Abstract
Stress-induced gastric ulcer is an important life-threatening condition, while the molecular basis of its development is incompletely understood. Toll-like receptor 4 (TLR4), an innate immune pattern recognition receptor, can induce pro-inflammatory transcription, aggravating a stress ulcer. The present study found that TLR4 played a protective role in a mouse model of water immersion (23 °C) restraint stress. Wild-type (WT) and TLR4−/− male mice were respectively divided into five groups (5 per group), and exposed to the stressor for 0, 0.5, 1, 2, or 4 hours. Gastric ulcer index, determined post mortem, increased with time in both types of mice but was greater in TLR4−/− mice. Furthermore, increased serum cortisol and corticosterone concentrations were observed in WT mice only, and such increases were detected only in WT mice 4 h after lipopolysaccharide (LPS) treatment (2 mg/kg, intraperitoneal injection). Moreover, the administration of cortisol alleviated the gastric injury in TLR4−/− mice. Western blotting showed expression in the adrenal of P450scc (CYP11A1), the first rate-limiting enzyme in the synthesis of steroids, was increased 4 h after water immersion restraint stress or LPS treatment in WT mice, but was conversely decreased in TLR4−/− mice after either stressor. Furthermore, in adrenal glands of TLR4−/− mice, structural distortion of mitochondria (which contain CYP11A1) was found with electron microscopy, and lack of lipid-storing droplets was found using light microscopy on adrenal cryosections stained with Oil red O. These data indicate that TLR4 plays a protective role in stress-induced gastric ulcer that is exerted via impacting synthesis of glucocorticoid in the adrenal gland.
Introduction
Stress may affect different physiological functions of the gastrointestinal tract including gastric secretion, motility, permeability and barrier function, visceral sensitivity, and mucosal blood flow (Nardone & Compare, Citation2014). Many stressful situations could lead to gastric ulcers, especially in an intensive care unit where stress-induced ulcer is a common and major complication, often causing hemorrhage or even death (Ali & Harty, Citation2009). The mechanisms underlying ulcer development are multifactorial and not fully understood. Damage to the gastric mucosa occurs through a series of events starting with the breakdown of protective defenses, followed by the aggravation of ulceration by physiological factors, including hypo-perfusion and increased release of catecholamines and pro-inflammatory cytokines (Quenot et al., Citation2009).
Endogenous glucocorticoid (predominantly corticosterone in rats) released during acute stress has gastro-protective effects against stress-induced gastric injury (Filaretova et al., Citation2004). The hypothalamic-pituitary-adrenocortical (HPA) axis is rapidly activated in response to acute stress (Aguilera, Citation2011), and pretreatment with corticotropin-releasing factor (CRF) or preconditioning with a mildly stressful stimulus has gastroprotective functions that involve corticosterone (Filaretova et al., Citation2008, Citation2012). Moreover, in an adrenalectomized rat model of stress, gastric mucosal damage increases as a function of decreased corticosterone level (Yigiter et al., Citation2010). Moreover, intra-hypothalamic dexamethasone implantation which induces long-lasting stress-induced glucocorticoid deficiency results in aggravation of stress-induced gastric injury (Filaretova & Filaretov, Citation1992). The gastroprotective effects of glucocorticoids during acute stress involve multiple mechanisms, including the maintenance of glucose homeostasis, gastric mucosal blood flow, mucus production, and attenuation of gastric motility and microvascular permeability (Filaretova et al., Citation2004; Kuo et al., Citation2015; Podvigina et al., Citation2009), as well as changes in the gastric microcirculation (Filaretova et al., Citation1999). Although circulating corticosterone is considered to be the main glucocorticoid in the regulation of stress in rodents, cortisol also has a corresponding response to stimulation by stress in mice (Gong et al., Citation2015). Whether it impacts stress-induced gastric ulcer in mice remains to be determined.
TLR4 is an innate immune pattern recognition receptor, which is part of the Interleukin-1 Receptor/Toll-like Receptor Superfamily containing a toll-like/IL-1 Receptor (TIR) domain and a Leucine-rich repeat motif in the extracellular domain (Nardone & Compare, Citation2014). As the receptor for lipopolysaccharide (LPS), TLR4 can be activated by the LPS-lipopolysaccharide-binding protein (LBP)-myeloid differentiation protein 2 (MD2) complex, thus passing this signal to the downstream nuclear factor (NF)-kb pathway (Akira & Takeda, Citation2004). Several studies have reported that downregulation of NF-kb or inflammation mitigates gastric ulcer (Kang et al., Citation2014; Mahmoud-Awny et al., Citation2015; Sinha et al., Citation2015). Hence it has been speculated that TLR4 signaling may promote stress-induced gastric injury because of its function in modulating immune activity. However, previous research reported that TLR4 activation can stimulate the HPA axis (Monroe & Simons, Citation1991). TLR4 signaling can also induce the upregulation of CRF (CRH) gene expression in paraventricular nucleus neurons in the hypothalamus (Loum-Ribot et al., Citation2006). Thus TLR4 activation is considered to give protection against stress-induced gastric ulcer through mediation by the synthesis and release of glucocorticoid.
