Abstract
Physical effort is a common cost of acquiring rewards, and decreased effort is a feature of many neuropsychiatric disorders. Stress affects performance on several tests of cognition and decision making in both humans and nonhumans. Only a few recent reports show impairing effects of stress in operant tasks involving effort and cognitive flexibility. Brain regions affected by stress, such as the medial prefrontal cortex and amygdala, are also implicated in mediating effortful choices. Here, we assessed effort-based decision making after an acute stress procedure known to induce persistent impairment in shuttle escape and elevated plasma corticosterone. In these animals, we also probed levels of polysialyted neural cell adhesion molecule (PSA-NCAM), a marker of structural plasticity, in medial frontal cortex and amygdala. We found that animals that consistently worked for high magnitude rewards continued to do so, even after acute shock stress. We also found that PSA-NCAM was increased in both regions after effortful choice experience but not after shock stress alone. These findings are discussed with reference to the existing broad literature on cognitive effects of stress and in the context of how acute stress may bias effortful decisions to a rigid pattern of responding.
Introduction
To choose optimally, we must conduct cost-benefit analyses wherein the cost of emitting a reward-seeking action is weighed against the value of the reward. Common costs of reward-seeking actions include delay, risk and effort. Effort-based decision making has been modeled in rodents with effort requirements ranging from physical to attentional (Izquierdo & Belcher, Citation2012), to cognitive (Hosking et al., Citation2014). Tests of effort discounting have shown that, as the effort requirement to obtain a high magnitude reward increases, animals will shift behavior toward lower magnitude rewards with lower effort requirements (Ghods-Sharifi et al., Citation2009). A common feature of several neuropsychiatric disorders is decreased effort. This occurs in depression (Treadway et al., Citation2012) and schizophrenia (Fervaha et al., Citation2013; Gold et al., Citation2013). Similarly, effort is impaired in neurological disorders such as Parkinson’s disease (Friedman et al., Citation2010) and multiple sclerosis (Lapierre & Hum, Citation2007). Because both animals and humans engage in decisions with each option associated with different effort requirements and reward magnitudes, a particularly useful assay of effort-based decision making should involve competing options. Such an assessment would differ from other measures of motivation (e.g. lever pressing according to a progressive ratio requirement) in that animals are allowed to self-titrate the amount of effort they are willing to expend in the context of choice.
It is well established that effortful choices (and effort discounting) are modulated by dopamine signaling (Denk et al., Citation2005; Salamone & Correa, Citation2012), and by the engagement of basolateral amygdala (BLA), nucleus accumbens and medial frontal cortex (Floresco & Ghods-Sharifi, Citation2007; Hauber & Sommer, Citation2009; Ghods-Sharifi et al., Citation2009; Schweimer & Hauber, Citation2005). The brain regions involved in effortful choice are also affected by stress. Specifically, models of both brief and chronic uncontrollable stress cause dendritic retraction in a subregion of medial frontal cortex, infralimbic cortex (Cook & Wellman, Citation2004; Holmes & Wellman, Citation2009; Izquierdo et al., Citation2006) and exposure to aversive stimuli, such as in fear conditioning, increases dendritic spines in the BLA (Heinrichs et al., Citation2013). Stress also affects performance on several tests of cognition and decision making ranging from altered memory formation (Sun & Alkon, Citation2014) in humans to impaired reversal learning in rodents (Campeau et al., Citation2011). Recent reports show effects of stress in an operant task-assessing effort (Bryce & Floresco, Citation2016; Shafiei et al., Citation2012) and alterations in cognitive flexibility following exposure to a rat model of post-traumatic stress disorder (George et al., Citation2015). With these few exceptions, the effects of stress on effortful decision making have not been extensively reported.
Outlining the conditions under which effortful choice behavior is impacted after stress could aid in refining therapeutic targets for depression and anxiety. For this reason, we investigated the effects of an acute stress regimen that is known to induce learned helplessness in rats as measured by impaired shuttle escape wherein rats are required to cross a barrier repeatedly to terminate shock (Minor et al., Citation2001). We assessed shuttle escape latencies and corticosterone levels in shock-stressed animals. For the latter, we measured free corticosterone, the main glucocorticoid in rodents that is increased by a variety of stressors (McEwen, Citation2007; Lu et al., Citation2015; Wu et al., Citation2015). One group of rats was tested on an effortful choice task before and after shock stress, using a paradigm we have previously employed (Stolyarova et al., Citation2015). Finally, levels of polysialyted neural cell adhesion molecule (PSA-NCAM), a marker of structural plasticity, in medial frontal cortex and amygdala were also measured. PSA-NCAM is expressed in inhibitory interneurons and is well positioned to gate the excitability of projection neurons both in amygdala and in frontal cortex (Gascon et al., Citation2007; Gomez-Climent et al., Citation2011; Nacher et al., Citation2013).
Stress may bias animals away from the effortful, high reward choice – producing a work-averse phenotype – if learned helplessness were the dominant behavioral phenotype. Alternatively, as others have shown (Morgado et al., Citation2015), stress may instead narrow the behavioral repertoire and render animals more exploitative once a preferred response has been selected. Based on previous work, we predicted that PSA-NCAM expression would increase in medial frontal cortex and amygdala after stress.
