Abstract
Catecholamines (CAs) are mainly produced by sympathoadrenal system but their de novo production has been also observed in adipose tissue cells. The aim of this work was to investigate whether immune challenge induced by lipopolysaccharide (LPS) modulates biosynthesis of CAs in mesenteric adipose tissue (MWAT), as well as whether previous exposure to immobilization (IMO) stress could modulate this process. Sprague–Dawley rats were exposed to single (2 h) or repeated (2 h/7 days) IMO and afterwards injected with LPS (i.p., 100 μg/kg body weight) and sacrificed 3 h later. LPS did not alter CA biosynthesis in MWAT in control rats. Single and repeated IMO elevated CAs and expression of CA biosynthetic enzymes in MWAT, including adipocyte and stromal/vascular fractions (SVF). Repeated IMO followed by LPS treatment led to the up-regulation of CA-biosynthetic enzymes expression, elevation of CAs in SVF but depletion of norepinephrine and epinephrine in adipocyte fraction. Prior IMO caused a marked LPS-induced macrophage infiltration in MWAT as evaluated by F4/80 expression. A positive correlation between expression of tyrosine hydroxylase and F4/80 suggests macrophages as the main source of LPS-induced CA production in MWAT. Furthermore, prior exposure to the single or repeated IMO differently affected immune responses following LPS treatment by modulation of inflammatory cytokine expression. These data suggest that stress might be a significant modulator of immune response in MWAT via stimulation of the macrophage infiltration associated with cytokine response and de novo production of CAs.
Introduction
Exposure of an organism to challenging stimuli induces activation of the sympathoadrenal system (SAS) and the hypothalamic-pituitary-adrenal (HPA) axis. This is manifested by exaggerated release of catecholamines (CAs) and glucocorticoids to mediate an appropriate response to stress. However, CAs are not only the SAS-specific molecules. Significant evidence exists regarding CA production within various cell types, though with an unclear function. CA biosynthesis has been reported in cardiomyocytes (Tillinger et al., Citation2006), endothelial cells (Sorriento et al., Citation2012), immune cells (Flierl et al., Citation2008; Laukova et al., Citation2013) and also in adipocytes (Vargovic et al., Citation2011). Prolonged or repeated exposure to stressors leads to increased CA production not only in sympathetic nerve terminals and adrenal medulla (Kvetnansky et al., Citation2009) but also in adipocytes (Vargovic et al., Citation2013) and immune cells (Laukova et al., Citation2013; Nguyen et al., Citation2011), further suggesting a potential role of these mediators in specific cellular processes.
Among all types of white adipose tissues, mesenteric adipose depot (MWAT) displays the highest catecholamine content and stress-inducible CA production (Vargovic et al., Citation2011, Citation2013). Immunoendocrine activity of this visceral fat tissue has been implicated in the development of metabolic as well as inflammatory bowel disease (Bertin et al., Citation2010; Gambero et al., Citation2007). Visceral adiposity is strongly related to metabolic dysfunction, including insulin resistance and systemic inflammation (Barbarroja et al., Citation2010; Miyazaki et al., Citation2002; Sam et al., Citation2009). Because of high production of adipokines and other immunoregulatory molecules, MWAT regulates local and systemic inflammatory response (Batra et al., Citation2012; Gambero et al., Citation2007).
Stress episodes are important risk factors for the development and reactivation of intestinal inflammation in rodents and humans (Stam et al., Citation1997). In animals, acute and chronic stress increases gastrointestinal tract (GIT) paracellular permeability resulting in visceral hypersensitivity (Ait-Belgnaoui et al., Citation2005), bacterial translocation associated with increased uptake of lipopolysaccharide (LPS) (Zareie et al., Citation2006) and exacerbation of experimental colitis (Gué et al., Citation1997). LPS molecule is recognized by CD14 which subsequently interacts with toll-like receptor 4 (TLR4). Both receptors are present on monocytes/macrophages but also on adipocytes (Khazen et al., Citation2005). TLR4 activation further promotes release of acute phase proteins, induction of nitric oxide synthase activity and synthesis, and increased release of cytokines via activation of nuclear factor-κB pathway. In addition, induction and release of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1 from immune cells and their interaction with corresponding receptors in the central nervous system (CNS) activates the HPA axis (Dunn, Citation1992) and secretion of stress hormones to the periphery (Dunn, Citation1992). Moreover, an elevation of plasma CAs has also been demonstrated during endotoxicosis (Jones & Romano, Citation1984).
Thus, in the present study, we aimed to investigate the effect of LPS treatment on stress-induced CA biosynthesis in MWAT, as well as in cell fractions obtained from MWAT. Additionally, the expression of several inflammatory cytokines and macrophage marker F4/80 were analyzed and correlated with the production of CAs.
Materials and methods
Animals
Male Sprague–Dawley rats, 4 months old (300–350 g, Charles River, Suzfeld, Germany) were used in our experiments. Animals were housed 3–4 per cage in a controlled environment (23 ± 1 °C, 12 hours light–dark cycle, lights on at 6 AM). The Ethics Committee of the Institute of Experimental Endocrinology (Slovak Academy of Sciences, Bratislava, Slovakia) approved all experimental procedures with animals used in this study in protocol no. Ro-3400/13-221.
