Abstract
Many modalities of cognition are affected in schizophrenia. The most common findings include dysfunctions of episodic and working memory and of executive functions. Although an inverse correlation between cortisol level and memory function has been proven, few studies have focused on the relationship between cortisol level and cognitive impairment in patients with schizophrenia. In an open, naturalistic, prospective study, consecutively hospitalized males diagnosed with first-episode schizophrenia, hypothalamic–pituitary–adrenal axis activity (afternoon cortisol levels, post-dexamethasone cortisol levels) was evaluated before and at the end of acute treatment. Psychopathology was assessed using the positive and negative syndrome scale (PANSS). Cognitive functions (memory, attention, psychomotor, verbal fluency, and executive functions) were tested after symptom alleviation using a neurocognitive test battery. In the total sample (n = 23), significant decreases in total PANSS score (including all subscales), afternoon cortisol levels, and post-dexamethasone cortisol levels occurred during the course of treatment. It was found that higher afternoon cortisol levels at the beginning of treatment were significantly related to impaired performance in memory functions. Afternoon cortisol levels were not significantly associated with other measured cognitive functions. No correlation was discovered between cognitive functions and post-dexamethasone cortisol levels. The determination of afternoon cortisol levels may serve to detect potential candidates for specific cognitive intervention immediately after the first psychotic breakthrough.
Introduction
Cognitive impairment is one of the core symptoms of schizophrenia, already clearly manifested in the first episode (Addington & Addington, Citation2002; Bilder et al., Citation2000; Townsend et al., Citation2000). The specific cognitive domains in which the deficit is markedly distinct are episodic and working memory and executive functions (Nuechterlein et al., Citation2004; Reichenberg & Harvey, Citation2007; Tuma & Lenderova, Citation2001).
It has been shown that the emergence of psychosis is preceded by a higher incidence of excessively stressful events, increased stress exposition in overall history (Read et al., Citation2005), increased distress, and inability to handle life events (Horan et al., Citation2005). In the last decade, considerable effort was directed towards understanding the biological mechanism of increased sensitivity to stress in psychosis. Based on the results of these efforts, some authors suggested that more attention should be focused on the hypothalamic-pituitary-adrenal axis (HPA axis) (Corcoran et al., Citation2003; Phillips et al., Citation2006; Walker et al., Citation2008).
Schizophrenia patients exhibit HPA axis function alterations (Bradley & Dinan, Citation2010). These dysfunctions are not simply a correlate of depression or other generalized pathological symptoms (for which HPA axis dysfunction is typical). They have a specific profile, probably based on a different genetic background or a different developmental trajectory of stress abnormalities (Borges et al., Citation2013). Studies specifically focused on the relation between cortisol levels and schizophrenia describe a patient tendency to have higher basal cortisol levels than healthy controls. Higher cortisol levels are also associated with an increase in the severity of psychotic symptoms (Corcoran et al., Citation2012; Garner et al., Citation2011; Mittal & Walker, Citation2011; Walder et al., Citation2000). It has been established that antipsychotic treatment reduces ACTH and cortisol secretion and decreases the number of non-suppressors in the dexamethasone suppression test (DST) (Ceskova et al., Citation2006).
An inverse correlation between glucocorticoids and cognition was shown in a number of animal studies (Wolf, Citation2003) and studies of healthy populations (Lee et al., Citation2007; Lupien et al., Citation2005, Citation2007; McAllister-Williams & Rugg, Citation2002), as well as in studies of schizophrenia patients (Walder et al., Citation2000). Most previous studies were conducted on chronic patients. To the best of our knowledge, only one study has been published investigating the relationship between the HPA axis and cognition in patients with first-episode psychosis (Aas et al., Citation2011). The authors found that a more blunted cortisol-awakening response was associated with a more severe deficit in verbal memory.
Investigation of this phenomenon in patients with first-episode schizophrenia (FES) provides the opportunity to examine this issue disengaged from possible confounding factors caused by chronic illness or long-term psychopharmacological treatment. It can also provide data that will help to facilitate the design and development of more effective preventive and treatment strategies.
The aim of this study was to investigate a possible link between cortisol levels, psychopathology, and particular domains of cognitive function in a population of patients with FES.