Method
Subjects
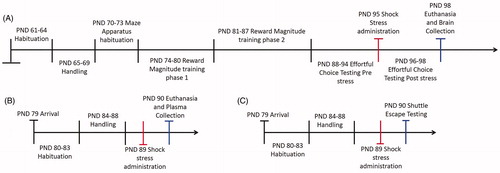
Subjects were 60 adult male Sprague-Dawley rats (Charles River Laboratories, Hollister, CA), singly housed. Sixteen rats were assessed for corticosterone 24 h following shock stress (n = 8) or restraint-only control (n = 8) conditions. A separate group of rats was assessed for their shuttle escape latencies 24 h following shock stress (n = 8) or restraint-only control (n = 8) conditions. Twelve rats were assessed for effortful choice behavior before and after shock stress (n = 12) and then brains were collected for PSA-NCAM expression assay in medial frontal cortex and amygdala. And finally, another group of sixteen rats was assessed for PSA-NCAM expression in these regions 24 h following shock stress (n = 8) or restraint-only control (n = 8). Animals arrived at the housing facility at postnatal day (PND) 60 for the effortful choice training and testing, PND 79 for corticosterone and shuttle escape testing (procedures described below). Animals that did not receive behavioral training in the maze apparatus were handled at a later PND than maze-trained animals so that tissue could be collected at approximately the same PND for all animals (including those that underwent maze testing), to minimize any effect of maturation on PSA-NCAM expression. All animals were handled for 5 d after a brief acclimation period in the vivarium. The vivarium was maintained under a 12/12 h light/dark cycle at 22 °C. All behavioral testing took place 5–7 d per week between 08:00 and 16:00 h during the rats’ inactive period. Shuttle escape and corticosterone groups were euthanized at PND 90, and rats that were given effortful choice testing and assessed for PSA-NCAM expression were euthanized at PND 98. All animals weighed between 300–310 g at the time of brain collection. A timeline of all procedures is shown in . All procedures were approved by the Chancellor’s Animal Research Committee at the University of California, Los Angeles.
Figure 1. Timeline of events. (A) Timeline of events for animals that underwent effortful choice testing, shock stress, and were analyzed for PSA-NCAM. (B) Timeline of events for animals that underwent no behavioral training and were analyzed instead for plasma corticosterone after shock stress. (C) Timeline of events for animals that received shock stress and were tested for shuttle escape latencies. Red tick marks denote shock stress administration days. Blue tick marks denote testing or euthanasia days.
Food restriction
Rats were left undisturbed for 3 d after arrival to our facility to acclimate to the vivarium. The rats (n = 12) to be assessed on effortful choice were food restricted to no less than 85% of their free-feeding body weight to ensure motivation to work for food for 5 d prior to and during behavioral testing, while water was always available ad libitum. On the last two days of food restriction prior to behavioral training, rats were fed 20 ½ froot loops in their home cage to accustom them to those rewards. Weights were monitored daily to ensure a healthy body weight.
Behavioral apparatus
Rats were tested on a task previously described in detail (Stolyarova & Izquierdo, Citation2015; Stolyarova et al., Citation2015). This apparatus was an 8-arm maze with the four arms nearest the start arm permanently blocked, leaving a start arm and three choice arms available to the animals. Three possible courses of action, each associated with different effort requirements and reward magnitudes were available: one arm of the maze was randomly designated as a low effort/reward (LER), another as a medium effort/reward (MER), and the third as a high effort/reward (HER) arm. The arms of the maze extended from the central arena 25 cm in diameter. The arms themselves were 50 cm long and 12 cm wide. The positions of extramaze cues remained constant throughout all phases of the experiment. The arm assignment was counterbalanced across animals and held constant between sessions. The arm containing the low reward was unimpeded by a barrier, but in order to obtain the medium or high reward, rats were required to climb a 20 cm or 30 cm barrier, respectively. Rats were required to climb straight up the side (90°) and down at an angle to the food reward located at the end of the goal arm. “Froot loops” (Kellogg NA Co., Battle Creek, MI) were given as food rewards during testing: a “high reward” consisted of four ½ froot loops (i.e. two froot loops), a “medium reward” consisted of two ½ froot loops (1 froot loop) and a “low reward” consisted of one ½ froot loop. Between trials, the rat was removed from the maze and placed in large, clear Plexiglas holding chamber with a 1651 cm2 base and 38.1-cm walls.
Shock stress
Restraint and tail shock stress occurred in clear-Plexiglas restraining tubes, measuring 23 cm in length and 6 cm in diameter. Adjustable front walls prevented the rats from moving forward in the tubes. The rat’s tail extended through the rear door of each tube and was taped to plastic rods. Unscrambled shock was delivered from one of four constant-current shock generators (Lafayette Instrument Co, Lafayette, IN, Model 82400) to electrodes attached to the rat’s tail with electrode paste and tape. Each tube was housed in a sound-attenuating box (44 × 64 × 36 cm) outfitted with an exhaust fan that masked outside noise. Illumination was provided by a 7-W houselight located in the center of the rear wall of the sound-attenuating box. Shock stress occurred 24 h prior to the first of 3 d of post-testing and consisted of 100, 1.0 mA tail shocks of 1–8 s variable duration with a variable ITI of 20–120 s. Shock stress sessions lasted 1.83 h. All animals that underwent effortful choice testing received shock stress. Restraint-only control animals used in the shuttle escape and corticosterone experiments were given the same treatment as shock stress, but shocks were not delivered.
Shuttle escape
Escape testing took place in a (45 cm ×20 cm ×20 cm) shuttle box (BRS-LVE model 146-40). The shuttle box was divided into two equal compartments by a metal barrier that had an 8 × 7 cm center opening flush with the grid floor. The floor consisted of 2-mm diameter stainless-steel rods spaced 1.1 cm apart center to center. Continuous scrambled shock was delivered to the grid floor from a Grason-Stadler (Series 700, West Concord, MA) shock generator. The floor pivoted in the center and a response was recorded when a 300 g rat's front paws touched the center grid in a compartment. Two 6-W lamps located in the center of each end wall provided constant illumination. The shuttle box was housed in a sound-attenuating chest, containing an exhaust fan that masked extraneous noise.
