Abstract
Stress and related disorders are in the focus of interest and glutamate is one of the most important neurotransmitters that can affect these processes. Glutamatergic neurons are characterized by vesicular glutamate transporters (VGluT1-3) among which vGluT3 is unique contributing to the non-canonical, neuromodulatory effect of glutamate. We aimed to study the role of vGluT3 in stress axis regulation and related anxiety during the early postnatal period using knockout (KO) mice with special focus on sex differences. Anxiety was explored on postnatal day (PND) 7-8 by maternal separation-induced ultrasonic vocalization (USV). Stress-hormone levels were detected 60 min after intraperitoneal lipopolysaccharide (LPS) injection 7 days later. Both genotypes gained weight, but on PND 14-15 KO mice pups had smaller body weight compared to wild type (WT). vGluT3 KO mice reacted to an immune stressor with enhanced adrenocorticotropin (ACTH) and corticosterone secretion compared to WT. Although there was a tendency for enhanced anxiety measured by more emitted USV, this did not reach the level of significance. The only sex-related effect was the enhanced corticosterone reactivity in male pups. For the HPA axis regulation in neonates vGluT3 expression seems to be dispensable under basal conditions, but is required for optimal response to immune stressors, most probably through an interaction with other neurotransmitters. Disturbance of the fine balance between these systems may result in a borderline enhanced anxiety-like behavior in vGluT3 KO pups.
Introduction
Glutamate is the most abundant excitatory neurotransmitter in the central nervous system and plays a principal role in neural activation. The dysfunction of glutamatergic neurotransmission can lead – among other outcomes – to the development of inadequate stress-adaptation and stress-related mental disorders, such as anxiety and depression.
The connection between glutamate and the regulation of the hypothalamo-pituitary-adrenocortical (HPA) axis, one of the main components of stress responses, is well established (Evanson & Herman, Citation2015). Pharmacological studies have shown that peripheral administration of glutamate receptor agonists is followed by a rapid rise in adrenocorticotropin (ACTH, the hypophyseal component of the HPA axis) levels (Zelena et al., Citation1999). On the other hand, acute stress exposure or administration of glucocorticoids (in rodents corticosterone, the peripheral component of the HPA axis) induces activity-dependent glutamate release in several brain areas e.g. hippocampus, amygdala and prefrontal cortex (Popoli et al., Citation2012).
However, little information is available about the contribution of glutamate to stress-reactivity during the early postnatal period, although it is well known that the HPA axis of the pups works differently from adults (Zelena et al., Citation2015). A lot of evidence suggests that young animals are vulnerable to environmental influences, such as stressors (e.g. challenges to the immune system) and these early life experiences have a great impact on pathological changes in adulthood. Despite the enhanced prevalence of anxiety disorders in women compared to men, studies of female subjects are limited (Donner & Lowry, Citation2013). Numerous human as well as animal studies have indicated that the stress reactivity is different between sexes, and rodents showed sex-dependent behavioral and hormonal responses after glutamate antagonist treatment (Jain & Zelena, Citation2011).
Glutamatergic neurons are characterized by three types of glutamate transporters (vGluT1-3). These transporters are responsible for the vesicular filling of glutamate allowing its release to the synaptic cleft after neural activation. These vGluT isoforms have been genetically targeted to develop different mouse strains to investigate the functional role of different types of vGluTs (Wallen-Mackenzie et al., Citation2010). vGluT3 was characterized in 2002. It is highly homologous with vGluT1 and 2, but their expression patterns in the brain are clearly different (Wallen-Mackenzie et al., Citation2010). vGluT3 is restricted to a smaller number of neurons and is coexpressed in most cases with other neurotransmitters, such as serotonin (5-hydroxytryptamine, 5HT) in the raphe nuclei, acetylcholine in the interneurons of the striatum and basal forebrain and gamma-aminobutyric acid (GABA) in the interneurons of hippocampus and cortex. Thus, in these neurons glutamate can act in a non-canonical way as a modulator or cotransmitter. In contrast, vGluT1 and 2 can be found almost in the whole brain in cortical and deep structures. Although the complete lack of vGluT1 or 2 is lethal (Callaerts-Vegh et al., Citation2013), and downregulation of these transporters in heterozygous mice was reported to induce behavioral abnormalities associated with psychiatric disorders. Moreover, other studies clearly showed changes in vGluT1/2 expression after acute, chronic, or perinatal stressors. Heterozygous vGluT1/2 mice would be interesting for studies of stress regulation during the perinatal period, but their widespread occurrence suggests that they would influence the whole brain with presumably opposing consequences on different parts. Therefore we concentrated on vGluT3, which distribution is more restricted, to get more specific effects. Previous studies using vGluT3 KO mice suggested a highly anxious phenotype (Amilhon et al., Citation2010), suggesting a possible contribution of vGluT3 in stress regulation, which was never tested before.