Stress procedures
Immobilization (IMO) was used as physical and emotional stressor. The rats were immobilized on a wooden board by taping the limbs with surgical tape and restricting the motion of the head as originally described by Kvetnansky & Mikulaj (Citation1970). IMO was performed at the same time of the day (between 8:00 A.M. and noon) for all experiments. For single IMO (1×IMO), rats were immobilized one time for 2 h and subsequently sacrificed 3 h after the termination of the stress. For repeated IMO, rats were immobilized for 2 h daily for 7 consecutive days (7×IMO) and sacrificed by decapitation 3 h after termination of the stress. For stimulation of immune system, intraperitoneal injection (i.p.) of lipopolysaccharide (LPS) from Escherichia coli K-235 (L2143, Sigma-Aldrich, St. Louis, MO) was used. We chose sub-septic dose of 100 μg/kg per body weight, which has been shown to induce a moderate systemic inflammatory response accompanied by significantly elevated plasma levels of pro-inflammatory cytokines within 3–6 h after injection (Morrison & Ryan, Citation1992). Control rats were injected with saline at the same conditions. In stressed animals, LPS or saline were injected immediately after the termination of the stress stimulus and the rats were sacrificed after 3 h. MWAT was immediately used for collagenase digestion and separation into adipocytes and stromal/vascular fraction (SVF).
Separation of adipose tissue cells
Mesenteric adipose tissue (200–300 mg) was minced and incubated for 40–60 min at 37 °C with a gentle constant agitation (100–120 rpm) in 0.5 ml of Medium 199 (Gibco, Invitrogen, Carlsbad, CA) containing 0.5% BSA, 3 mM CaCl2 (medium + BSA + CaCl2) and 1 mg.ml−1 of type I collagenase (Sigma-Aldrich, St. Louis, MO) until digestion was completed. Adipocytes were present in the floating layer of the cell suspension. After a brief centrifugation (1 min at 100 × g) the infranatant and pellet, both containing stromal/vascular fraction (SVF), were removed, centrifuged (10 min at 500 × g) and thereafter washed with medium + BSA + CaCl2. Adipocyte and SVF fractions were washed two times and filtered through 210 μm nylon mesh filter (Small Parts, Logansport, IN). The expression of F4/80, a macrophage marker, was used to evaluate the presence of macrophages in all fractions of MWAT.
Catecholamine determination
Catecholamines were analyzed using 3-CAT Research RIA kits (Labor Diagnostica Nord, Nordhorn, Germany) according to the manufacturer’s protocol. Values were normalized to protein in homogenate determined by bicinchoninic acid (BCA) Protein Assay (Thermo Scientific, Rockford, IL) modified for samples with high content of lipids according to Morton & Evans (Citation1992).
RNA isolation and relative quantification of mRNA levels by real time RT-PCR
Total RNA from whole mesenteric adipose tissue, adipocytes and SVF was isolated with TRI Reagent (MRC Ltd., Cincinnati, OH) as described previously (Vargovic et al., Citation2011). Reverse transcription and real time PCR were performed as described previously (Vargovic et al., Citation2013). For all PCR amplifications, aliquots containing 20 ng of total RNA were used. Tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT) were amplified using specific TaqMan probes (Applied Biosystems, Foster City, CA) (Rn00562500_m1 for TH, Rn01495588_m1 for PNMT) and Maxima Probe/ROX qPCR Master Mix according to the manufactureŕs protocol in 20 μl of total volume. Genes encoding vesicular monoamine transporter 1 (VMAT1), 18S ribosomal subunit, IL-6, IL-10, TNFα and F4/80 were amplified using SYBR Green Mastermix (ThermoFischer Scientific, Vilnius, Lithuania) with specific primers (5 pmol) in 20 μl of final volume. The primer sequences are displayed in . Data were analyzed with SDS software version 2.3 (Applied Biosystems, Foster City, CA) and inspected to determine artifacts (loading errors, threshold errors, etc.). Baseline levels for each gene were computed automatically. Count numbers (Ct values) were exported to an Excel spreadsheet and analyzed according to the ΔΔCT method described by Livak & Schmittgen (Citation2001). 18S rRNA was used as an internal control.
Table 1. Primers used in quantitative real time RT-PCR.