Shuttle-escape testing occurred 24 h after shock stress administration. The test consisted of five trials during which a rat had to cross from one side of the central barrier to the other to terminate shock (FR-1 trials). These trials occurred on a fixed-time 60-s schedule. FR-1 trials were followed by 25 FR-2 trials during which a rat had to cross from one side of the central barrier and then return to terminate shock. Shock terminated automatically if the appropriate response contingency was not met within 40 s of shock onset on a given trial. Escape latencies were recorded on each trial. Shock intensity was set at 0.6 mA with FR-2 trials occurring on a variable time 60-s schedule (range: 20–230 s); however, 3 min intervened between FR-1 and FR-2 trials (Haracz et al., Citation1988). Administration of FR-1 and FR-2 trials is based on previous work on helplessness in rats (Minor & LoLordo, Citation1984). Impaired performance was not evident in rats pre-exposed to inescapable shock when the task required less effort (FR-1 trials), but instead manifested once the response requirement was increased to FR-2. Furthermore, a 3-min delay was used because it increased the magnitude of the FR-2 escape deficit.
Plasma corticosterone levels
Twenty-four hours following shock stress, animals (n = 8 shock stress, n = 8 restraint-only control) were euthanized with 0.8 mL of Euthasol (Virbac Animal Health, 390 mg pentobarbital sodium and 50 mg phenytoin sodium per mL) decapitated, and trunk blood was collected in heparin-coated tubes. Samples were spun at 15,000 rpm for 15 min, after which plasma was collected and stored at −80 °C until analysis by ELISA (Cat # ADI-900-097, Enzo Life Sciences, Farmingdale, NY). Samples were diluted 1:9 with TBS, after which assays were performed according to the manufacturer’s instructions.
Effortful choice training and testing
Habituation
A habituation and training protocol adapted from (Walton et al., Citation2002) was used to habituate the rats to the maze and familiarize them with the froot loops. During the acclimation phase, five ½ froot loops were placed into each arm of the maze (4 arms, 20 total ½ froot loops). Each rat was individually placed into the maze and allowed to explore and eat froot loops freely. Criterion for advancement to the next phase was consumption of 20 ½ froot loops within 15 min
Reward magnitude training (Phase 1)
After a brief habituation period, one goal arm was baited with four ½ froot loops (HR arm), another with two ½ froot loops (MR arm), and the third arm with one ½ froot loop (LR arm). Rats were allowed to sample freely from all three arms for 10 trials. No barriers were present at this phase. Each trial lasted until the rat finished all the froot loops. Trials were separated by a 30-s intertrial interval (ITI), during which time the rats were placed in a holding chamber and the maze was wiped clean with 70% ethanol to prevent the rat’s use of odor-guided choice. The order of arm visits was recorded. Criterion for advancement to the next phase was completion of 10 trials within 30 min.
Reward magnitude training (Phase 2)
Here, animals were allowed to visit only one arm per trial. Rats were removed from the maze as soon as the arm was chosen and the reward was consumed. Animals were given 10 trials per day separated by a 30 s ITI. Criterion for advancement to “choice trials with barriers” was choice of HR arm on 80% or more of the trials for two consecutive days.
Effortful choice testing prestress
During this phase, rats were required to climb barriers for higher rewards. The LER arm continued to be unimpeded by a barrier, but in order to obtain the medium (MER) or high (HER) reward, rats were required to climb a 20 cm or 30 cm barrier, respectively. Thirteen trials were administered per day. Each day of testing consisted of 10 free and three forced choice (one for each arm) trials, administered at the beginning. Thus, the structure of the testing was as follows: forced choice trials (1 through 3), followed by ten free choice trials (4 through 13). On forced choice trials, all goal arms except one were blocked. The order of arm presentation during forced choice trials was counterbalanced between days. Upon eating the food reward, the rat was placed in a holding chamber for the 30 s ITI, and maze wiped clean with 70% ethanol. Rats were tested daily until stable baseline choice performance was established (choice preferences on free choice trials did not differ across three consecutive days).
Effortful choice testing poststress
Rats were retested the day after stress for three consecutive days. Testing was identical to the effortful choice testing.
Tissue dissection and ELISAs
Rats were euthanized 24 h after the last day of effortful choice testing (n = 12) or 24 h after shock stress/restraint-only control (n = 16) with an overdose of 0.8 mL of Euthasol and decapitated. The brains were immediately extracted and 2-mm-thick coronal sections of medial frontal cortex and amygdala were rapidly dissected, using a brain matrix, over wet ice at 4 °C. Frontocortical dissections included medial sectors of the frontal cortex, but excluded most of medial orbital and all of ventral and lateral subregions. Amygdala dissections were not specific to the basolateral nucleus, but instead included a large dissection of most nuclei. To prepare the tissues for the assays 0.3 mL (medial frontal cortex) or 0.2 mL (amygdala) of PBS (0.01 mol/L, pH 7.2) containing a protease and phosphatase inhibitor cocktail (aprotinin, bestatin, E-64; leupeptin, NaF, sodium orthovanadate, sodium pyrophosphate, β- glycerophosphate; Thermo Scientific, Rockford, IL) was added to each sample. Each tissue was minced, homogenized, sonicated with an ultrasonic cell disrupter, and centrifuged at 5000 g at 4 °C for 10 min. Supernatants were removed and stored at +4 °C until ELISA assays were performed (within 24 h). PSA-NCAM protein levels were determined using a commercially- available ELISA kit (Cat# 67-ABC0027B, ALPCO Diagnostics, Salem, NH). The assays were performed according to the manufacturer’s instructions. The sensitivity of the assays is 0.25 ng/mL for PSA-NCAM, and the detection range is 0.25–16 ng/mL. Bradford protein assays were also performed to determine total protein concentrations in each sample. The concentration of PSA-NCAM is presented as ng/mg of total protein accounting for dilution factor. Correlational analyzes were conducted between each rat’s percentage of HER, MER and LER choices and PSA-NCAM content in medial frontal cortex and amygdala.