Here we investigated the role of vGluT3 in acute regulation of the HPA axis in connection with the appearance of innate anxiety during the early postnatal period using KO mice with special focus on sex differences. To assess anxiety short (10 min) maternal separation-induced ultrasonic vocalization (USV) was recorded in PND7-8 animals (Varga et al., Citation2015). Stress reactivity of the same animals was examined after intraperitoneal (i.p.) lipopolysaccharide (LPS, model of bacterial infection) injection 7 days later.
Methods
Animals
vGluT3 KO and wild type (WT; C57BL/6J background) mice were obtained from heterozygous mating of local breeding pairs at the Institute of Experimental Medicine, Budapest, Hungary. Pups were kept with their mothers under a standard 12 h light–dark cycle (lights on at 6 am), with food and water available ad libitum, and their body weights were recorded during the tests. All the experiments were carried out between 9 h and 14 h.
On PND 7-8 (both sexes, 21 litters, 3–11 pups/litter) pups were examined in USV test (details see later; n = 10–13/group), marked and replaced back with their mothers. A week later (PND 14-15) LPS (Sigma-Aldrich, St. Louis, MO; O55:B5; 100 μg/ml/kg, dissolved in saline) or saline was injected i.p. to the same animals (n = 3–8/group). One hour later blood samples were taken for hormone measurements (ACTH, corticosterone) and tail tissue was collected for verification of VGluT3 genotype by PCR. Thus, the animals were blindly assigned into different treatment groups and the genotype was determined only after the experiment. The data of homozygous animals only are presented. All manipulations of the animals were approved by the local committee for animal health and care and performed according to the European Communities Council Directive recommendations for the care and use of laboratory animals (2010/63/EU).
Ultrasonic vocalization
Pups were brought to a soundproof room and placed in a 600 ml glass beaker without bedding and heating. USV was followed for 10 min as previously described (Varga et al., Citation2015). Briefly, individual calls were detected using an ultrasonic-sensitive frequency division detector (CIEL electronique, Koenigslutter, Germany, CDB205 R2) fixed on a holder 12 cm above the bottom of the glass beaker coupled to a computer. Vocalizations were recorded using a free Audacity 2.0.5. software and stored on a personal computer. Data were automatically counted in the power spectrum 30–50 kHz (typically occuring after maternal separation, (Varga et al., Citation2015)) using a USV Counter software (Budapest, Hungary) (developed by S. Zsebők). Total number and total duration of calls per session were measured. In addition, USV frequency was calculated as the total number/10 min.
Hormone measurements
Blood was collected after decapitation in ice-cold plastic tubes, was centrifuged and the serum was separated and stored at −20 °C until analysis. ACTH and corticosterone were measured by radioimmunoassay in 50 μl or 10 μl unextracted serum, respectively, as described earlier (Varga et al., Citation2015). The intra-assay coefficients of variations were 4.7% and 7.5%, respectively for the two hormones. All the samples from a particular experiment were measured in one radioimmunoassay.