Western blot analysis
Individual adipocyte fractions were sonicated in 0.6 ml of 20 mM Tris pH 7.0, 5 mM PMSF (Pefabloc SC + Compete, Roche Diagnostic, Basel, Switzerland), incubated on ice for 1 h and centrifuged for 15 min (16,100 × g, 4 °C) in order to extract the cytosolic fraction. Western blot was performed as described previously (Laukova et al., Citation2010). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal and loading control since it was not affected by experimental conditions. The following primary antibodies were used: Monoclonal anti-TH (1:3,000; MAB5280, Chemicon International, Temecula, CA), polyclonal anti-PNMT (1:200, CA-401 bMTr ab, Protos Biotech Corp., New York, NY) and monoclonal anti-GAPDH (1:5000, MAB374, Chemicon International, Temecula, CA). Membranes were incubated with corresponding horseradish peroxidase-linked secondary antibodies (TH with anti-mouse Ab diluted 1:5000; PNMT with anti-rabbit Ab diluted 1:5000) for 1 h at room temperature. The signals were detected using Femto Supersignal reagent (Pierce, Rockford, IL) and visualized by exposure to Amersham Hyperfilm (Amersham Biosciences, Little Chalfont, England). Optical density of individual bands (o.d/mm2) was quantified by PC BASE 2.08e software (Raytest Inc., Dusseldorf, Germany) and normalized to GAPDH.
Ex vivo effect of LPS
A direct effect of LPS on the adipocyte fraction was also evaluated ex vivo by incubating freshly isolated adipocytes in the presence of LPS added to the media. Adipocyte fractions of control animals were washed four times and incubated in medium + BSA + CaCl2 containing LPS (1 μg/mL) for 2 h at 37 °C with gentle agitation. After incubation, samples were washed two times with medium + BSA + CaCl2 and adipocyte suspension was stored at −80 °C.
Statistical analysis
Statistical differences among two experimental groups were determined by T-test, while statistical differences among more than two groups were determined by one-way and two way analysis of variance (ANOVA) followed by Bonferonni post hoc tests [SigmaStat, version 3.1, Systat Software, Inc., San Jose, CA]. Results are presented as mean ± S.E.M. and each value represents 4–6 rats. Values of *p < 0.05, **p < 0.01, ***p < 0.001 defined the statistical significance versus control group and values of +p < 0.05, ++p < 0.01, +++p < 0.001 for LPS versus saline groups.
Results
Effect of IMO and LPS administration on catecholamine levels and expression of CA biosynthetic enzymes in adipocyte fraction
In unstressed rats, LPS administration did not affect intracellular CA levels or mRNA expression of CA biosynthetic enzymes TH and PNMT or the vesicular monoamine transporter (VMAT1, ). Single IMO led to elevation of intracellular NE (F2,11 = 58.50, p < 0.001, ) and EPI (F2,10 = 34.02, p < 0.001), while LPS administration did not evoke any additional changes in CA levels induced by single IMO. Levels of all three CAs were unchanged after repeated IMO. However, LPS administration following the last IMO increased DA levels (F1,10 = 4.82, p < 0.05), while NE and EPI concentrations were barely detectable or under detection limit ().
Figure 1. Effect of LPS on catecholamine biosynthetic pathway in adipocyte fraction of mesenteric adipose tissue of rats exposed to a single (1 × IMO) or repeated (7 × IMO) immobilization. C: control rats; Sal: saline injected; LPS: lipopolysaccharide injected. Dopamine (DA), norepinephrine (NE) and epinephrine (EPI) concentrations in adipocytes. Gene expression of catecholamine biosynthetic enzymes, tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT), as well as vesicular monoamine transporter (VMAT1) determined by real time RT-PCR. Values are normalized to internal control (18 S rRNA) and presented as fold change relative to control values evaluated by ΔΔCt method. Each column is displayed as mean ± S.E.M. and represents an average of 5–6 animals. Values of *p < 0.05, **p < 0.01, ***p < 0.001 defined the statistical significance versus control groups and values of +p < 0.05, ++p < 0.01, +++p < 0.001 LPS versus saline groups of the same stress interval.
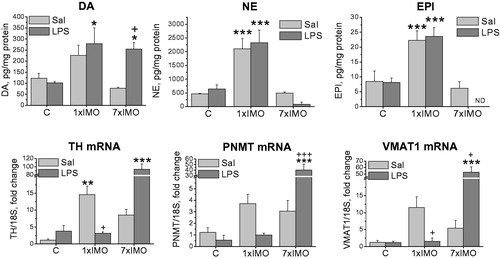
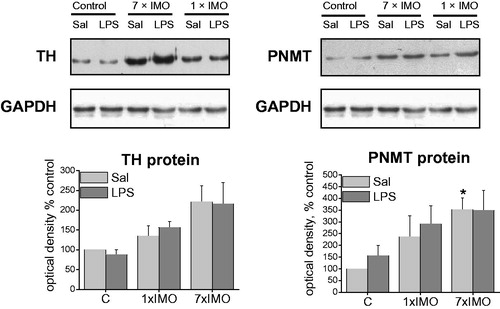
IMO significantly affected the gene expression of TH (F2,24 = 27.95, p < 0.001, ), PNMT (F2,24 = 9.05, p < 0.001) and VMAT1 (F2,24 = 28.156, p < 0.001). Single IMO elevated TH (F2,13 = 8.55, p < 0.01) and PNMT (F2,11 = 6.04, p < 0.05) mRNAs, but subsequent exposure to LPS completely blocked these changes. Repeated IMO alone did not induce TH, PNMT or VMAT1 expressions, but following LPS injection produced a marked elevations of TH (F2,29 = 31.25, p < 0,001), PNMT (F2,29 = 9.05, p < 0.001) and VMAT1 (F2,22= 29.70, p < 0.001) mRNAs. In order to confirm whether mRNA levels reflect the amount of translated protein, TH and PNMT protein levels were analyzed by Western blot (). While TH protein levels did not show any significant changes, the PNMT protein level was elevated in saline treated rats exposed to the repeated IMO (F2,10 = 4.50, p < 0.05).