Methods
Study design
The research was designed as an open, naturalistic, prospective pilot study. The study was approved by the local ethics committee (the Faculty Hospital Brno) and complies with the requirements of the Declaration of Helsinki.
Subjects
Male patients consecutively hospitalized between January 2006 and May 2010 at the Psychiatric Hospital in Brno were included if they (1) were experiencing their first admission for FES; (2) provided written informed consent; and (3) were drug-naive or had a maximum of 4 weeks of cumulative exposure to antipsychotic treatment before admission. All patients were diagnosed with FES independently by at least two psychiatrists according to ICD-10 (Research criteria). ICD-10 diagnoses were made on the basis of information from the patient and their relatives (including long-term social withdrawal and positive family history) and a comprehensive assessment of symptoms and history. Estimation of illness duration was based on the clear manifestation of the first psychotic symptoms. The history of previous psychopharmacological treatment was carefully documented in interviews with each patient and the patient’s relatives. Subjects identified as having any neuroendocrine disorders (e.g. Cushing’s syndrome), a history of traumatic brain injury, or neurological disorders, or who abused addictive substances or took antidepressants or steroids which are known to affect HPA axis function, were excluded from the study.
Clinical assessment
The psychopathology (positive and negative schizophrenia symptoms, general psychopathology) of the patients was evaluated using the positive and negative syndrome scale (PANSS) at the same time points (before and at the end of acute treatment). Administering the scale at two time points enabled the evaluation of treatment responses (using delta expressing the percentage change in each subscale during the treatment, i.e. delta PANSS = 100 × [(score at the end of acute treatment − score at the beginning of treatment)/score at the beginning of treatment]. Patients whose values had improved by at least 30% in total PANSS score were classified as responders. This criterion was used in order to make it possible to compare the results with older studies. Patients were assessed by a trained psychiatrist (RP).
Endocrine assessment
Endocrine data were obtained in parallel with clinical assessment, i.e. before and at the end of acute treatment. Procedure: At 4 p.m., blood samples were collected to determine afternoon cortisol levels (basal cortisol levels). On the same day at 11 p.m., patients orally took 1 mg of dexamethasone. The next day at 4 p.m., blood samples were collected again to determine post-dexamethasone cortisol levels (PDCL). Cortisol levels were measured using a chemiluminescent immunoassay. Cortisol values greater than 138 nmol/l (5 mg/dl) in any of post-dexamethasone collections were labeled as DST non-suppression (e.g. Carroll et al., Citation1981).
Measuring cortisol levels in parallel with clinical assessment enabled the evaluation of cortisol level dynamics during the acute treatment (using cortisol levels delta, i.e. delta cortisol levels = 100 × [(cortisol level at the end of acute treatment − cortisol level at the beginning of treatment)/cortisol level at the beginning of treatment] and its association with treatment response.
Psychological assessment
Neuropsychological assessment was performed using a neurocognitive test battery composed of separate standardized tests chosen with regards to the examined group and difficulty level. Patients were assessed once, by the end of the first week (range 3–7 d) after admission, after the alleviation of psychotic symptomatology when patients were able to undergo the assessment. The battery was administered in the same room, at the same time of day, in convenient physical conditions, and always with the same instructions.
The administration of the neuropsychological test battery and testing of cognitive functions took two hours on average. The selected tests were oriented towards psychomotor (Trail Making Test A – TMT-A), attention distribution (Trail Making Test B – TMT-B), working memory (Auditory Verbal Learning Test, subtests 1–5 – AVLT 1–5), delayed memory and memory recall (Auditory Verbal Learning Test, subtest 6 – AVLT 6), verbal fluency (Verbal Fluency Test – VFT), and executive functions (Wisconsin Card Sorting Test – WCST). To compare patient results with healthy population results in tests of cognitive functions, we used the standard norms for the healthy population postulated in the manuals of these tests. Rough test scores were converted to z-scores based on the mean and standard deviation of the results of a healthy population. The degree of cognitive impairment was determined quantitatively by comparison with the statistical norm for corresponding age. Results ranging from −0.01 to −0.5 standard deviation (SD) were labeled as a mild cognitive impairment. Moderate cognitive impairment ranged from −0.51 to −1.5 SD. Performances worse than −1.5 SD were marked as a severe cognitive impairment (as defined in e.g. Harvey & Keefe, Citation2009).