Taken together, the above findings indicate that the relationship between the TLR4 and stress ulcer is still unclear. Our preliminary study has shown activation of the TLR4 downstream NF-kB pathway could accelerate the pathogenesis of stress-induced gastric damage (Jia et al., Citation2007a). Herein, we hypothesized that activation of TLR4 also could enhance glucocorticoid synthesis, thus playing a protective role in the pathological ulceration process. In this study, functions of TLR4 in gastric injury and synthesis of glucocorticoid during stress and effects of cortisol on stress ulcer in mice were examined in a combined water immersion and restraint stress model.
Methods
Animals and management
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Second Military Medical University in Shanghai (IACUC-2012-XuSG) in accordance with the U.S.A. National Institutes of Health guidelines for the care and use of laboratory mice. TLR4 knockout (TLR4−/−; C57BL/10ScNJU) and wild-type (WT;C57BL/6_129) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, U.S.A.). The TLR4−/− mice were genetically identified in the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, Jiangsu, China). Male WT and TLR4−/− mice bred under specific pathogen-free conditions aged 6–8 weeks were used for experiments. Mice were housed three to five per cage and acclimatized to standard laboratory conditions (lights on between 08:00 h and 19:00 h, temperature 20 ± 1 °r, free access to food and water) for 7 days before the experiment.
Induction of ulcers by exposure to combined water immersion and restraint stress
WT and TLR4−/− mice were fasted for 24 h before experiments but given free access to tap water, and kept in two different cages with adequate capacity respectively. WT and TLR4−/− mice were restrained in perforated centrifuge tubes (50 ml) and immersed in 23 °C water in a 20-cm deep constant temperature water tank to the depth of the xiphoid process for 0, 0.5, 1, 2, and 4 h as described previously (Jia et al., Citation2007b). After the indicated times, mice were surgically anesthetized by intraperitoneal injection of sodium pentobarbital (1% w/v, 50 mg/kg), and blood samples were collected from the inferior vena cava and the stomach was removed to assess gastric damage. Blood samples were allowed to clot for 30 min at room temperature before centrifugation for 15 min at 1000 × g. Serum was removed and assayed immediately or stored at −80 °C until assay.
Evaluation of the gastroprotective effects of exogenous cortisol
WT and TLR4−/− mice were randomized (n = 5 per group) and injected subcutaneously 15 min prior to stress exposure with 4 mg/kg cortisol (0.4 mg/ml, Tocris Bioscience, Minneapolis, MN) or isotonic solvent (dimethylsulfoxide in saline, 1:200) in the treatment and vehicle groups, respectively. A control group was untreated. After 4 h of water immersion restraint stress (Jia et al., Citation2007b), mice were anesthetized with sodium pentobarbital (as above) for blood sampling and stomach removal for the estimation of cortisol level and gastric injury, respectively.
Endotoxemia model
WT and TLR4−/− mice were randomized (n = 5 per group) and injected intraperitoneally with LPS (0.2 mg/ml, 2 mg/kg) (Sigma-Aldrich Corp., St. Louis, MO) or isotonic saline and killed 4 h later to determine the effect of TLR4 (receptor for LPS) on the regulation of endogenous glucocorticoids.
Corticotropin (adrenocorticotropic hormone, ACTH) test
WT and TLR4−/− mice were randomized and injected intraperitoneally with corticotropin (5 IU/ml, 50 IU/kg) (Shanghai NO.1 Biochemical & Pharmaceutical Co., LTD, Shanghai, China) or isotonic saline and killed after 4 h to measure serum cortisol and corticosterone.
Assessment of gastric damage
Stomachs were removed and filled with 2 ml of 1% formalin and immersed in formalin for 24 h. The stomachs then were cut along the length of the greater curvature and examined for mucosal lesions. The total length (mm) of linear hemorrhagic erosions was measured macroscopically as the ulcer index (UI) (mm) by an investigator blinded to the treatment conditions (Shimozawa et al., Citation2006). Part of each stomach tissue sample was used for pathological examination.
Measurement of serum cortisol, corticosterone, LPS, and lipopolysaccharide-binding protein (LBP) concentrations
Samples were allowed to clot for 30 min at room temperature in a serum separator tube and centrifuged for 15 min at 1000 × g. Cortisol and corticosterone concentrations in serum samples were measured using a cortisol EIA Kit (sensitivity: 0.11 ng/ml; coefficient of variation: intra-assay range 5.4%–9.2%, and inter-assay range 9.3%–21.2%) (R&D Systems, Minneapolis, MN) and a Corticosterone Parameter Assay Kit (sensitivity: 0.047 ng/ml; coefficient of variation: intra-assay range 4.5%–7.5%, and inter-assay range 5.6%–7.1%) (R&D Systems, Minneapolis, MN). LPS and LBP concentrations in serum samples were measured using mouse LPS enzyme-linked immunosorbent assay kits (sensitivity: 0.039 ng/ml; coefficient of variation: intra-assay <8%, and inter-assay <10%) (Cusabio, Wuhan, China) and the mouse LBP enzyme-linked immunosorbent assay kits (sensitivity: 3.12 ng/ml; coefficient of variation: intra-assay <8%, and inter-assay <10%) (Cusabio, Wuhan, China).