Figure 2. Expression of tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT) protein in adipocyte fractions, detected by Western blot. For TH, 30 μg and for PNMT 60 μg of soluble protein fraction was loaded into the gel lanes. Each column is displayed as mean ± S.E.M. and represents an average of 4–6 animals. Values of *p < 0.05, **p < 0.01, ***p < 0.001 defined the statistical significance versus control groups and values of +p < 0.05, ++p < 0.01, +++p < 0.001 LPS versus saline groups of the same stress interval.
Effect of IMO and LPS on inflammatory pathway in adipocyte fraction
The expression of the pro-inflammatory cytokines IL-6 and TNF-α were determined to confirm the LPS-induced inflammatory response in adipocyte fraction of MWAT (). LPS significantly stimulated expression of IL-6 (F1,26 = 6.50, p < 0.05) and TNF-α (F1,31 = 8.83, p < 0.01) in control (unstressed) rats. Single IMO elevated expression of IL-6 (F2,26 = 13.23, p < 0.05) and TNF-α mRNA (F2,31 = 6.54, p < 0.05) in vehicle-treated rats but not in the LPS-treated rats. While the repeated IMO did not alter expression of IL-6 and TNF-α, the subsequent LPS treatment caused a significant raise of TNF-α (F1,30 = 5.14, p < 0.05).
Figure 3. Levels of mRNAs encoding several immunological factors in adipocyte fractions. Interleukin 6 (IL-6), tumor necrosis factor (TNF-α), interleukin 10 (IL-10) and marker of differentiated macrophages (F4/80) in control rats (C) and in rats exposed to a single (1 × IMO) and repeated (7 × IMO) immobilization with subsequent injection of saline (Sal) or lipopolysaccharide (LPS). Each column is displayed as mean ± S.E.M. and represents an average of 5–6 animals. Values of *p < 0.05, **p < 0.01, ***p < 0.001 defined the statistical significance versus control groups and values of +p < 0.05, ++p < 0.01, +++p < 0.001 LPS versus saline groups of the same stress interval.
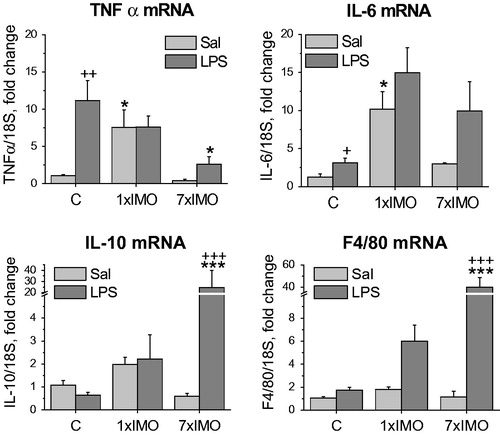
Anti-inflammatory cytokine interleukin 10 (IL-10) was unchanged by stress in saline-treated rats, but animals exposed to the repeated IMO exhibited a pronounced LPS-induced increase of IL-10 mRNA (F2,31 = 12.05, p < 0.001).
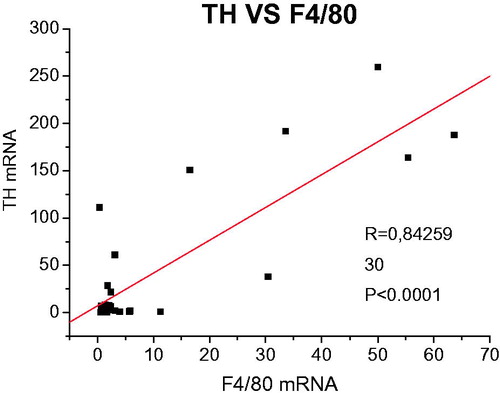
The marker of differentiated macrophages F4/80 was monitored in order to investigate macrophages infiltration, as well as to assess the contamination of adipocyte fraction with adherent macrophages. F4/80 mRNA exhibited a marked elevation (F1,29 = 24.59, p < 0.001) after stimulation with LPS in rats previously exposed to the repeated IMO. In addition, an association between CA production and presence of macrophages in the adipocyte fraction was confirmed by correlation of TH and F4/80 expressions ().
Direct effect of LPS on adipocytes
Ex vivo incubation of adipocytes with LPS () was performed to evaluate a direct effect of LPS on adipocytes via TLR-4 and to avoid any indirect effects caused by an intensive inflammatory response and activation of sympathoadrenal system and HPA axis in vivo. Two hours of incubation with LPS (10 μg/ml) induced 4-fold increase of IL-6 (p < 0.01) and 2-fold increase of TNF-α mRNA (p < 0.01). The expression of CA biosynthetic enzymes (TH, PNMT) and VMAT1 was significantly downregulated after incubation with LPS (TH, p < 0.05; PNMT p < 0.01; VMAT1 p < 0.01).