Statistical analysis
Data were expressed as means ± SEM and analyzed using STATISTICA 12.0 software package (StatSoft, Inc., Tulsa, OK) by analysis of variance (ANOVA) using repeated measure (body weight; factors ‘genotype’, ‘sex’ and ‘time’), two (USV; factor ‘genotype’ and ‘sex’) or three way ANOVA (LPS; factors ‘treatment’, ‘genotype’, and ‘sex’). Post-hoc comparison was made by the Newman–Keuls method and the results were presented on the figures. Correlation was calculated by the Pearson method. p values under 0.05 were considered significant, while between 0.05 and 0.1 were considered to indicate trends.
Results
Weight
At the time of USV testing (PND 7-8) there was no difference between the genotypes in the body weight. However, at the time of LPS-stressor (PND 14-15) KO mice were significantly smaller than WTs (genotype: F(1,41) = 3.71, p < 0.05), with a more pronounced effect in females (). Nevertheless, both genotypes gained weight (time: F(1,41) = 690.02, p < 0.01, no interaction with genotype or sex). There was no significant difference between sexes at any of the measured time points.
Table 1. Weight of the vGluT3 knockout mice pups.
Anxiety-like behavior (USV)
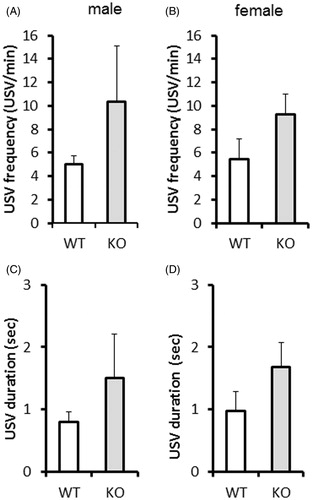
As a sign of enhanced innate anxiety there was a tendency for higher USV frequency (genotype: F(1,28)=3.82, p = 0.06) () and duration (genotype: F(1,28)=3.05, p = 0.09) () in KO pups compared to WT. We could not find any differences between sexes.
Figure 1. Maternal separation-induced ultrasound vocalization (USV) on postnatal day (PND) 7-8 in neonatal vGluT3 knockout (KO) mice (n = 10–13/group). As a sign of enhanced anxiety there was a tendency for higher USV frequency (number of calls per minute; (A,B) and duration (sum during the entire 10 min observation period; C,D) in neonatal KO mice compared to wild type (WT). We could not find any differences between the sexes.
Stressor exposure (LPS)
Sixty minutes after a saline injection ACTH and corticosterone levels did not differ significantly between genotypes and sexes ( controls).
Figure 2. Changes in stress-hormone levels 60 min after intraperitoneal lipopolysaccharide (LPS, 100 μg/ml/kg) injection on postnatal day (PND) 14-15 in neonatal vGluT3 knockout (KO) mice (n = 3–8/group). (A,B) Adrenocorticotropin (ACTH, fmol/ml) levels increased in response to LPS injection in both genotypes with significantly greater elevations in KO animals compared to wild type (WT) without sex differences. (C,D) Corticosterone (pmol/ml) showed smaller elevation to LPS, but the KO mice showed greater elevation also in this case. Moreover, males had higher levels than females also among basal and stress-induced levels. *p < 0.05, **p < 0.01 versus WT; #p < 0.05, ##p < 0.01 versus saline.
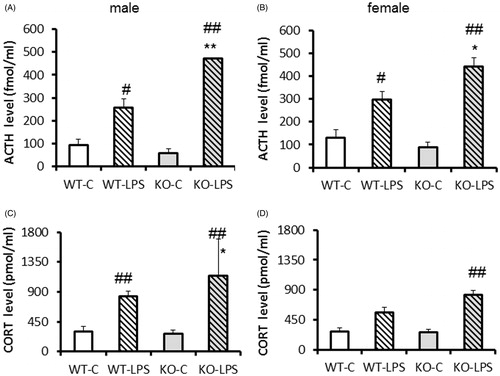
Serum ACTH levels increased in response to the immune stressor in both genotypes (treatment: F(1,32)=93.42, p < 0.01), with significantly greater elevations in KO animals compared to WT (genotype: F(1,32)=6.26, p < 0.05; genotype × treatment: F(1,32)=14.97, p < 0.05) (). ACTH levels did not show sex-dependent changes neither under control conditions nor after stress exposure and the sex did not influence the effect of treatment or genotype (no interactions). In post-hoc comparison the LPS-treated KO animals had higher ACTH levels than the WT in both sexes.