Statistical analyses
Since the sample was smaller, non-parametric methods were selected for the detection and description of variables of interest. Correlations among cortisol levels, parameters of cognition, and psychopathology were determined using Spearman’s correlation coefficient. To verify the results of correlation between afternoon cortisol levels at the beginning of treatment and memory functions, Mann–Whitney U tests were used. Comparisons of repeated measurements (PANSS scores and cortisol levels before and at the end of acute treatment) were performed using the Wilcoxon matched-pair signed-rank test. A Bonferroni correction was calculated to adjust for multiple comparisons.
All statistical analyses were performed using Statistica software version 12 (Statistica software version 12, IBM, StatSoft CR; http://www.statsoft.cz/). Results were interpreted conventionally, on the basis of their statistical significance. Results were generally considered significant if the risk of incorrect rejection of the null hypothesis was not higher than 5%; in other words, relationships were significant at the 5% level (p ≤ 0.05).
Results
Sample characteristics
The sample consisted of 23 men, average age 23.52 years, education duration 12 years, and illness duration approximately 3 months. The average duration of index hospitalization was 43 d. The average length of illness was 3.1 months. All patients received antipsychotic medication; the average chlorpromazine equivalent was 224.5 mg (). In order to make the sample as homogenous as possible, risperidone was the first choice of medication.
Table 1. Sociodemographic and clinical characteristics of the sample (n = 23).
Clinical data
During the treatment process, we observed a significant reduction in the average total PANSS score (Z = −4.2, p < 0.01) (more details in ). Of the 23 patients, three were classified as non-responders according to the delta PANSS; 20 (87% of the reference sample) were responders.
Table 2. PANSS before and at the end of acute treatment.
Endocrine data
Average cortisol levels (afternoon and post-dexamethasone) before and at the end of treatment are shown in (normal level ranges from 83 to 441 nmol/l). There was a significant decrease in the afternoon cortisol level (Z = 3.16, p <0.01) and in the post-dexamethasone cortisol level (Z = 2.03, p = 0.04) during the treatment.
Table 3. Cortisol levels before and at the end of acute treatment.
Cognitive data
On an average, the most significant impairment occurred in the AVLT 6 subtest measuring delayed memory and memory recall (m = −0.92; SD = 1.26). Moderate cognitive impairment of another memory component (working memory) was revealed in the AVLT 1–5 subtests (m = −0.68; SD = 0.94). Memory impairment is also indirectly related to another cognitive function, verbal fluency, which is measured by the VFT and which also showed moderate cognitive impairment (m = 0.7; SD = 1.35). Executive functions were measured by WCST, from which we focused on three indexes: perseverative responses (m = −0.13; SD = 1.47), perseverative errors (m = −0.15; SD = 1.39), and conceptual level of responses (m = −0.17; SD = 1.03); the cognitive impairment indicated by these items can be characterized as mild. TMT-A detected moderate levels of average psychomotor deficit (m = 0.4; SD = 0.93). In the TMT-B subtest, focusing on attention, patients achieved the same results as the healthy population (m = 0.01; SD = 0.51); their performance in this cognitive domain was thus normal ( and ).
Figure 1. Results from cognitive testing *Norms for corresponding age category; TMT-A: Trail Making Test: subtest A; TMT-B: Trail Making Test: subtest B; AVLT 1–5: Auditory Verbal Learning Test: subtests 1–5; AVLT 6: Auditory Verbal Learning Test: subtest 6; VFT: Verbal Fluency Test; WCST (PR): Wisconsin Card Sorting Test: perseverative responses; WCST (PE): WCST (PR): Wisconsin Card Sorting Test: perseverative errors; WCST (CLR)–WCST (PR): Wisconsin Card Sorting Test: conceptual level of responses. Average score of healthy population is in paragraph 0.
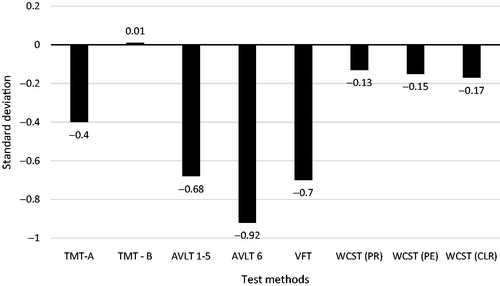
Table 4. Results from cognitive testing (z-score).