Western blotting
Whole adrenal glands were lysed in protein extraction buffer [20 mM Tris (pH7.5), 150 mM NaCl, 1%Triton X-100, 1 mM EDTA] followed by centrifugation at 12,000 × g for 15 min at 4 °C. The supernatant was removed and total protein was determined (Bradford assay). Samples containing 40 μg protein were loaded, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (90 V), and blotted onto nitrocellulose membrane. The blots were probed with anti-CYP11A1 antibody (1/1000; Cell Signaling Technology) and with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1/2000; Santa Cruz Biotechnology). After extensive washing, protein bands were visualized by chemiluminescence staining. Blots were also probed with anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1/1000; Sigma–Aldrich) to confirm equal loading of protein. Expression of proteins was then calculated semiquantitatively as integrated optical density (IOD) ratio of CYP11A1 to GAPDH.
Electron microscopy
Adrenal glands of WT and TLR4−/− mice (3 per group) without any treatment were removed and fixed in 0.1-M phosphate buffer (pH 7.3) with 2% (v/v) formaldehyde and glutaraldehyde. Tissue sections were post-fixed for 90 min (2% OsO4 in 0.1M cacodylate buffer, pH 7.3), dehydrated in a graded series of ethanol (30%, 50%, 70%, 80%, 90% and 100%) for 15 min at each step, and embedded in epoxy resin. Ultrathin sections (70–90 nm) were stained with uranyl acetate and lead citrate and examined at 80 kV using a CM 10 electron microscope (Philips, Best, Netherlands) to assess mitochondrial structure in cells of the zona fasciculata, which produce glucocorticoids from pregnenolone via action of lCYP11A1 located in the inner membrane of mitochondria to convert cholesterol to pregnenolone (Halkerston et al., Citation1961; Koritz & Kumar, 1970; Stone & Hechter, 1955).
Adrenal neutral lipid visualization
Frozen sections (7 μm, transverse sections) were prepared on a Leica CM3050-S cryostat. Cryosections were routinely stained with Oil red O for neutral lipid visualization. Nuclei were detected using a hematoxylin stain.
Statistical analysis
Data were analyzed by two-way ANOVA, one-way ANOVA, or Tukey’s test of variance using SPSS v.20.0 software (SPSS Inc., Chicago, IL). Differences between groups were calculated using the least significant differences method, and significance level was defined as p < .05. Results are expressed as mean ± SEM.
Results
TLR4 deficiency exacerbates stress-induced gastric mucosal injury and modulates serum glucocorticoid concentrations
Gastric mucosa. Gastric mucosal injury was exacerbated in WT mice as a function of water immersion restraint time. Cellular swelling, hypochromia of the cytoplasm, stenosis, and erythropenia in the mucosa appeared starting 0.5 h after stress application. Atrophy and exfoliation of mucosal cells, and increased gastric stromal cell erosion was observed at 1 h. After 2 h, mucosal erosion became more severe, and ulceration appeared at 4 h. The pathological changes induced by water immersion restraint stress were more severe in TLR4−/− than in WT mice (), and more ulcers were observed grossly in the gastric mucosa of the mutants after 4 h (), which was associated with a higher UI at successive time points (two-way ANOVA, Fmice-type = 145.8, pmice-type < .001; Ftime = 215.2, ptime < .001; Fmice-type × time = 26.8, pmice-type × time < .001; one-way ANOVA after two-way ANOVA, df = (9,40), F = 123.7, p < .001; Tukey’s test, pWT0h/TLR4−/−0 h = 1.000, pWT0.5 h/TLR4−/−0.5 h = 1.000, pWT1h/TLR4−/−1 h < .001, pWT2h/TLR4−/−2 h < .001, pWT4h/TLR4−/−4 h < .001) ().
Figure 1. Effect of TLR4 deficiency on stress-induced gastric mucosal injury. (A) Histological analysis of gastric mucosa of WT and TLR4−/− mice (n = 5 each) exposed for indicated times to water immersion restraint stress. (B) Gastric mucosa of mice (n = 5 each) stressed for 4 h. (C) Stress-induced gastric mucosal damage quantified as gastric ulcer index (UI); these values, along with serum cortisol and corticosterone concentrations, are shown as the mean ± SEM (n = 5 mice per group). *p < .05 (one-way ANOVA, Tukey’s test) versus stressed WT mice at each time point.