Statistical analyses
Software package SPSS (SAS Institute, Inc., Version 16.0, Cary, NC) was used for statistical analyses. A repeated-measures analysis of variance (ANOVA) with stress condition as a between-subjects factor and trial block as a within-subjects factor was conducted for shuttle escape latencies. Following significant interactions, post hoc t-tests are reported. T-tests were conducted for corticosterone levels between shock stress and restraint-only controls. To calculate “stable performance” on effortful choice testing (prestress), we determined no effect of day on separate repeated-measures ANOVAs on HER, MER, and LER across 3 d. Repeated-measures ANOVAs for effortful choice behavior with day and stress status (pre–post) as within-subjects factors were also conducted. Amygdala and medial frontal cortex PSA-NCAM levels were analyzed using ANOVAs with effortful choice testing/stress, no effortful choice testing/stress, and no effortful choice testing/no stress as between-subjects factors. Finally, Pearson correlation coefficients are reported for associations between PSA-NCAM and effortful choice behavior. Statistical significance was noted when p values were less than 0.05, and a trend toward significance was noted when p values were 0.05–0.08.
Results
Shuttle escape behavior
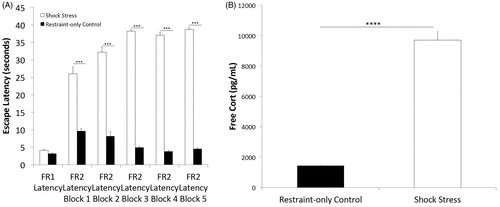
FR-1 escapes were characterized by running from one side of the shuttle box to the other in order to terminate shock. There were no significant differences in this kind of escape latency between shock stress and restraint-only control animals (unpaired t-test, t(14) = 1.574, p = .138, means = 4.12 and 3.15 s, respectively). When analyzing FR-2 escape latencies, where rats were required to run from one side of the shuttle box to the other, and back again in order to terminate shock, a repeated-measures ANOVA with stress condition as a between-subjects factor revealed a significant main effect of group such that shock stress rats were slower to escape (f(1,14) = 246.035 p < .0001), and a significant group by trial block interaction (f(4,56) = 10.914 p < .0001), but no significant within-subject effect of trial block (f(4,56) = 1.694 p = .164). This suggests impaired performance was manifested only with an increased effort requirement. Post hoc analysis revealed simple main effects of stress condition at each trial block (unpaired t-test, all p’s <.01) ().
Figure 2. Effects of shock stress on shuttle escape performance and plasma corticosterone. (A) Shock stress significantly impaired shuttle escape performance, increasing escape latencies across all five trial blocks (1–5) each constituting five trials (25 trials total) on the FR-2 schedule. (B) Shock stress significantly increased plasma levels of free corticosterone. Error bars denote mean + SEM. ****p< .0001 and ****p <.00001.
Plasma corticosterone levels
Free corticosterone was elevated in shock stress animals 24 h after stress, consistent with previous reports showing elevation of corticosterone after stress at this time point (Ling & Jamali, Citation2003), indicating that our shock stress was stressful. We found significantly increased plasma corticosterone levels in our shock stress animals (mean = 9704 pg/mL, standard error = 1096) relative to restraint-only controls (mean = 1438 pg/mL, standard error = 15) (unpaired t-test, t(14) = 7.539, p < .00001) ().
Effortful choice behavior
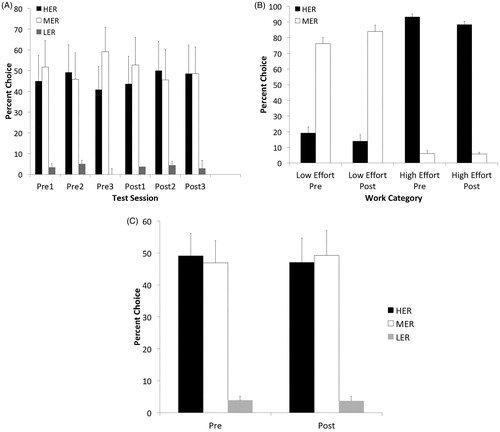
A repeated-measures ANOVA was conducted for the HER, MER and LER choice. This test revealed shock stress had no effect on the established pattern of LER, MER and HER choices. On post-stress day, mean percentages of HER, MER, and LER choices were not different from baseline (means = 40.83%, 59.17% and 0%, respectively) . A repeated-measures ANOVA for each choice with day (3 d) and stress status (pre–post) as within-subjects factors was conducted (shown as 3-d mean in ). This revealed no effect of stress (f(1,10) = 0.77 p = .401), day (f(2,20 = 1.394, p = .271), and no significant stress by day interaction (f(2,20) = 0.499, p = .614) for the HER choice. A separate repeated-measures ANOVA revealed no effect of stress (f((1,10) = .290, p = .602), day (f(2,20) = 2.456 p = .111), and no significant stress by day interaction (f(2,20) = 0.661, p = .527) for the MER choice. Another repeated-measures ANOVA revealed no effect of stress (f(1,10) = 0.214, p = .653), day (f(2,20) = 1.023, p = .377), and no significant stress by day interaction (f(2,20) = 0.342, p = .715) for the LER choice.