Correlation between cortisol levels and cognitive functions
Afternoon cortisol levels at the beginning of treatment and cognition
A significant negative correlation was observed only in the AVLT 1–5 subtests measuring working memory (r = −0.42; p = 0.047) (see for a scatter plot of this correlation). Trend-level correlation was present for results in the AVLT 6 subtest measuring delayed memory and memory recall (r = −0.4; p = 0.058).
Figure 2. Scatter plot and linear regression of afternoon cortisol level at the beginning of treatment and z-scores of AVLT 1–5 subtests.
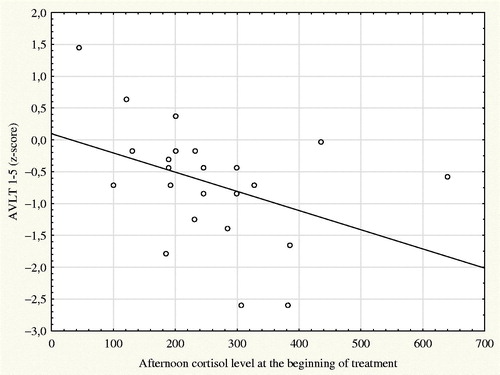
To verify the results of this correlation, we analyzed data after splitting the patients into two groups depending on whether their afternoon cortisol levels were above or below the median of the measured values (Me = 233 nmol/L). The group of patients above the median had worse results in AVLT 1–5 subtests (Z = −1.75; p = 0.078) and significantly worse results in the AVLT 6 subtest (Z = −2.08; p = 0.037).
Other test results did not significantly correlate with afternoon cortisol levels at the beginning of treatment. In post hoc analysis, no significant correlations were found between data from cognitive testing and age, education, response to acute treatment, illness duration, severity of illness, or dose of medication, with the exception of WCST PR and WCST PE, which correlated with dose of medication. Analyses correlating individual cognitive tests are presented with and without Bonferroni correction in .
Table 5. Correlation between afternoon cortisol levels at the beginning of treatment and cognitive functions.
Post-dexamethasone cortisol levels at the beginning of treatment and cognition
No statistically significant correlation between post-dexamethasone cortisol levels and cognitive test results was found.
Correlation between cortisol levels and treatment response
No statistically significant correlations between afternoon and post-dexamethasone cortisol levels at the beginning of treatment or their changes during the treatment (delta cortisol levels) and any of the delta PANSS scores were observed.
Dexamethasone suppression test
Five patients (21.7%) were classified as non-suppressors at the beginning of treatment; at the end of acute treatment, all patients were classified as suppressors. At the beginning of treatment, the suppressor group did not show any statistically significant difference from the non-suppressor group in terms of age, illness duration, PANSS score, or any of the monitored cognition parameters. Further, no statistically significant differences between these two groups were found in afternoon cortisol levels; only post-dexamethasone cortisol levels were significantly higher in the group of non-suppressors.
Discussion
The present study demonstrates that total PANSS score (including all subscales), afternoon cortisol concentrations, and post-dexamethasone cortisol levels decrease during the course of treatment in patients with first-episode schizophrenia. Higher afternoon cortisol levels at the beginning of treatment were associated with impaired performance in memory functions.
All the patients included in the study were men. The main reason for this was at first logistic; later, we saw some advantages in this more homogenous sample. A recent study by Montalvo et al. (Citation2013), which was not published in full text, found a gender difference in the relationship between the HPA axis and working memory in subjects with early psychosis.
Most patients were antipsychotic drug-naive on admission. During index hospitalization, all patients were treated openly with monotherapy using atypical antipsychotics and individualized dosing. We started to use risperidone in FES in 1992, when risperidone was the second atypical antipsychotic available in the Czech Republic (after clozapine). We used the same treatment algorithm to minimize confounders related to the treatment. The good risk/benefit ratio of risperidone was recently confirmed (Zhang et al., Citation2013); other antipsychotics have been used based on clinical judgment. In severe, exceptional cases, the ICD 10 duration criterion was not fulfilled. There was a significant decrease of psychopathology in the average scores at the end of the treatment. Unfortunately, samples of group comparisons (responders versus non-responders and suppressors versus non-suppressors) were too small to make any reliable conclusion.