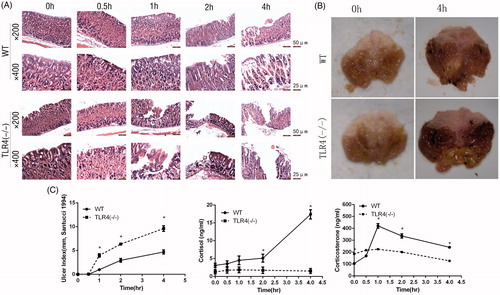
Serum cortisol. Serum cortisol concentrations were increased in WT mice starting from 2 h after the application of the stressor and peaked at the 4-h time point (two-way ANOVA, Fmice-type = 109.4, pmice-type < .001; Ftime = 29.8, ptime < .001; Fmice-type × time = 30.7, pmice-type × time < .001; one-way ANOVA after two-way ANOVA, df = (9,40), F = 39.0, p < .001; Tukey’s test, pWT0h/WT0.5 h = 1.000, pWT0.5 h/WT1h = .39, pWT1h/WT2h = .001, pWT2h/WT4h = .005). In contrast, cortisol concentration remained lower in TLR4−/− mice during exposure to stress (one-way ANOVA after two-way ANOVA, df = (9, 40), F = 39.0, p < .001; Tukey’s test, pWT0h/TLR4−/−0 h = .8, pWT0.5 h/TLR4−/−0.5 h = .8, pWT1h/TLR4−/−1 h = .3, pWT2h/TLR4−/−2 h = .042, pWT4h/TLR4−/−4 h < .001) ().
Serum corticosterone. Serum corticosterone concentrations were increased by the stressor from 0.5 h, peaked at 1 h in WT mice and then decreased in both types of mice (two-way ANOVA, Fmice-type = 84.4, pmice-type < .001; Ftime = 86.9, ptime < .001; Fmice-type × time = 62.3, pmice-type × time < .001; one-way ANOVA after two-way ANOVA, df = (9,40), F = 75.7, p < .001; Tukey’s test, pWT0h/WT0.5 h = .006, pWT0.5 h/WT1h < .001, pWT1h/WT2h < .001, pWT2h/WT4h < .001, pTLR4−/−0 h/TLR4−/−0.5 h = .6, pTLR4−/−0.5 h/TLR4−/−1 h = 1.000, pTLR4−/−1 h/WT2h = .9, pTLR4−/−2 h/WT4h = .001). The corticosterone concentrations showed a higher basal value in TLR4−/− mice but increased less than in the WT with exposure to the stressor (one-way ANOVA after two-way ANOVA, df = (9, 40), F = 75.7, p < .001; Tukey’s test, pWT 0 h/TLR4−/− 0 h < .001, pWT 0.5 h/TLR4−/− 0.5 h = .08, pWT 1 h/TLR4−/− 1 h < .001, pWT 2 h/TLR4−/− 2 h < .001, pWT 4 h/TLR4−/− 4 h < .001) ().
Administration of cortisol alleviates stress-induced gastric mucosal injury
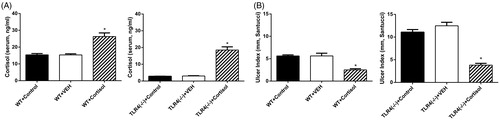
As expected, serum cortisol concentrations were increased in WT and TLR4−/− mice upon injection of exogenous cortisol as compared to the vehicle-injected and uninjected control groups (WT: F = 2.98, df = (2, 12) p = .0001; TLR4−/−: F = 67.18, df = (2, 12), p < .0001) () and was associated with a lower UI (WT: F = 19.02, df = (2, 12) p = .0002; TLR4−/−: F = 60.13, df = (2, 12), p < .0001) (). Hence, cortisol administration alleviated stress-induced gastric mucosal damage, in both WT and TLR4−/− mice.
Figure 2. Effect of cortisol administration on stress-induced ulceration. WT and TLR4−/− mice were evaluated for (A) serum cortisol concentrations, and (B) gastric ulcer index (UI) after pretreatment with cortisol. Results represent mean ± SEM (n = 5 mice per group). *p < .05 (one-way ANOVA, Tukey’s test) versus vehicle group (dimethylsulfoxide in saline, 1:200) and control groups (without any treatment).
LPS and TLR4 modulate serum glucocorticoid concentrations upon exposure to stress
Serum LPS and LBP concentrations increased markedly in WT mice after 4 h of exposure to water immersion restraint stress (WT: F = 20.04, p = .0021; TLR4−/−: F = 6.03, p = .039. All df are (3, 16)) ().
Figure 3. Effect of stress, lipopolysaccharide (LPS), or corticotropin on serum LPS, lipopolysaccharide-binding protein (LBP), and glucocorticoid concentrations. (A) Serum LPS and LBP concentrations after a 4-h exposure to water immersion restraint stress or not. (B) Serum LPS, cortisol, and corticosterone concentrations in mice treated with LPS or vehicle control (isotonic saline) for 4 h. (C) Serum cortisol and corticosterone concentrations in WT and TLR4−/− mice after a corticotropin (5 IU/ml, 50 IU/kg) test. Results represent mean ± SEM (n = 5). *p < .05 (one-way ANOVA, Tukey’s test) versus vehicle group. #p < .05 (one-way ANOVA, Tukey’s test) between linked groups.