Figure 3. Effects of shock stress on effortful choice behavior. (A) Rats were assessed on effortful choices before (pre) and after (post) shock stress. Shock stress had no effect on the pattern of HER, MER, and LER choices. (B) “High effort rats”, defined as HER preferring animals, and “Low effort rats”, defined as MER preferring animals, were not differentially affected by shock stress (pre = before shock stress; post = after shock stress). (C) Mean percent choice across 3 d prior to shock stress (Pre) and 3 d post shock stress (Post). Error bars denote mean + SEM.
HER and MER preference
Rats’ choice performance was analyzed on an “effort score”. This was a sum of their three prestress session preferences. For example, an animal would have an effort score of 300, had the animal chosen the HER on 100% of trials across 3 d. Using this method, we were able to generate criteria for HER-preferring rats (>250 and above HER) and MER-preferring rats (>220 and above MER, instead of HER). Few animals chose the LER with any consistency. We then designated the animals “high effort” rats (n = 5) and “low effort” rats (n = 7), by binning them according to their prestress preferences. Though there were such individual differences in their preference for HER and MER, no differences emerged poststress. High-effort rats chose the HER arm on 93.33% of trials prior to shock stress and on 88.33% of trials postshock stress. Similarly, Low-effort rats chose the MER arm on 76.19% of trials prior to stress and on 84% of trials post stress. A repeated-measures ANOVA for HER and MER choices with day and stress status (pre-post) as within-subjects factors was conducted for both high-effort rats and low-effort rats. For low-effort rats, this revealed no effect of stress (f(1,6) = 3.028 p = .132), day (f(2,12 = 0.596, p = .597), and no significant stress by day interaction (f(2,12) = 0.933, p = .420) for the HER choice. A separate repeated-measures ANOVA revealed no effect of stress (f((1,6) = 3.323, p = .118), day (f(2,12) = 0.585 p = .572), and no significant stress by day interaction (f(2,12) = 1.044, p = .382) for the MER choice. For high-effort rats, this revealed no effect of stress (f(1,4) = 1.054 p = .363), day (f(2,8 = 1.605, p = .259), and no significant stress by day interaction (f(2,8) = 0.153, p = .861) for the HER choice. A separate repeated-measures ANOVA revealed no effect of stress (f((1,4) = 0.096, p = .772), day (f(2,8) = 0.585 p = .759), and no significant stress by day interaction (f(2,8) = 0.681, p = .533) for the MER choice ().
PSA-NCAM levels
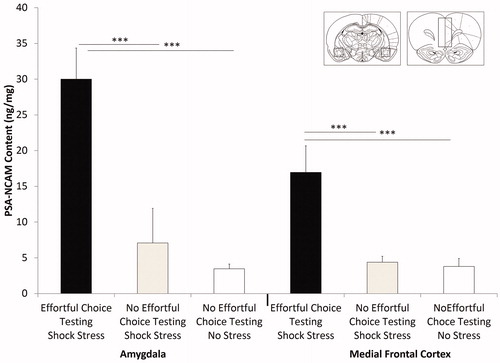
Tissue from medial frontal cortex was analyzed using ANOVA with effortful choice testing/stress, no effortful choice testing/stress, and no effortful choice testing/no stress as levels of the between-subjects factor (i.e. group). This analysis revealed that there was a main effect of group (f(2,24) = 7.938, p = .002). Post hoc tests revealed that PSA-NCAM was increased in animals that received effortful choice testing and stress (p = .009). Amygdalar tissue was similarly analyzed with effortful choice testing/stress, no effortful choice testing/stress, and no effortful choice testing/no stress as three levels of the between-subjects factor. This test also revealed a main effect of group (f(2,23) = 14.638 p < .001). Post hoc tests revealed that PSA-NCAM was higher in effortful choice testing/stressed animals (p = .001). Amygdalar PSA-NCAM expression trended toward a moderate positive correlation with HER choices (r = 0.55, p = .08). Similarly, amygdalar PSA-NCAM expression trended toward a moderate negative correlation with MER choices (r = −0.56, p = .07). ( inset shows matched coronal sections with approximate dissection boundaries).
Figure 4. Effects of shock stress and effortful choice testing on PSA-NCAM. Rats that had effortful choice experience and shock stress exhibited significantly higher levels of PSA-NCAM in both the amygdala and medial frontal cortex relative to rats with no effortful choice testing and no stress and those that received shock stress alone. Inset shows matched coronal sections with approximate dissection boundaries for amygdala (left) and medial frontal cortex (right). Error bars denote mean + SEM. ***p < .0001.
Discussion
Our acute stress procedure increased plasma corticosterone over six-fold and resulted in profound deficits in shuttle escape performance. Yet, despite the severity of this stressor, we found that rats exhibited an identical pattern of effortful choices as prior to stress. Consistent with a previous finding in another strain of rats (Stolyarova & Izquierdo, Citation2015), we found that rats that had effortful choice experience and behavioral training (that included food restriction), expressed elevated levels of PSA-NCAM in both medial frontal cortex and amygdala. Also as in the previous study (Stolyarova & Izquierdo, Citation2015), this effect was greatest in the amygdala. Interestingly, the level of PSA-NCAM expressed in rats with both effortful choice and stress experience was greater than what we have previously reported for adult animals with just effortful choice experience alone (though different strains). Conversely, PSA-NCAM, a marker that was previously found to be dysregulated after more chronic restraint stress (Nacher et al., Citation2013; Pham et al., Citation2003) was unchanged in both medial frontal cortex and amygdala after our acute stress procedure alone.