The changes of serum corticosterone during stress were similar to those seen with ACTH (genotype: F(1,34) = 3.99, p = 0.05; treatment: F(1,34)=68.93, p < 0.01; genotype × treatment: F(1,34) = 5.14, p < 0.05) (). Although after control treatment there was no significant difference between males and females, after the LPS injection the effect of sex became significant with higher values in males (sex: F(1,34) = 5.22, p < 0.05; sex × treatment: F(1,34) = 4.48, p < 0.05). In males post-hoc comparison revealed higher corticosterone levels in LPS-treated KO animals than in WTs.
We could not find any significant correlation between the studied USV and hormone parameters neither among control conditions, nor after stressor exposure.
Discussion
Our data demonstrated that during the early postnatal period the vGluT3 KO mice react to an immune stressor with enhanced ACTH and corticosterone secretion compared to WT. Although there was a tendency for enhanced anxiety measured by USV, it did not reach the level of significance. The only sex-related effect was the enhanced corticosterone reactivity of male pups.
Converging lines of evidence from animal and human studies suggested that in adulthood glutamate was a major player in HPA axis regulation (Evanson & Herman, Citation2015). Here we were concentrating on a naturally occurring stressor, the sickness behavior-inducing LPS, which shares many features with major depression (anhedonia, cognitive dysfunction, anxiety, fatigue, etc.) (Al-Amin et al., Citation2016). Further relevance of this model is that early-life infections is considered as a risk factor for the development of neuropsychiatric disturbances in adulthood, e.g. schizophrenia. Based on the stimulatory nature of glutamate, one would expect that decreased glutamate release would lower rather than increase endocrine stress response. However, vGluT1 and 2, but not vGluT3 were found in the hypothalamic part of the HPA axis, namely, in neurones innervating the corticotropin-releasing hormone synthesizing neurones of the paraventricular nucleus of the hypothalamus (PVN) (Herzog et al., Citation2004). Thus, it is more probable that vGluT3 influences its cotransmitters in serotonergic, cholinergic or GABAergic neurones, which have an effect on the HPA axis regulation. Indeed, vGluT3 was specifically implicated in promoting the release of these neurotransmitters, as its transcript is massively expressed in the soma of 5-HT neurons from early embryonic life to adulthood modulating the 5-HT transmission in the raphe (Amilhon et al., Citation2010). Although the presence of vGluTs on other parts of the HPA axis was not confirmed, yet, emerging evidence suggests that glutamate could be an extracellular signal mediator (in an autocrine and/or paracrine way) in peripheral tissues including pituitary and adrenal gland (Hinoi et al., Citation2004).
The main source of 5-HT in the brain is the raphe nuclei and electrical stimulation of the dorsal raphe nucleus (DR) induces ACTH release (Weidenfeld et al., Citation2002), while after its electrical lesion the hormonal stress-response is smaller (Jorgensen, Citation2007). Moreover, the DR seems to be essential in the glucocorticoid negative feedback during acute stressor-exposure (Vincent & Jacobson, Citation2014). Systemic administration of 5-HT1A receptor antagonist diminished restraint-stressor-induced corticosterone elevation only in adult male rats, but not in females, suggesting a sex-dependent regulatory role of 5-HT innervation (Goel et al., Citation2014). Moreover, 5-HT can contribute to sex-related differences in stress-related disorders, like anxiety disorders, and may provide a potential mechanism how neonatal LPS treatment has sex-dependent late consequences on anxiety-like behavior (Donner & Lowry, Citation2013). Although during the neonatal period there was no difference between WT and vGluT3 KO mice in the brainstem 5-HT content, but its metabolism was impaired, suggesting that in VGluT3 KO mice an acute 5-HT release can be more effective (Miot et al., Citation2012).