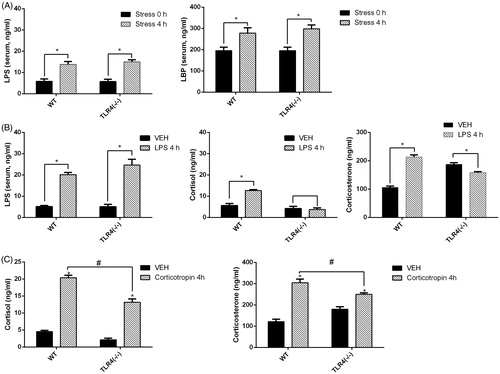
Cortisol and corticosterone concentrations were measured in mice pretreated with LPS (1% w/v, 2 mg/kg) for 4 h. Serum LPS concentration increased compared to the vehicle-treated group in both WT and TLR4−/− mice (WT: F = 123.4, p < .0001; TLR4−/−: F = 35.14, p = .0004. All df are (3, 16)), while cortisol concentrations were elevated in LPS-treated as compared to vehicle-treated WT but not TLR4−/− mice (WT: F = 33.03, p = .0004; TLR4−/−: F = .091, p = .77. All df are (3, 16)). The corticosterone concentrations were elevated also in WT, but declined in TLR4−/− mice compared to the vehicle-treated group (WT: F = 146.4, p < .0001; TLR4−/−: F = 18.37, p = .003. All df are (3, 16)) ().
The corticotropin test showed the concentrations of cortisol and corticosterone increased in both types of mice, but the increase was less in TLR4−/− mice ().
Together, these findings indicate that the stress-induced increases in glucocorticoid concentration are due to increased LPS/TLR4 signaling.
TLR4−/−deficiency causes disorganization of mitochondrial structure, lack of lipid droplets, and down expression of CYP11A1 in adrenal cortex
In WT mice, the oval mitochondria of zona fasciculata cells were filled with parallel, tubulovesicular cristae in a homogeneous arrangement. In contrast, mitochondria in these cells in TLR4−/− mice were round and had disorganized cristae, and vacuoles were present in the mitochondria matrix (). In addition, with light microscopy, obvious reduction in density of lipid-storing droplets was seen in Oil red O-stained whole adrenal frozen section in unstressed TLR4−/− mice ().
Figure 4. Ultrastructure, lipid storage, and steroidogenic enzyme content in WT and TLR4−/− mouse adrenal glands. (A) Electron micrographs of adrenocortical cells in the zona fasciculata. Mitochondria (MIT), smooth endoplasmic reticulum, and liposomes (LIP) are visible in the cytoplasm of WT and TLR4−/− mice. Note the round shape, disorganized arrangement of tubulovesicular cristae, and presence of metrical vacuoles in the mutant. Scale bar 1 μm. (B) Cryosections of whole adrenal gland stained with Oil red O in WT and TLR4−/− mice without any treatment showing fewer red-stained lipid-droplets in TLR4 mutant mice. Scale bar 200 μm. (C) Western blotting of adrenal gland protein samples and the corresponding integrated optical density (IOD) ratio values (CYP11A1/glyceraldehyde 3-phosphate dehydrogenase [GAPDH]). The expression of CYP11A1 (P450scc) increased 4 h after stress or lipopolysaccharide (LPS) treatment in WT but decreased in TLR4−/− mice. Results represent mean ± SEM (n = 5 mice per group). *p < .05 versus WT vehicle group (isotonic saline). #p < .05 (one-way ANOVA, Tukey’s test) versus TLR4−/− vehicle group (isotonic saline).
![Figure 4. Ultrastructure, lipid storage, and steroidogenic enzyme content in WT and TLR4−/− mouse adrenal glands. (A) Electron micrographs of adrenocortical cells in the zona fasciculata. Mitochondria (MIT), smooth endoplasmic reticulum, and liposomes (LIP) are visible in the cytoplasm of WT and TLR4−/− mice. Note the round shape, disorganized arrangement of tubulovesicular cristae, and presence of metrical vacuoles in the mutant. Scale bar 1 μm. (B) Cryosections of whole adrenal gland stained with Oil red O in WT and TLR4−/− mice without any treatment showing fewer red-stained lipid-droplets in TLR4 mutant mice. Scale bar 200 μm. (C) Western blotting of adrenal gland protein samples and the corresponding integrated optical density (IOD) ratio values (CYP11A1/glyceraldehyde 3-phosphate dehydrogenase [GAPDH]). The expression of CYP11A1 (P450scc) increased 4 h after stress or lipopolysaccharide (LPS) treatment in WT but decreased in TLR4−/− mice. Results represent mean ± SEM (n = 5 mice per group). *p < .05 versus WT vehicle group (isotonic saline). #p < .05 (one-way ANOVA, Tukey’s test) versus TLR4−/− vehicle group (isotonic saline).](/cms/asset/03c44cd0-9f59-47c6-9eff-a4a573905e21/ists_a_1224843_f0004_c.jpg)
Western blotting of the protein samples from the adrenal glands showed the expression of CYP11A1 protein was increased 4 h after water immersion restraint stress or LPS treatment in WT but was decreased in TLR4−/− mice (ANOVA, WT: F = 5.88, df = (2, 12), p = .017; TLR4−/−: F = 24.41, df = (2, 12), p < .0001) (). Thus, in the absence of TLR4, adrenocortical mitochondrial structure and CYP11A1 protein expression were compromised, which may be an underlying cause of low secretion of glucocorticoids during stress.