Table 2. Ex vivo effect of lipopolysaccharide (LPS).
Catecholamine biosynthesis and inflammatory response in the stromal/vascular fraction and in the entire MWAT
MWAT contains a number of other cell types collectively known as the stromal/vascular fraction (SVF), several of which can produce catecholamines de novo (e.g. macrophages and lymphocytes). Although this study is primarily focused to analyze the adipocyte fraction we also compared the SVF fraction with the entire MWAT samples to uncover the source of CA biosynthesis and immune responses in MWAT.
In the SVF fraction of control rats, LPS did not affect CA levels or CA biosynthetic enzymes expression (). Single IMO elevated TH mRNA levels (F2,24 = 7.67, p < 0.01) leading to an elevation of dopamine (F2,24 = 7.62, p < 0.05) and epinephrine (F2,24 = 6.28, p < 0.05). No additional changes in CA levels were observed following LPS treatment. Repeated IMO alone did not change CA levels or expression of CA-biosynthetic enzymes, however, subsequent LPS injection caused the elevation of NE (F2,24 = 4.39, p < 0.05) and dopamine (F2,24 = 7.62, p < 0.01), as well as the increased gene expression of TH (F2,24 = 7.67, p < 0.05) and PNMT (F2,23 = 3.39, p < 0.05).
Figure 5. Catecholamine biosynthetic pathway and inflammatory mediators in stromal/vascular fraction of mesenteric adipose tissue of rats exposed to a single (1 × IMO) or repeated (7 × IMO) immobilization. C: control rats; Sal: saline injected; LPS: lipopolysaccharide injected. Dopamine (DA), norepinephrine (NE) and epinephrine (EPI) concentrations. Gene expression of catecholamine biosynthetic enzymes, tyrosine hydroxylase (TH), phenylethanolamine N-methyltransferase (PNMT), vesicular monoamine transporter VMAT1, tumor necrosis factor (TNF-α), interleukin 10 (IL-10) and marker of differentiated macrophages (F4/80). Values are normalized to internal control (18 S rRNA) and presented as fold change relative to control values evaluated by ΔΔCt method. Each column is displayed as mean ± S.E.M. and represents an average of 5–6 animals. Values of *p < 0.05 defined the statistical significance versus control groups and values of +p < 0.05, LPS versus saline groups of the same stress interval.
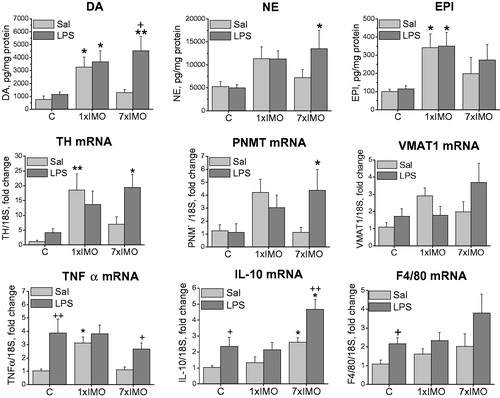
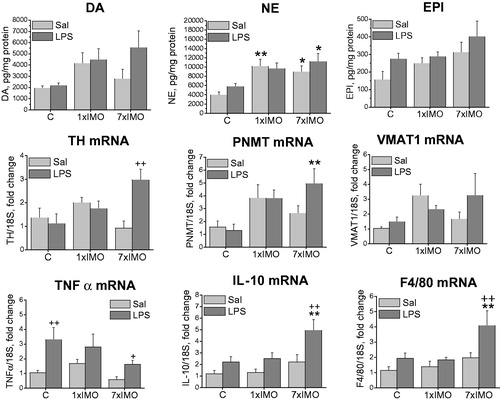
In the entire MWAT samples, LPS or IMO alone did not affect expression of CA producing enzymes but combination of repeated IMO and LPS induced a rise in TH (F2,24 = 6.49, p < 0.01), PNMT (F2,24 = 6.08, p < 0.01) and VMAT1 mRNA. NE was elevated in MWAT following a single (F2,24 = 10.98, p < 0.01) or repeated (F2,24 = 10.98, p < 0.05) IMO. Expression of the pro-inflammatory cytokines TNF-α and IL-10 exhibited similar pattern of changes in the SVF and entire MWAT as observed in the adipocyte fraction (). Primarily in control rats, LPS administration elevated TNF-α (for SVF: F1,24 = 12.84, p < 0.01; for MWAT: F1,24 = 12.12, p < 0.01), IL-10 (for SVF: F1,24 = 12.84, p < 0.05) and F4/80 (for SVF: F1,24 = 6.79, p < 0.05) mRNA expression. Prior exposure to a single IMO prevented the response to LPS, while repeated IMO restored the increase of TNF-α (for SVF: F2,24 = 3.78, p < 0.05; for MWAT: F2,24 = 12.12, p < 0.05) as well as IL-10 (for MWAT: F1,24 = 6.83, p < 0.01; for SVF: F1,24 = 15.02, p < 0.01) expression. Repeated IMO in combination with LPS also increased expression of F4/80 suggesting a rise in infiltration of macrophages in MWAT (F1,24 = 7.92, p < 0.01).