During the treatment, a significant decrease in elevated afternoon (basal) cortisol levels towards the normal range occurred in all patients. The observed data corresponds with the literature (Bradley & Dinan, Citation2010). Acute treatment in our sample resulted in a significant decrease of post-dexamethasone cortisol levels. These data are in agreement with our previous results (Ceskova et al., Citation2006).
Although the average scores of the patients in our study were, except for the attention test performance, worse than the average performance of the healthy population stated in the norms of the tests, these deficits were less pronounced than in other studies focused on exploring the depth of cognitive impairment in FES patients (Addington & Addington, Citation2002; Bilder et al., Citation2000; Townsend et al., Citation2000). A possible explanation could be the relatively short illness duration (mean 3.1 months).
Analyses suggest that increased afternoon cortisol levels at the beginning of treatment are associated with impaired performance in tasks requiring the involvement of working memory, short-term verbal memory (AVLT 1–5), and possibly also with delayed memory and memory recall (AVLT 6). The correlation of 0.42 explains only 17.6% of the variation and future studies are needed to confirm whether this correlation may be considered as potentially clinically relevant.
If a Bonferroni correction was applied to account for the eight different comparisons shown in , the critical p-value would reduce from 0.05 to 0.00625. In this case, the correlation between AVLT 1–5 and afternoon cortisol levels at the beginning of treatment (p = 0.047) would no longer reach statistical significance.
Our results are consistent with other studies focused on exploring the relationship between cortisol levels and memory functions in animals (Wolf, Citation2003), healthy individuals (Lee et al., Citation2007; McAllister-Williams & Rugg, Citation2002), and schizophrenia patients (Newcomer et al., Citation1998; Ritsner & Strous, Citation2010; Walder et al., Citation2000), but in contradiction with the study aimed at studying relationship between HPA axis activity and cognition in patients with first-episode psychosis (Aas et al., Citation2011). The authors found in patients (not in a control group) a significant correlation only between cortisol awakening response and a more severe deficit in verbal memory; they found no correlation between daytime cortisol levels and cognitive functioning. The partially different results could be due to the timing of cortisol assessment (awakening, 12:00 noon, and 8:00 p.m.), methods (salivary cortisol assessment), or the patients included in the study (patients with first-episode psychosis, mean age 30 years, range 18–65). Our sample was more homogenous, only males with FES, and the study was a naturalistic one, done under the conditions of routine clinical practice. However, our findings indicate a significant connection between memory functions and cortisol levels, which is interesting, particularly in connection with studies documenting that high cortisol levels are associated with a smaller hippocampal volume, even in first-episode psychosis (Borges et al., Citation2013).
No significant correlations were found between afternoon and post-dexamethasone cortisol levels, including changes in their levels and delta PANSS scores. The second-generation antipsychotics have been shown to significantly reduce cortisol levels even in healthy controls, suggesting that this effect may be independent from the effect of the antipsychotic medication on the psychotic symptoms (Cohrs et al., Citation2006).
Limitations of our research undoubtedly included the small number of patients enrolled, the gender homogeneity, the open design of the study, and the lack of control groups. Another limitation was connected with the selection of the tests for the neurocognitive test battery. We realize that the chosen tests do not measure only one cognitive domain and, therefore, that some measured domains are reflected in the results of more tests. The fact that we obtained the cognitive data only by single measurements is also quite a significant disadvantage. We had the advantage of a relatively homogeneous sample in terms of age and sex and in terms of no or only brief antipsychotic therapy prior to study entry.
Conclusions
Although this was a preliminary study with some methodological limitations, the results suggest that higher afternoon cortisol levels are connected with impaired performance in memory functions, specifically working memory, delayed memory, and memory recall. A very simple laboratory test used in routine clinical practice, such as the assessment of afternoon cortisol levels, probably has a greater predictive value than DST in relation to memory and it might identify suitable candidates for possible early and specific pro-cognitive intervention. Furthermore, antipsychotics may reduce elevated plasma cortisol levels independent of the treatment response.
Funding information
This research was supported by the project CEITEC – Central European Institute of Technology (CZ.1.05/1.1.00/02.0068) from the European Regional Development Fund.
Disclosure statement
The senior author (E. C.) received a speakerˈs honoraria from Janssen-Cilag, Angelini and Lundbeck CR. All others authors report no conflicts of interest.