Discussion
The activation of the inflammatory response and ensuing release of pro-inflammatory cytokines are features of stress-induced peptic ulcers (McGettrick & O’Neill, Citation2004). The innate inflammatory/immune response is activated after exposure to stressful stimuli. Cold restraint stress can induce a rapid activation of nuclear factor (NF)-κB in rats, the inhibition of which alleviates inflammation and injury (Jia et al., Citation2007a). The deletion of the NF-κB p50 subunit can alleviate gastric injury by inhibiting NF-κB activation and consequent inflammation (Ye et al., Citation2013). We previously demonstrated that the activation of the mitogen-activated protein kinase (MAPK) p38 by reactive oxygen species may also play a role in stress-induced gastric damage in rats (Jia et al., Citation2007b). TLR4 protein is a positive regulator of inflammation (Szumilas et al., Citation2013). Signaling pathways activated by Toll-like receptors (TLRs) in the innate immune system are linked to the induction of pro-inflammatory cytokines. NF-κB, p38, and the MAPK family member c-Jun N-terminal kinase are the major factors activated by TLR4 (O’Neill, Citation2006) which, along with its co-receptor MD-2 and some inflammatory mediators, is upregulated by chronic mild stress (Garate et al., Citation2011). Hence it is a plausible hypothesis that TLR4 may increase gastric injury through the activation of inflammatory pathways in stress ulcer. However, we found just the opposite. In this study, it was observed that water immersion restraint stress-induced gastric ulceration was more severe in TLR4−/− than in WT mice. Hence, in addition to promoting inflammation, TLR4 signaling provides a gastroprotective function under stress conditions.
Many studies have found that glucocorticoid (corticosterone in rodents) release in response to acute stress protects the gastric mucosa against stress-induced ulceration (Filaretova et al., Citation1998, Citation2014;Citation Hernandez et al., Citation1984; Yigiter et al., Citation2010). In the present study, corticosterone release was not stimulated during acute stress in TLR4−/− mice, which accorded with some other reports (Vakharia & Hinson, Citation2005; Zacharowski et al., Citation2006). Serum cortisol concentration showed a similar trend to that of corticosterone during the acute water immersion restraint stress, while other research reported that although plasma corticosterone is considered the main glucocorticoid involved in the regulation of stress responses in rodents, the presence of plasma cortisol and whether its level can be used as an indicator of stress has not been determined in rodents before (Gong et al., Citation2015). In the present study, furthermore, administering cortisol 15 min before exposure to stress alleviated gastric injury in both WT and TLR4−/− mice. This indicates that the release of glucocorticoid during acute stress plays an important role in indicating injury and protecting the gastric mucosa during acute stress in mice.
Previous research showed that TLR4 signaling is sufficient to cause glucocorticoid release from adrenal cells (Vakharia & Hinson, Citation2005). LPS from Gram-negative bacteria, which act via TLR4, stimulates glucocorticoid release (Zacharowski et al., Citation2006). In addition, translocation from the intestinal flora of bacterial LPS occurs during chronic mild stress (Garate et al., Citation2011). LBP is essential for the recognition of LPS by TLR4 (Palsson-McDermott & O’Neill, Citation2004), and it has been found that plasma LPS and LBP expression is upregulated after cold restraint stress, which has been linked to changes in intestinal permeability and bacterial translocation (Garate et al., Citation2011). In this study, we also found that serum concentrations of LPS and of LBP were upregulated after 4-h water immersion restraint stress. Also, the serum cortisol and corticosterone concentrations both increased 4 h after LPS treatment in this study in WT, but importantly not in the TLR4−/− mice. In contrast, the corticotropin test showed increased circulating glucocorticoid (corticosterone and cortisol) concentrations in both types of mice, although the response was less in TLR4−/− mice. Hence, TLR4 in the adrenal gland is not essential for a response to ACTH, but has an important role in regulating the extent of glucocorticoid release during acute stress.