Figure 6. Catecholamine biosynthetic pathway and inflammatory mediators in entire mesenteric adipose tissue samples of rats exposed to a single (1 × IMO) or repeated (7 × IMO) immobilization. C: control rats; Sal: saline injected; LPS: lipopolysaccharide injected. Dopamine (DA), norepinephrine (NE) and epinephrine (EPI) concentrations. Gene expression of catecholamine biosynthetic enzymes, tyrosine hydroxylase (TH), phenylethanolamine N-methyltransferase (PNMT), vesicular monoamine transporter VMAT1, tumor necrosis factor (TNF-α), interleukin 10 (IL-10) and marker of differentiated macrophages (F4/80). Values are normalized to internal control (18 S rRNA) and presented as fold change relative to control values evaluated by ΔΔCt method. Each column is displayed as mean ± S.E.M. and represents an average of 5–6 animals. Values of *p < 0.05 defined the statistical significance versus control groups and values of +p < 0.05, LPS versus saline groups of the same stress interval.
Discussion
We have recently demonstrated that CA biosynthesis in rat mesenteric adipocytes (Vargovic et al., Citation2011) is stimulated by repeated IMO stress (Kvetnansky et al., Citation2012; Vargovic et al., Citation2013). Since immune cells use endogenous CAs for autocrine/paracrinne signaling (Flierl et al., Citation2008), an analogous function of CAs might be proposed for adipocytes or other cells in mesenteric adipose tissue (MWAT). As MWAT contains many types of cells (immune cells, fibroblasts, endothelial cells, neuronal cells, adipose-derived adult stem cells) and many of them (macrophages, T and B cells, endothelial cells, adipocytes) are able to produce CAs de novo, as well as express adrenergic and dopaminergic receptors, it is very likely that crosstalk between these cell populations is also mediated by CAs.
In order to determine a relationship between CA production and immune response in MWAT, we designed experiment to reveal how inflammatory responses to the LPS affect CA-production in MWAT in unstressed or stressed animals. Basically, LPS induced appropriate inflammatory responses in MWAT, as evidenced by up-regulation of TNF-α and IL-6 expression, while de novo production of CAs remained unchanged. LPS in combination with prior repeated IMO elevated expression of CA producing enzymes, TH and PNMT and vesicular monoamine transporter VMAT1 resulting in elevated CA concentrations in stromal/vascular fraction or entire MWAT. In the adipocyte fraction, norepinephrine (NE) and epinephrine (EPI) levels were only elevated after a single IMO, but not after the repeated IMO. Furthermore, a marked depletion of NE and EPI () was observed in animals exposed to the repeated IMO and subsequent LPS treatment, though dopamine levels as well as the expression of TH and PNMT were markedly up-regulated at the same time. This could be explained by a concomitant increase of NE and EPI turnover leading to the time-framed depletion followed by exaggerated expression of TH, PNMT and VMAT1. This further indicates the ability of MWAT to replenish intracellular CA stores. Increased NE turnover in association with increased splenic nerve activity and elevated plasma NE and EPI has been reported after LPS administration (MacNeil et al., Citation1997). However, we did not observe depletion of CAs in SVF, or entire MWAT as opposed to adipocyte samples. This could be explained by a presence of additional CA producing systems within MWAT exhibiting separate regulatory mechanism of CA production, storage and preservation for further degradation by monoamine oxidases. Depletion of CAs in adipocyte fraction suggests increased turnover or degradation of CAs in adipocytes, while SVF which contains mainly CAs produced by immune cells did not exhibit this phenomenon. Completely different situation is observed in the entire MWAT, where NE concentration increased in response to single or repeated IMO. However, these NE levels represents primarily NE supplies originated in sympathetic nerve endings, which therefore may not be associated with expression of CA producing enzymes in MWAT samples per se (i.e. non-neuronal cell-specific CAs).
Although our previous work described de novo production of CAs directly in adipocytes (Kvetnansky et al., Citation2012; Vargovic et al., Citation2011, Citation2013), a recent data (Ebke et al., Citation2014) shed light on the key question of CA origin in the MWAT. Specifically, adipocyte fractions obtained by collagenase digestion usually do not display enough purity due to an incomplete elimination of macrophages from floating layer of adipocytes which contains lipid-engorged macrophages, macrophages in contact with lipid droplets and sheath-like assemblies of macrophages surrounding the adipocytes (Ebke et al., Citation2014). Therefore, we included the analysis of differentiated macrophage marker F4/80 which revealed an association with the expression of CA biosynthetic enzymes. While this finding suggests the LPS-driven production of CAs by infiltrated macrophages, it does not disprove the importance of adipocyte-specific CA biosynthetic pathway. Although we have observed CAs and CA-synthesizing enzymes presented also in primary adipocyte culture, it purity could be also debatable. While the question of the origin of endogenous CAs in MWAT remains still unanswered, this work suggests an effect of the LPS-driven immune response on de novo synthesis of CA in MWAT. The regulation of this synthesis represents a fast response to stress and suggests the involvement of stress mediators, such as sympathoadrenal CAs and/or glucocorticoids, in this process. Enhancing effect of repeated IMO on LPS-mediated stress response is most likely due to a heterotypic character of consequent exposure to LPS-mediated inflammatory stress.