The involvement of the cholinergic system in regulating stress-reaction is also evident. For example intra-PVN acetylcholine injection stimulated ACTH release and dietary reduction of choline content has been shown to diminish the stress-reactivity without any influence on the resting levels. In the striatum it was confirmed that cholinergic signaling requires vGluT3 (Nelson et al., Citation2014) and on other brain areas acetylcholine and vGluT3 were also highly co-expressed (Nickerson Poulin et al., Citation2006). As acetylcholine inhibits the production of pro-inflammatory cytokines (Ofek & Soreq, Citation2013), an important component of LPS-induced HPA axis regulation, we might assume, that reduced cholinergic tone contributes to enhanced HPA axis reactivity to an immune challenge in vGluT3 KO animals.
The reduced GABAergic innervation observable in vGluT3 KO animals (Noh et al., Citation2010) may also contribute to their enhanced stress-reactivity, because of known inhibitory GABAergic tone on the HPA axis (Herman et al., Citation2004). However, this assumption needs confirmation.
Knocking out a glutamate-related gene might have strong impacts on development. As the maternal separation-induced USV is substantially influenced by the maturity of the pups (Varga et al., Citation2015), first we examined the physiological differences between genotypes. At the time of USV we could not detect any difference in the body weight suggesting similar development in WT and KO animals until this timepoint. Thus, the observed USV differences were due to innate anxiety rather than secondary to developmental changes. We recorded the maternal separation-induced USV for 10 min, although most authors used even shorter periods (Takahashi et al., Citation2009), but our previous results in rat pups confirmed 10 min as optimal timing (Varga et al., Citation2015). However, we could detect only trends for genotype effects, despite previously published data measuring USV for 20 min (Amilhon et al., Citation2010). Nevertheless, in line with previously mentioned enhanced 5-HT effect and reduced GABAergic innervation of VGluT3 KO mice, both lack of 5-HT and administration of GABA receptor agonists diminish USV (Takahashi et al., Citation2009). Moreover, acetylcholine was suggested to be a possible common neurochemical substrate underlying production of USV in mammals (Brudzynski & Bihari, Citation1990). Thus, it seems that an interaction among these players may result in a fine-tuning of anxiety-related USV. In adults clear sex differences in emitted USV were repeatedly reported due to different social behavior of males and females (von Merten et al., Citation2014). In pups we could not find any description of sex-dependent alteration in maternal separation-induced USV and our present data did not reveal any sex-related effects either. Moreover, despite the stress-related nature of anxiety we were unable to find any correlation between USV and hormonal stress-reactivity to an immune challenge. Although we cannot exclude that it was due to the specifics of the LPS treatment, but other reports also suggested that HPA axis regulation and anxiety-like behavior often dissociate (Zelena et al., Citation2015).
All in all, for the HPA axis regulation in neonates vGluT3 expression seems to be dispensable under basal conditions, but is required for optimal response to immune stressors, in agreement with changes in other parameters during hypoxic stress (Miot et al., Citation2012), most probably through an interaction with 5-HT, acetylcholine and/or GABA neurotransmissions. Disturbance of the fine balance among all these systems may result in a borderline enhanced anxiety-like behavior in vGluT3 KO pups. The late consequences of these alterations in adulthood require further studies. The 5-HT system may contribute to the observed sex differences.
Funding information
This study was supported by ERC 2011 Advanced grant 294313, Országos Tudományos Kutatási Alap (OTKA) grant K 101645 to JH and by the National Research, Development and Innovation Office (NKFIH) grant PD-115730 to SZs Hungary.
Disclosure statement
We declare that there are no conflicts of interest in the conduct and reporting of research. The agencies had no further role in study design, in the collection, analysis or interpretation of the data.
References
- Al-Amin MM, Sultana R, Sultana S, Rahman MM, Reza HM. (2016). Astaxanthin ameliorates prenatal LPS-exposed behavioral deficits and oxidative stress in adult offspring. BMC Neurosci 17:11.
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, et al. (2010). VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci 30:2198–210.
- Brudzynski SM, Bihari F. (1990). Ultrasonic vocalization in rats produced by cholinergic stimulation of the brain. Neurosci Lett 109:222–6.