Stress in effort
The pattern of effortful choices exhibited by rats supports an exploitative, rigid pattern of responding following acute stress. Indeed, our animals required several weeks of training to reach stable performance, which may have rendered their behavior habitual. Despite experiencing forced trials for each choice prior to each session, rats exhibited strong spatial response preferences. In humans, it has been shown that stress can render behavior habitual (Schwabe & Wolf, Citation2009, Citation2011), impairs goal-directed control of behavior, and this effect also occurs in rodents (Dias-Ferreira et al., Citation2009). Therefore, it is possible that our stress manipulation-biased animals toward a perseverative pattern of responding.
As stated in the introduction, stress has been found to produce effects on a wide range of cognitive functions (Campeau et al., Citation2011; Sun & Alkon, Citation2014; George et al., Citation2015). We were particularly interested in the potential effect of stress on cost-benefit (effortful) decision-making, a cognitive process that is often affected in neuropsychiatric conditions (Salamone et al., Citation2016). Given that the stressed rats exhibited work aversion in our shuttle escape task (when experiencing an increased response requirement from FR-1 to FR-2), and given there have been previous reports of stress impairing effort in an operant lever-pressing task (Bryce & Floresco, Citation2016; Shafiei et al., Citation2012), a lack of a work averse pattern for reward was somewhat surprising. However, critical task differences offer a potential explanation. and Bryce and Floresco (Citation2016) and Shafiei et al. (Citation2012) gave animals a choice between a low-value reward and high-value reward alternative, while the former value was kept constant, the effort requirement for the high reward increased as a function of trial block, within a single session. Unlike our task, those methods required the rat to track the effort requirement changes and adjust choice behavior more dynamically. To control for differences in processing cost changes and value updating, we trained animals to choose between three possible courses of action, each associated with different but fixed effort requirements and reward magnitudes. The present task, with increased option space, may better mimic decision-making problems faced by animals in their natural environment, yet this paradigm limited our ability to introduce dynamic changes to the effort costs. It also remains to be determined how more naturalistic settings with some semblance of foraging (maze tasks) versus automated platforms might influence outcomes.
Rigid choices following stress may be attributed to an inability to successfully represent or remember the variability of options to choose from and/or, an inability to cope with ambiguity or uncertainty. In humans, acute stress interferes with a computationally expensive, model-based choice strategy (Otto et al., Citation2013). In our study, the outcome values were constant through time, conversely in the progressive ratio task employed by Shafiei et al., (Citation2012) the value of the high reward/effort option varied as a function of trial block. Their results demonstrate that following an hour-long stress, rats chose HR options as frequently as they did during the last block of prestress session, suggesting that their choice behavior was guided by the most recently-experienced option. Stressed animals may be unable to retrieve the more elaborate representations of their reward environment, rendering their choice behavior more sensitive to the most recent outcome. Therefore, reduced temporal complexity of the reward environment in our task with stable outcome values may have contributed to the inflexible nature of choice behavior. As described earlier, our finding is instead consistent with the idea that stress renders animals more rigid in their behavioral repertoire and exploitative in their decision making (Morgado et al., Citation2015).
Our stress manipulation was previously used as a rat model of learned helplessness (Haracz et al., Citation1988; Minor & LoLordo, Citation1984). It is noteworthy that with lack of options to choose from, the shock stress did greatly reduce physical effort in the absence of food rewards (in the shuttle escape context). Importantly, learned helplessness is context-specific and occurs readily if the shock apparatus and shuttle box have a common odor cue (Minor & LoLordo, Citation1984). However, shock stress and effortful choice testing took place in entirely different contexts, and the maze apparatus was cleaned prior to every test session and between trials within sessions. It is possible that a robust effect of stress on effort-related choice behavior would be dependent on some level of contextual overlap with the stress apparatus.
An additional consideration is rat strain. Our subjects were Sprague-Dawley rats, whereas others have used Long-Evans rats (Shafiei et al., Citation2012). Although we are not aware of a systematic investigation of strain effects on decision making paradigms following exposure to stress, at least one previous study revealed different biochemical responses to an acute stress following chronic unpredictable mild stress between the two strains (Bielajew et al., Citation2002).
A final consideration is that rats rarely chose the low effort option in our task. Given that wheel running exercise, a form of physical exertion similar to barrier climbing, is known to be reinforcing (Belke & Wagner, Citation2005; Kagan and Berkun, Citation1954; Thompson et al., Citation2015), this presents the possibility that rats experienced the barrier climbing to be similarly reinforcing. Since animals consumed all of the food on all trials, it is clear that motivation to procure food also contributed to our results, yet the present data do not rule out the act of foraging by climbing itself as contributing a unique source of enrichment. Rats subjected to a shift from a low to a higher work requirement for food exhibit elevated ventral striatal dopamine release (Segovia et al., Citation2011) in line with idea that physical workloads may be a source of reward and enrichment. Future work should investigate how the potential reinforcing components of foraging or work output may mitigate the effects of stress.