References
- Aas M, Mondelli V, Toulopoulou T, Reichenberg A, Di Forti M, Fisher H. (2011). Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol Med 41:463–76.
- Addington J, Addington D. (2002). Cognitive functioning in first-episode schizophrenia. J Psychiatry Neurosci 27:188–92.
- Bilder R, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, et al. (2000). Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 157:549–59.
- Borges S, Gayer-Anderson C, Mondelli V. (2013). A systematic review of the activity of hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology 38:603–11.
- Bradley AJ, Dinan TG. (2010). A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol (Oxford) 24:91–118.
- Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, et al. (1981). A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry 38:15–22.
- Ceskova E, Kasparek T, Zourkova A, Prikryl R. (2006). Dexamethasone suppression test in first episode schizophrenia. Neuro Endocrinol Lett 27:433–7.
- Cohrs S, Roher C, Jordan W, Meier A, Huether G, Wuttke W, Rüther E, Rodenbeck A. (2006). The atypical antipsychotics, olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology 185:11–18.
- Corcoran CM, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. (2003). The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 29:671–92.
- Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. (2012). HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res 135:170–4.
- Garner B, Phassouliotis C, Phillips LJ, Markulev C, Butselaar F, Bendall S, Yun Y, McGorry PD. (2011). Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. J Psych Res 45:249–55.
- Harvey PD, Keefe RSE. (2009). Clinical neuropsychology of schizophrenia. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. Oxford: Oxford University Press. p 507–22.
- Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, Mintz J. (2005). Stressful life events in recent-onset schizophrenia: reduced frequencies and altered subjective appraisals. Schizophr Res 75:33–374.
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. (2007). Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry 64:810–18.
- Lupien SJ, Fiocco A, Wan Maheu F, Lord C, Schramek T, Tu MT. (2005). Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology 30:225–42.
- Lupien SJ, Maheu F, Fiocco A, Schramek TE. (2007). The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn 5:209–37.
- McAllister-Williams RH, Rugg MD. (2002). Effects of repeated cortisol administration on brain potential correlates of episodic memory retrieval. Psychopharmacology 160:74–83.
- Mittal VA, Walker EF. (2011). Minor physical anomalies and vulnerability in prodromal youth. Schizophr Res 129:116–21.
- Montalvo I, Creus M, Solé M, Feliu T, Franch J, Vilella E, Gutiérrez-Zotes A, Labad J. (2013). Sex differences in the relationship between cortisol awakening response and working memory in subjects with early psychosis [Abstract]. Eur Neuropsychopharmacol 23:439–40.
- Newcomer JW, Hershey T, Bardgett ME, Csernansky JG, Craft S, Gagliardi AE, Vogler G. (1998). Glucocorticoid interactions with memory function in schizophrenia. Psychoneuroendocrinology 23:65–72.
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. (2004). Identification of separable cognitive factors in schizophrenia. Schizophr Res 72:29–39.
- Phillips LJ, McGorry PD, Garner B, Thompson KN, Pantelis C, Wood SJ, Berger G. (2006). Stress, the hippocampus and the hypothalamic–pituitary–adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry 40:725–41.
- Read J, van Os J, Morrison AP, Ross CA. (2005). Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand 112:330–50.
- Reichenberg A, Harvey PD. (2007). Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull 133:833–58.
- Ritsner MS, Strous RD. (2010). Neurocognitive deficits in schizophrenia are associated with alterations in blood levels of neurosteroids: a multiple regression analysis of findings from a double-blind, randomized, placebo-controlled, crossover trial with DHEA. J Psychiatr Res 44:75–80.
- Townsend LA, Malla KA, Norman RM. (2000). Cognitive functioning in stabilized first-episode psychosis patients. Psychiatry Res 104:119–31.
- Tuma I, Lenderova Z. (2001). Schizofrenie a kognitivní funkce. Psychiatrie 4:275–82.
- Walder DJ, Walker EF, Lewine RJ. (2000). Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol Psychiatry 48:1121–32.
- Walker E, Mittal V, Tessner K. (2008). Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 4:189–216.
- Wolf OT. (2003). HPA axis and memory. Best Pract Res Clin Endocrinol Metab 2:287–99.
- Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Corell CU. (2013). Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropssychopharmacol 16:1205–18.