Conversion of cholesterol to pregnenolone in mitochondria is the first, rate-limiting, and hormonally regulated step in the synthesis of all steroid hormones (Halkerston et al., Citation1961; Koritz & Kumar, Citation1970; Stone & Hechter, Citation1955). Adrenal steroidogenesis is controlled by ACTH which initiates several enzymatic processes starting with conversion of cholesterol to pregnenolone mediated by the key side chain cleavage enzyme (CYP11A1), and then synthesis of particular steroids which takes place in the endoplasmic reticulum and mitochondria (Miller & Auchus, Citation2011). Forms of CYP11A1 engineered to lack the mitochondrial leader or that are targeted to the endoplasmic reticulum are inactive, demonstrating that the mitochondrial environment is required for activity (Black et al., Citation1994). In the present study, mitochondria in adrenocortical cells of TLR4−/− mice showed ultrastructural defects as compared to those of WT mice. Furthermore, the expression of CYP11A1 protein increased after 4 h stress and by LPS treatment in WT mice, but was decreased in TLR4−/− mice. Moreover, lipid-storing droplets constituting the substrates for steroidogenesis were reduced in TLR4−/− mice in Oil red O-stained cryosections of whole adrenal gland. These changes may underlie the differences in the synthesis and secretion of corticosterone and cortisol by adrenocortical cells between WT and TLR4−/− mice.
Exposure to low concentrations of endotoxin produces long-term changes in HPA axis activity (Shanks et al., Citation2000). In particular, early-life exposure to Gram-negative LPS increases corticosterone secretory pulse frequency and amplitude (Vakharia & Hinson, Citation2005). Moreover, preconditioning with mild stress provides gastroprotection to rats during acute stress (Filaretova et al., Citation2008). In another study, increasing glucocorticoid receptor expression induced a weaker response to restraint stress (Reichardt et al., Citation2000). Thus, it can be conjectured that long-term exposure to stressful environmental stimuli may promote the neuroendocrine/neuroimmune response during acute stress events, and that this form of adaptation is lost in the absence of TLR4, thereby decreasing protection against the adverse consequences of acute stress.
In summary, TLR4 deficiency resulted in aggravated gastric ulceration that was associated with a reduction in glucocorticoid release during acute stress, an effect that was alleviated by treatment with exogenous cortisol. TLR4 thus plays a gastroprotective role in stress-induced gastric ulceration that is exerted via modulation of glucocorticoid secretion by impacting the ultrastructure and the synthetic function of the adrenal gland through LPS/TLR4 signaling. In addition, as well as corticosterone, cortisol also plays an important role during acute stress in mice.
Disclosure statement
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Aguilera G. (2011). HPA axis responsiveness to stress: implications for healthy aging. Exp Gerontol 46:90–5.
- Akira S, Takeda K. (2004). Toll-like receptor signalling. Nat Rev Immunol 4:499–511.
- Ali T, Harty RF. (2009). Stress-induced ulcer bleeding in critically ill patients. Gastroenterol Clin North Am 38:245–65.
- Black SM, Harikrishna JA, Szklarz GD, Miller WL. (1994). The mitochondrial environment is required for activity of the cholesterol side-chain cleavage enzyme, cytochrome P450scc. Proc Natl Acad Sci USA 91:7247–51.
- Filaretova L, Bagaeva T, Morozova O. (2012). Stress and the stomach: corticotropin-releasing factor may protect the gastric mucosa in stress through involvement of glucocorticoids. Cell Mol Neurobiol. 32:829–36.
- Filaretova L, Maltcev N, Bogdanov A, Levkovich Y. (1999). Role of gastric microcirculation in the gastroprotection by glucocorticoids released during water-restraint stress in rats. Chin J Physiol 42:145–52.
- Filaretova L, Podvigina T, Bagaeva T, Morozova O. (2014). From gastroprotective to ulcerogenic effects of glucocorticoids: role of long-term glucocorticoid action. Curr Pharm Des 20:1045–50.
- Filaretova LP, Bagaeva TR, Amagase K, Takeuchi K. (2008). Contribution of glucocorticoids to protective influence of preconditioning mild stress against stress-induced gastric erosions. Ann N Y Acad Sci 1148:209–12.
- Filaretova LP, Filaretov AA. (1992). [The effect of large doses of corticosteroids on stomach ulcer formation: a new hypothesis] [Article in Russian]. Fiziol Zh SSSR Im I M Sechenova 78:77–83.
- Filaretova LP, Filaretov AA, Makara GB. (1998). Corticosterone increase inhibits stress-induced gastric erosions in rats. Am J Physiol 274:G1024–30.
- Filaretova LP, Podvigina TT, Bagaeva TR, Tanaka A, Takeuchi K. (2004). Mechanisms underlying the gastroprotective action of glucocorticoids released in response to ulcerogenic stress factors. Ann N Y Acad Sci 1018:288–92.
- Garate I, Garcia-Bueno B, Madrigal JL, Bravo L, Berrocoso E, Caso JR, Mico JA, Leza JC. (2011). Origin and consequences of brain Toll-like receptor 4 pathway stimulation in an experimental model of depression. J Neuroinflammation 8:151. PubMed PMID:22053929.
- Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH. (2015). Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 10:e0117503.
- Halkerston ID, Eichhorn J, Hechter O. (1961). A requirement for reduced triphosphopyridine nucleotide for cholesterol side-chain cleavage by mitochondrial fractions of bovine adrenal cortex. J Biol Chem 236:374–80.