Synaptic remodeling (PSA-NCAM)
PSA-NCAM was elevated in both medial frontal cortex and amygdala in animals that experienced effortful choice testing, consistent with previous work from our lab (Stolyarova & Izquierdo, Citation2015), but not after stress alone. PSA-NCAM is a cell adhesion molecule that is linked to morphological changes in the hippocampus (Seki & Arai, Citation1993), and previous work has demonstrated that conditions that induce dendritic reorganization, such as repeated restraint stress, also induce PSA-NCAM expression in the hippocampus (Sandi et al., Citation2001). Given that PSA-NCAM expression is restricted to amygdalar interneurons (Nacher et al., Citation2013) and that stress has effects on amygdalar interneurons (Wolff et al., Citation2014), PSA-NCAM was an appropriate marker of stress-related changes in the present experiment. There is previous work demonstrating that chronic stress reduces amygdalar PSA-NCAM expression (Cordero et al., Citation2005), yet acute stress may have a different mechanism of effect.
Importantly, we used a within-subject design wherein all of the animals that received effortful choice testing in the maze apparatus also received shock stress (there was not an effortful choice testing/no-stress group). The present data therefore cannot rule out whether it was effortful choice experience alone, or effortful choice interacting with stress that caused increased PSA-NCAM expression. The lack of effect of stress alone on PSA-NCAM could be due to our acute manipulation, as previous reports of stress impacting this molecule used chronic stress (Cordero et al., Citation2005). Our observation of increased PSA-NCAM levels in animals that received effortful choice testing suggests a potential role of environmental enrichment in ameliorating the effects of stress on plasticity in the amygdalar inhibitory network. It would be interesting to test whether reward experience introduced prior to or during stress exposure can counteract changes in PSA-NCAM levels.
Given that our lab previously showed that PSA-NCAM expression in frontal cortex was significantly correlated with HER choices (Stolyarova & Izquierdo, Citation2015), it is surprising that we found a trend toward this effect in the amygdala, not medial frontal cortex. Importantly, that finding was reported for adolescent Long-Evan rats, and the animals used in this work were Sprague-Dawley adults. This points to a need for systematic evaluation of maturation, strain, and sex differences on this molecule.
Future work should investigate the role of habits in effortful choice behavior and assess synaptic remodeling measures in the striatum. We also found a bimodal distribution in the preference of effortful choices between rats. Previous work on a progressive ratio effortful choice task (Randall et al., Citation2012) attributed similar effects to individual differences in dopamine-related signal transduction. A neural mechanism for these individual differences related to preferences in effort allocation should be explored further.
Conclusions
We found that animals that consistently worked for high-magnitude rewards continued to do so, even after experiencing acute shock stress. This evidence supports the idea that stress narrows the behavioral repertoire and renders animals more exploitative once a preferred response is selected, consistent with Morgado et al. (Citation2015). This observed pattern of responses may manifest only if effort requirements are fixed and if animals have considerable training and experience with the task. Our data are generally also consistent with the view that a single stressful episode can produce perseverative, rigid behavioral tendencies to reward, resistance to fear extinction (George et al., Citation2015; Moench et al., Citation2015, Schwabe & Wolf, Citation2009, Citation2011), and a compulsive, not impulsive, behavioral phenotype particularly if experienced during development (Brydges et al., Citation2015). It should be noted that this rigid pattern of responding is not observed after milder acute stressors (Butts et al., Citation2013). Finally, we also found that PSA-NCAM was increased in both amygdala and medial frontal cortex after effortful choice and shock experience but not after shock stress alone, suggesting that there is appreciable synaptic remodeling in the areas that modulate stress effects on motivated, reward seeking behavior.
Acknowledgements
The authors would like to thank Ariel Lee and Nina Christie for their assistance with conducting behavioral testing and Andrew Thompson for useful feedback on the manuscript.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Belke TW, Wagner JP. (2005). The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes 68:165–172.
- Bielajew C, Konkle AT, Merali Z. (2002). The effects of chronic mild stress on male Sprague-Dawley and long evans rats: I. biochemical and physiological analyses. Behav Brain Res 136:583–592.
- Bryce CA, Floresco SB. (2016). Perturbations in effort-related decision-making driven by acute stress and corticotropin-releasing factor. Neuropsychopharmacology 41:2147–2159.
- Brydges NM, Holmes MC, Harris AP, Cardinal RN, Hall J. (2015). Early life stress produces compulsive-like, but not impulsive, behavior in females. Behav Neurosci 129:300–308.
- Butts KA, Floresco SB, Phillips AG. (2013). Acute stress impairs set-shifting but not reversal learning. Behav Brain Res 252:222–229.
- Campeau S, Liberzon I, Morilak D, Ressler K. (2011). Stress modulation of cognitive and affective processes. Stress 14:503.
- Cook S, Wellman CL. (2004). Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60:236–248.
- Cordero MI, Rodriguez JJ, Davies HA, Peddie CJ, Sandi C, Stewart MG. (2005). Chronic restraint stress down-regulates amygdaloid expression of polysialylated neural cell adhesion molecule. Neuroscience 133:903–910.
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. (2005). Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology (Berl) 179:587–96.
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325:621–625.
- Fervaha G, Foussias G, Agid O, Remington G. (2013). Neural substrates underlying effort computation in schizophrenia. Neurosci Biobehav Rev 37:2649–2665.
- Floresco SB, Ghods-Sharifi S. (2007). Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex 17:251–260.
- Friedman JH, Alves G, Hagell P, Marinus J, Marsh L, Martinez-Martin P, Goetz CG, et al. (2010). Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson's disease. Mov Disord 25:805–822.
- Gascon E, Vutskits L, Kiss JZ. (2007). Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons. Brain Res Rev 56:101–118.
- George SA, Rodriguez-Santiago M, Riley J, Abelson JL, Floresco SB, Liberzon I. (2015). Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behav Brain Res 286:256–264.
- Ghods-Sharifi S, St Onge JR, Floresco SB. (2009). Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci 29:5251–5259.
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. (2013) Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry 74:130–136.