It is known that exposure to heterotypic stressors often alters the subsequent responsiveness of stress systems. For example, exposure to chronic restraint modifies the hypothalamic–pituitary–adrenal (HPA) axis response to subsequent acute stressors, with adaptive response to the homotypic but sensitization to a heterotypic stressor (Dronjak et al., Citation2004; Spiga et al., Citation2009). Similarly, acute and chronic stress sensitizes the inflammatory response to subsequent inflammatory challenges (Maier, Citation2003). However, we observed inhibitory effect of stress adaptation on the inflammatory response (). While a single IMO increased expression of the pro-inflammatory cytokines (IL-6, TNF-α) in accordance with Johnson et al. (Citation2005), no changes after a subsequent LPS exposure have been observed. On the contrary, repeated IMO restored the response to LPS as evidenced by elevated TNF-α mRNA. Increased expression of IL-10 in rats exposed to both repeated IMO and LPS suggests additional anti-inflammatory responses taking place in MWAT. These data also indicate a rising number of alternatively activated macrophages (M2 population) in MWAT, which are associated with catecholamine production in adipose tissue (Nguyen et al., Citation2011). This could explain our observations of rising CA production, infiltration of macrophages monitored by F4/80 expression and IL-10 expression in MWAT in animals exposed to the repeated IMO followed by the LPS treatment suggesting a link between de novo CA synthesis and anti-inflammatory response.
It also appears there might be an association between CA-production in MWAT cells and inflammatory responses elicited by a direct incubation of adipocyte fraction with LPS ex vivo. Inflammatory responses were confirmed by elevated expression of TNF-α and IL-6 as previously observed (Berkowitz et al., Citation1998), alongside with down-regulation of genes involved in CA production. As mentioned above, according to Nguyen et al. (Citation2011) CA production in adipose tissue is probably associated with anti-inflammatory M2 macrophage populations, while LPS exposure might shift the cell programing into a pro-inflammatory response and/or inhibition of anti-inflammatory response. It is possible that LPS-mediated attenuation of anti-inflammatory status may consequently affect CA production. Furthermore, Lukewich & Lomax (Citation2013) reported a decreased CA response in adrenal medulla after LPS administration, where LPS via toll-like receptors (TLR-4) activation and nuclear factor kappa B-dependent pathway reduced adrenal chromaffin cell excitability. However, increased NE turnover following LPS treatment reduced the pro-inflammatory while enhanced the anti-inflammatory response (Elenkov et al., Citation1995; Suberville et al., Citation1996), indicating a physiological mechanism to limit the magnitude of an inflammatory response. Taking into account our results and the available literature, it appears that local de novo production of CAs in MWAT could be positively associated with infiltration of alternatively activated M2 subclass of macrophages and subsequent production of anti-inflammatory mediators such as IL-10, as well as production of CAs participating in a local immunomodulation (Barnes et al., Citation2015). Since it has been demonstrated that stress exposure increases intestine permeability and consequently leads to the bacteria and LPS uptake (Zareie et al., Citation2006), activity of co-localized MWAT could also account for the local modulation of the inflammatory response.
In summary, these data confirmed our previous observation that repeated exposure to psychological stressors induces CA production in mesenteric adipose tissue. In addition, subsequent exposure to LPS as a heterotypic stressor produces a significant response of the MWAT CA system that is accompanied by exaggerated infiltration of macrophages and modulation of immune responses.
Funding information
This research was supported by Slovak Research and Development Agency (No. APVV-0088-10) and VEGA Grants (2/0067/14, 2/0180/15).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. (2005). Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 113:141–7.
- Barbarroja N, Lopez-Pedrera R, Mayas MD, Garcia-Fuentes E, Garrido-Sanchez L, Macías-González M, El Bekay R, et al. (2010). The obese healthy paradox: is inflammation the answer? Biochemistry 430:141–9.
- Barnes MA, Carson MJ, Nair MG. (2015). Non-traditional cytokines: how catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 72:210–19.
- Batra A, Heimesaat MM, Bereswill S, Fischer A, Glauben R, Kunkel D, Scheffold A, et al. (2012). Mesenteric fat – control site for bacterial translocation in colitis? Mucosal Immunol 5:580–91.
- Berkowitz DE, Brown D, Lee KM, Emala C, Palmer D, An Y, Breslow M. (1998). Endotoxin-induced alteration in the expression of leptin and beta3-adrenergic receptor in adipose tissue. Am J Physiol 274:E992–7.
- Bertin B, Desreumaux P, Dubuquoy L. (2010). Obesity, visceral fat and Crohn's disease. Curr Opin Clin Nutr Metab Care 13:574–80.