- Hernandez DE, Adcock JW, Nemeroff CB, Prange AJ, Jr. (1984). The role of the adrenal gland in cytoprotection against stress-induced gastric ulcers in rats. J Neurosci Res 11:193–201.
- Jia YT, Ma B, Wei W, Xu Y, Wang Y, Tang HT, Xia ZF. (2007a). Sustained activation of nuclear factor-kappaB by reactive oxygen species is involved in the pathogenesis of stress-induced gastric damage in rats. Crit Care Med 35:1582–91.
- Jia YT, Wei W, Ma B, Xu Y, Liu WJ, Wang Y, Lv KY, et al. (2007b). Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J Immunol 179:7808–19.
- Kang JW, Yun N, Han HJ, Kim JY, Kim JY, Lee SM. (2014). Protective effect of flos lonicerae against experimental gastric ulcers in rats: mechanisms of antioxidant and anti-inflammatory action. Evid Based Complement Alternat Med 2014:596920.
- Koritz SB, Kumar AM. (1970). On the mechanism of action of the adrenocorticotrophic hormone. The stimulation of the activity of enzymes involved in pregnenolone synthesis. J Biol Chem 245:152–9.
- Kuo T, Mcqueen A, Chen TC, Wang JC. (2015). Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol 872:99–126.
- Loum-Ribot E, Lafon P, Chaigniau M, Tramu G, Corio M. (2006). Glucocorticoids down-regulate lipopolysaccharide-induced de novo production of neurotensin mRNA in the rat hypothalamic, paraventricular, corticotrophin-releasing hormone neurons. Neuroimmunomodulation 13:170–8.
- Mahmoud-Awny M, Attia AS, Abd-Ellah MF, El-Abhar HS. (2015). Mangiferin mitigates gastric ulcer in ischemia/reperfused rats: involvement of PPAR-γ, NF-κB and Nrf2/HO-1 signaling pathways. PLoS One 10:e0132497.
- McGettrick AF, O’Neill LA. (2004). The expanding family of MyD88-like adaptors in Toll-like receptor signal transduction. Mol Immunol 41:577–82.
- Miller WL, Auchus RJ. (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151.
- Monroe SM, Simons AD. (1991). Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull 110:406–25.
- Nardone G, Compare D. (2014). The psyche and gastric functions. Dig Dis 32:206–12.
- O’Neill LA. (2006). How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol 18:3–9.
- Palsson-Mcdermott EM, O’Neill LA. (2004). Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153–62.
- Podvigina TT, Bobryshev PY, Bagaeva TR, Mal’tsev NA, Levkovich YI, Filaretova LP. (2009). The effects of desensitization of capsaicin-sensitive afferent neurons on the microcirculation in the stomach in rats depend on the blood glucocorticoid hormone level. Neurosci Behav Physiol 39:559–64.
- Quenot JP, Thiery N, Barbar S. (2009). When should stress ulcer prophylaxis be used in the ICU? Curr Opin Crit Care 15:139–43.
- Reichardt HM, Umland T, Bauer A, Kretz O, Schutz G. (2000). Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol 20:9009–17.
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. (2000). Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA 97:5645–50.
- Shimozawa N, Okajima K, Harada N, Arai M, Ishida Y, Shimada S, Kurihara H, Nakagata N. (2006). Contribution of sensory neurons to sex difference in the development of stress-induced gastric mucosal injury in mice. Gastroenterology 131:1826–34.
- Sinha K, Sadhukhan P, Saha S, Pal PB, Sil PC. (2015). Morin protects gastric mucosa from nonsteroidal anti-inflammatory drug, indomethacin induced inflammatory damage and apoptosis by modulating NF-kappaB pathway. Biochim Biophys Acta 1850:769–83.
- Stone D, Hechter O. (1955). Studies on ACTH action in perfused bovine adrenals: aspects of progesterone as an intermediary in corticosteroidogenesis. Arch Biochem Biophys 54:121–8.
- Szumilas D, Krysiak R, Okopien B. (2013). [The role of TLR4 receptor in development of inflammation and carcinogenesis in ulcerative colitis and pharmacotherapy of this disorder]. Wiad Lek 66:3–9.
- Vakharia K, Hinson JP. (2005). Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology 146:1398–402.
- Ye B, Zhou PY, Jia M, Cheng XS, Jia YT, Xu SG. (2013). Absence of NF-kappaB subunit p50 ameliorates cold immobilization stress-induced gastric ulcers. Biochem Biophys Res Commun 434:547–51.
- Yigiter M, Albayrak Y, Polat B, Suleyman B, Salman AB, Suleyman H. (2010). Influence of adrenal hormones in the occurrence and prevention of stress ulcers. J Pediatr Surg 45:2154–9.
- Zacharowski K, Zacharowski PA, Koch A, Baban A, Tran N, Berkels R, Papewalis C, et al. (2006). Toll-like receptor 4 plays a crucial role in the immune-adrenal response to systemic inflammatory response syndrome. Proc Natl Acad Sci USA 103:6392–7.