- Gomez-Climent MA, Guirado R, Castillo-Gomez E, Varea E, Gutierrez-Mecinas M, Gilabert-Juan J, Garcia-Mompo C, et al. (2011). The polysialylated form of the neural cell adhesion molecule (PSA-NCAM) is expressed in a subpopulation of mature cortical interneurons characterized by reduced structural features and connectivity. Cereb Cortex 21:1028–1041.
- Haracz JL, Minor TR, Wilkins JN, Zimmermann EG. (1988). Learned helplessness: an experimental model of the DST in rats. Biol Psychiatry 23:388–396.
- Hauber W, Sommer S. (2009). Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex 19:2240–2247.
- Heinrichs SC, Leite-Morris KA, Guy MD, Goldberg LR, Young AJ, Kaplan GB. (2013). Dendritic structural plasticity in the basolateral amygdala after fear conditioning and its extinction in mice. Behav Brain Res 248:80–84.
- Holmes A, Wellman CL. (2009). Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev 33:773–783.
- Hosking JG, Cocker PJ, Winstanley CA. (2014). Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology 39:1558–1567.
- Izquierdo A, Belcher AM. (2012). Rodent models of adaptive decision making. Methods Mol Biol 829:85–101.
- Izquierdo A, Wellman CL, Holmes A. (2006). Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26:5733–5738.
- Kagan J, Berkun M. (1954). The reward value of running activity. J Comp Physiol Psychol 47:108.
- Lapierre Y, Hum S. (2007). Treating fatigue. Int MS J 14:64–71.
- Ling S, Jamali F. (2003). Effect of cannulation surgery and restraint stress on the plasma corticosterone concentration in the rat: application of an improved corticosterone HPLC assay. J Pharm Pharm Sci 6:246–251.
- Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL, Zhang QJ, et al. (2015). Sex differences in the stress response in SD rats. Behav Brain Res 284:231–237.
- McEwen BS. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904.
- Minor TR, LoLordo VM. (1984). Escape deficits following inescapable shock: The role of contextual odor. J Exp Psychol Anim Behav Process 10:168–181.
- Minor TR, Rowe MK, Soames Job RF, Ferguson EC. (2001). Escape deficits induced by inescapable shock and metabolic stress are reversed by adenosine receptor antagonists. Behav Brain Res 120:203–212.
- Moench KM, Maroun M, Kavushansky A, Wellman C. (2015). Alterations in neuronal morphology in infralimbic cortex predict resistance to fear extinction following acute stress. Neurobiol Stress 3:23–33.
- Morgado P, Sousa N, Cerqueira JJ. (2015). The impact of stress in decision making in the context of uncertainty. J Neurosci Res 93:839–947.
- Nacher J, Guirado R, Castillo-Gomez E. (2013). Structural plasticity of interneurons in the adult brain: role of PSA-NCAM and implications for psychiatric disorders. Neurochem Res 38:1122–1133.
- Otto AR, Raio CM, Chiang A, Phelps EA, Daw ND. (2013). Working-memory capacity protects model-based learning from stress. Proc Natl Acad Sci USA 110:20941–20946.
- Pham K, Nacher J, Hof PR, McEwen BS. (2003). Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17:879–886.
- Randall PA, Pardo M, Nunes EJ, Lopez Cruz L, Vemuri VK, Makriyannis A, Baqi Y, et al. (2012). Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One7:e47934.
- Salamone JD, Correa M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485.
- Salamone JD, Yohn SE, Lopez-Cruz L, San Miguel N, Correa M. (2016). Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology. Brain 139:1325–1347.
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. (2001). Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience 102:329–339.
- Schwabe L, Wolf OT. (2009). Stress prompts habit behavior in humans. J Neurosci 29:7191–7198.
- Schwabe L, Wolf OT. (2011). Stress-induced modulation of instrumental behavior: from goal-directed to habitual control of action. Behav Brain Res 219:321–328.
- Schweimer J, Hauber W. (2005) Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem 12:334–342.
- Segovia KN, Correa M, Salamone JD. (2011). Slow phasic changes in nucleus accumbens dopamine release during fixed ratio acquisition: a microdialysis study. Neuroscience 196:178–188.
- Seki T, Arai Y. (1993). Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci 13:2351–2358.
- Shafiei N, Gray M, Viau V, Floresco SB. (2012). Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37:2194–2209.
- Stolyarova A, Izquierdo A. (2015). Distinct patterns of outcome valuation and amygdala-prefrontal cortex synaptic remodeling in adolescence and adulthood. Front Behav Neurosci 9:115.
- Stolyarova A, Thompson AB, Barrientos RM, Izquierdo A. (2015). Reductions in frontocortical cytokine levels are associated with long-lasting alterations in reward valuation after methamphetamine. Neuropsychopharmacology 40:1234–1242.
- Sun MK, Alkon DL. (2014). Stress: perspectives on its impact on cognition and pharmacological treatment. Behav Pharmacol 25:410–424.
- Thompson AB, Stolyarova A, Ying Z, ZHuang Y, Gómez-Pinilla F, Izquierdo A. (2015). Methamphetamine blocks exercise effects on Bdnf and Drd2 gene expression in frontal cortex and striatum. Neuropharmacology 99:658–664.
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. (2012). Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 121:553–558.
- Walton ME, Bannerman DM, Rushworth MF. (2002). The role of rat medial frontal cortex in effort-based decision making. J Neurosci 22:10996–1003.
- Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, Herry C, et al. (2014) Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509:453–458.
- Wu XY, Hu YT, Guo L, Lu J, Zhu QB, Yu E, Wu JL, et al. (2015). Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol Behav 145:118–121.