- Callaerts-Vegh Z, Moechars D, Van Acker N, Daneels G, Goris I, Leo S, Naert A, et al. (2013). Haploinsufficiency of VGluT1 but not VGluT2 impairs extinction of spatial preference and response suppression. Behav Brain Res 245:13–21.
- Donner NC, Lowry CA. (2013). Sex differences in anxiety and emotional behavior. Pflugers Arch 465:601–26.
- Evanson NK, Herman JP. (2015). Role of paraventricular nucleus glutamate signaling in regulation of HPA axis stress responses. Interdiscip Inf Sci 21:253–60.
- Goel N, Innala L, Viau V. (2014). Sex differences in serotonin (5-HT) 1A receptor regulation of HPA axis and dorsal raphe responses to acute restraint. Psychoneuroendocrinology 40:232–41.
- Herman JP, Mueller NK, Figueiredo H. (2004). Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci 1018:35–45.
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. (2004). Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience 123:983–1002.
- Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y. (2004). Glutamate signaling in peripheral tissues. Eur J Biochem 271:1–13.
- Jain SK, Zelena D. (2011). Gender specific influence of endogenous glutamate release on stress-induced fear in rats. Endocr Regul 45:13–21.
- Jorgensen HS. (2007). Studies on the neuroendocrine role of serotonin. Dan Med Bull 54:266–88.
- Miot S, Voituron N, Sterlin A, Vigneault E, Morel L, Matrot B, Ramanantsoa N, et al. (2012). The vesicular glutamate transporter VGLUT3 contributes to protection against neonatal hypoxic stress. J Physiol (Lond.) 590:5183–98.
- Nelson AB, Bussert TG, Kreitzer AC, Seal RP. (2014). Striatal cholinergic neurotransmission requires VGLUT3. J Neurosci 34:8772–7.
- Nickerson Poulin A, Guerci A, El Mestikawy S, Semba K. (2006). Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol 498:690–711.
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. (2010). Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci 13:232–8.
- Ofek K, Soreq H. (2013). Cholinergic involvement and manipulation approaches in multiple system disorders. Chem Biol Interact 203:113–19.
- Popoli M, Yan Z, McEwen BS, Sanacora G. (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13:22–37.
- Takahashi A, Yap JJ, Bohager DZ, Faccidomo S, Clayton T, Cook JM, Miczek KA. (2009). Glutamatergic and GABAergic modulations of ultrasonic vocalizations during maternal separation distress in mouse pups. Psychopharmacology (Berl) 204:61–71.
- Varga J, Fodor A, Klausz B, Zelena D. (2015). Anxiogenic role of vasopressin during the early postnatal period: maternal separation-induced ultrasound vocalization in vasopressin-deficient Brattleboro rats. Amino Acids 47:2409–18.
- Vincent MY, Jacobson L. (2014). Glucocorticoid receptor deletion from the dorsal raphé nucleus of mice reduces dysphoria-like behavior and impairs hypothalamic-pituitary-adrenocortical axis feedback inhibition. Eur J Neurosci 39:1671–81.
- von Merten S, Hoier S, Pfeifle C, Tautz D. (2014). A role for ultrasonic vocalisation in social communication and divergence of natural populations of the house mouse (Mus musculus domesticus). PLoS One 9:e97244.
- Wallen-Mackenzie A, Wootz H, Englund H. (2010). Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission? Ups J Med Sci 115:11–20.
- Weidenfeld J, Newman ME, Itzik A, Gur E, Feldman S. (2002). The amygdala regulates the pituitary-adrenocortical response and release of hypothalamic serotonin following electrical stimulation of the dorsal raphe nucleus in the rat. Neuroendocrinology 76:63–9.
- Zelena D, Makara GB, Jezova D. (1999). Simultaneous blockade of two glutamate receptor subtypes (NMDA and AMPA) results in stressor-specific inhibition of prolactin and corticotropin release. Neuroendocrinology 69:316–23.
- Zelena D, Stocker B, Barna I, Toth ZE, Makara GB. (2015). Vasopressin deficiency diminishes acute and long-term consequences of maternal deprivation in male rat pups. Psychoneuroendocrinology 51:378–91.