- Dronjak S, Jezova D, Kvetnansky R. (2004). Different effects of novel stressors on sympathoadrenal system activation in rats exposed to long-term immobilization. Ann N Y Acad Sci 1018:113–23.
- Dunn AJ. (1992). Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exp Ther 261:964–9.
- Ebke LA, Nestor-Kalinoski AL, Slotterbeck BD, Al-Dieri AG, Ghosh-Lester S, Russo L, Najjar SM, et al. (2014). Tight association between macrophages and adipocytes in obesity: implications for adipocyte preparation. Obesity (Silver Spring) 22:1246–55.
- Elenkov IJ, Haskó G, Kovács KJ, Vizi ES. (1995). Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J Neuroimmunol 61:123–31.
- Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. (2008). Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora's box? Mol Med 14:195–204.
- Gambero A, Marostica M, Abdalla Saad MJ, Pedrazzoli J Jr. (2007). Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm Bowel Dis 13:1357–64.
- Gué M, Del Rio-Lacheze C, Eutamene H, Théodorou V, Fioramonti J, Buéno L. (1997). Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 9:271–9.
- Jones SB, Romano FD. (1984). Plasma catecholamines in the conscious rat during endotoxicosis. Circ Shock 14:189–201.
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. (2005). Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135:1295–307.
- Khazen W, M’Bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C. (2005). Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett 579:5631–4.
- Kvetnansky R, Mikulaj L. (1970). Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology 87:738–43.
- Kvetnansky R, Sabban EL, Palkovits M. (2009). Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89:535–606.
- Kvetnansky R, Ukropec J, Laukova M, Manz B, Pacak K, Vargovic P. (2012). Stress stimulates production of catecholamines in rat adipocytes. Cell Mol Neurobiol 32:801–13.
- Laukova M, Vargovic P, Krizanova O, Kvetnansky R. (2010). Repeated stress down-regulates β(2)- and α (2C)-adrenergic receptors and up-regulates gene expression of IL-6 in the rat spleen. Cell Mol Neurobiol 30:1077–87.
- Laukova M, Vargovic P, Vlcek M, Lejavova K, Hudecova S, Krizanova O, Kvetnansky R. (2013). Catecholamine production is differently regulated in splenic T- and B-cells following stress exposure. Immunobiology 218:780–9.
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–8.
- Lukewich MK, Lomax AE. (2013). Toll-like receptor 4 activation reduces adrenal chromaffin cell excitability through a nuclear factor-κB-dependent pathway. Endocrinology 154:351–62.
- MacNeil BJ, Jansen AH, Janz LJ, Greenberg AH, Nance DM. (1997). Peripheral endotoxin increases splenic sympathetic nerve activity via central prostaglandin synthesis. Am J Physiol 273:R609–14.
- Maier SF. (2003). Bi-directional immune-brain communication: implications for understanding stress, pain, and cognition. Brain Behav Immun 17:69–85.
- Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, et al. (2002). Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 283:E1135–43.
- Morrison DC, Ryan JL (editors). (1992). Lipopolysaccharide-induced interferon. In: Bacterial endotoxic lipopolysaccharides. Chapter 7, Vol. 1: Immunopharmacology and pathophysiology. London: CRC Press. pp. 166–84.
- Morton RE, Evans TA. (1992). Modification of the bicinchoninic acid protein assay to eliminate lipid interference in determining lipoprotein protein content. Anal Biochem 204:332–4.
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, et al. (2011). Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480:104–8.
- Sam S, Haffner S, Davidson MH, D'Agostino RB Sr., Feinstein S, et al. (2009). Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care 32:932–7.
- Sorriento D, Santulli G, Del Giudice C, Anastasio A, Trimarco B, Iaccarino G. (2012). Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension 60:129–36.
- Spiga F, et al. (2009). Effect of vasopressin 1b receptor blockade on the hypothalamic–pituitary–adrenal response of chronically stressed rats to a heterotypic stressor. J Endocrinol 200:285–91.
- Stam R, Akkermans LM, Wiegant VM. (1997). Trauma and the gut: interactions between stressful experience and intestinal function. Gut 40:704.
- Suberville S, Bellocq A, Fouqueray B, Philippe C, Lantz O, Perez J, Baud L. (1996). Regulation of interleukin-10 production by beta-adrenergic agonists. Eur J Immunol 26:2601–5.
- Tillinger A, Novakova M, Pavlovicova M, Lacinova L, Zatovicova M, Pastorekova S, Krizanova O, Kvetnansky R. (2006). Modulation by 6-hydroxydopamine of expression of the phenylethanolamine N-methyltransferase (PNMT) gene in the rat heart during immobilization stress. Stress 9:207–13.
- Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R. (2011). Adipocytes as a new source of catecholamine production. FEBS Lett 585:2279–84.
- Vargovic P, Ukropec J, Laukova M, Kurdiova T, Balaz M, Manz B, Ukropcova B, Kvetnansky R. (2013). Repeated immobilization stress induces catecholamine production in rat mesenteric adipocytes. Stress 16:340–52.
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, et al. (2006). Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55:1553–60.