010). Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Dev Sci 13:662–73.
- Lang PJ, Bradley MM, Cuthbert BN. (1997). International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. NIMH Center for the Study of Emotion and Attention, available at http://www2.unifesp.br/dpsicobio/Nova_versao_pagina_psicobio/adap/instructions.pdf.
- Levine S. (1957). Infantile experience and resistance to physiological stress. Science 126:405.
- Machado TD, Dalle Molle R, Laureano DP, Portella AK, Werlang IC, Benetti Cda S, Noschang C, Silveira PP. (2013). Early life stress is associated with anxiety, increased stress responsivity and preference for “comfort foods” in adult female rats. Stress 16:549–56.
- Macmillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Duku EK, et al. (2001). Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry 158:1878–83.
- Mamun AA, Lawlor DA, O’Callaghan MJ, Bor W, Williams GM, Najman JM. (2007). Does childhood sexual abuse predict young adult’s BMI? A birth cohort study. Obesity (Silver Spring) 15:2103–10.
- Maniam J, Morris MJ. (2010). Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology 35:717–28.
- McClelland S, Korosi A, Cope J, Ivy A, Baram TZ. (2010). Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol Learn Mem 96:79–88.
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–8.
- Mellin AE, Neumark-Sztainer D, Story M, Ireland M, Resnick MD. (2009). Unhealthy behaviors and psychosocial difficulties among overweight adolescents: the potential impact of familial factors. J Adolesc Health 31:145–53.
- Metcalfe J, Mischel W. (1999). A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev 106:3–19.
- Miller-Matero LR, Armstrong R, McCulloch K, Hyde-Nolan M, Eshelman A, Genaw J. (2014). To eat or not to eat; is that really the question? An evaluation of problematic eating behaviors and mental health among bariatric surgery candidates. Eat Weight Disord 19:377–82.
- Noll JG, Zeller MH, Trickett PK, Putnam FW. (2007). Obesity risk for female victims of childhood sexual abuse: a prospective study. Pediatrics 120:e61–7.
- Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, et al. (2011). Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest 121:4161–9.
- Parker G. (1979). Reported parental characteristics in relation to trait depression and anxiety levels in a non-clinical group. Aust N Z J Psychiatry 13:260–4.
- Parker G, Roy K, Wilhelm K, Mitchell P, Austin MP, Hadzi-Pavlovic D. (1999). An exploration of links between early parenting experiences and personality disorder type and disordered personality functioning. J Pers Disord 13:361–74.
- Pederson CL, Wilson JF. (2009). Childhood emotional neglect related to posttraumatic stress disorder symptoms and body mass index in adult women. Psychol Rep 105:111–26.
- Pinheiro ABV, Lacerda EMA, Benzecry EH, Gomes MCS, Costa VM. (2005). Tabela para avaliação de consumo alimentar em medidas caseiras. Rio de Janeiro: Atheneu.
- Pompili M, Innamorati M, Lamis DA, Erbuto D, Venturini P, Ricci F, et al. (2014). The associations among childhood maltreatment, “male depression” and suicide risk in psychiatric patients. Psychiatry Res 220:571–8.
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31.
- Raikkonen K, Matthews KA, Kuller LH. (2007). Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care 30:872–7.
- Salum GA, Isolan LR, Bosa VL, Tocchetto AG, Teche SP, Schuch I, Costa JR, et al. (2011). The multidimensional evaluation and treatment of anxiety in children and adolescents: rationale, design, methods and preliminary findings. Rev Bras Psiquiatr 33:181–95.
- Silveira PP, da Silva Benetti C, Ayres C, Pederiva FQ, Portella AK, Lucion AB, Dalmaz C. (2006). Satiety assessment in neonatally handled rats. Behav Brain Res 173:205–10.
- Silveira PP, Portella AK, Benetti Cda S, Zugno AI, Scherer EB, Mattos CB, Wyse AT, et al. (2011). Association between Na(+), K(+)-ATPase activity and the vulnerability/resilience to mood disorders induced by early life experience. Neurochem Res 36:2075–82.
- Silveira PP, Portella AK, Crema L, Correa M, Nieto FB, Diehl L, Lucion AB, Dalmaz C. (2008). Both infantile stimulation and exposure to sweet food lead to an increased sweet food ingestion in adult life. Physiol Behav 93:877–82.
- Silveira PP, Portella AK, Assis SACN, Nieto FB, Diehl LA, Crema LM, Peres W, et al. (2010). Early life experience alters behavioral responses to sweet food and accumbal dopamine metabolism. Int J Develop Neurosci 28:111–18.
- Skilton MR, Moulin P, Terra JL, Bonnet F. (2008). Associations between anxiety, depression, and the metabolic syndrome. Biol Psychiatry 62:1251–7.
- Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, Barch DM. (2014). Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J Am Acad Child Adolesc Psychiatry 53:800–13.e10.
- Tomiyama AJ, Dallman MF, Epel ES. (2011). Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 36:1513–9.
- Tryon MS, DeCant R, Laugero KD. (2013). Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol Behav 114–115:32–7.
- Turner HM, Rose KS, Cooper MJ. (2015). Schema and parental bonding in overweight and nonoverweight female adolescents. Int J Obes 29:381–7.
- USDA. (2013). USDA National Nutrient Database for Standard Reference, Release 26. Nutrient Data Laboratory Home Page, available at http://www.ars.usda.gov/ba/bhnrc/ndl.
- Yokum S, Stice E. (2013). Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes 37:1565–70.
- Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y, Zhou QG, et al. (2010). Neuronal nitric oxide synthase alteration accounts for the role of 5-ht1a receptor in modulating anxiety-related behaviors. J Neurosci 30:2433–41.
