Abstract
Hypothalamic–pituitary–adrenal (HPA) axis functioning is characterized by the baseline production of cortisol following a circadian rhythm, as well as by the superimposed production of cortisol in response to a stressor. However, it is relatively unknown whether the basal cortisol circadian rhythm is associated with the cortisol stress response in children. Since alterations in cortisol stress responses have been associated with mental and physical health, this study investigated whether the cortisol circadian rhythm is associated with cortisol stress responses in 6-year-old children. To this end, 149 normally developing children (Mage = 6.09 years; 70 girls) participated in an innovative social evaluative stress test that effectively provoked increases in cortisol. To determine the cortisol stress response, six cortisol saliva samples were collected and two cortisol stress response indices were calculated: total stress cortisol and cortisol stress reactivity. To determine children’s cortisol circadian rhythm eight cortisol circadian samples were collected during two days. Total diurnal cortisol and diurnal cortisol decline scores were calculated as indices of the cortisol circadian rhythm. Hierarchical regression analyses indicated that higher total diurnal cortisol as well as a smaller diurnal cortisol decline, were both uniquely associated with higher total stress cortisol. No associations were found between the cortisol circadian rhythm indices and cortisol stress reactivity. Possible explanations for the patterns found are links with children’s self-regulatory capacities and parenting quality.
The hypothalamic–pituitary–adrenal (HPA) axis has as primary hormonal end product, the hormone cortisol (e.g. Lupien et al., Citation2009). Baseline HPA-axis functioning is characterized by a 24 h cortisol circadian rhythm consisting of high morning cortisol concentrations followed by a gradual decline till nadir (e.g. Edwards et al., Citation2001; Kirschbaum & Hellhammer, Citation1989). Superimposed on this rhythm, the HPA-axis also produces cortisol in response to stressors (e.g. Dickerson & Kemeny, Citation2004; Nicolson, Citation2007). Both of these normative patterns are characterized by individual differences (e.g. Karlamangla et al., Citation2013; Kudielka et al., Citation2009). This study investigated associations between these two aspects of HPA-axis functioning in normally developing children at the beginning of elementary school.
In many countries the beginning of elementary school marks the start of achievement monitoring, raising teachers’ and parents’ expectations. Furthermore, at this age impression management/self-presentation is used (first seen in 5-year-olds; Engelmann et al., Citation2012) and feelings of relief and regret develop (between age 4 and 7; Weisberg & Beck, Citation2012). Hence, assumingly at this age children become more exposed and sensitive to social evaluative stressors that can trigger a cortisol stress response (Dickerson & Kemeny, Citation2004).
Since alterations in cortisol stress responses have been associated with mental and physical health (e.g. Buske-Kirschbaum et al., Citation2003; Hankin et al., Citation2010; Luby et al., Citation2003) it is important to understand its correlates. Uncovering associations between the cortisol circadian rhythm and cortisol stress responses may reveal how certain aspects of circadian cortisol dynamics may in part be predictive of children’s physiological capacity to cope with stressors. Dynamics of the cortisol circadian rhythm may facilitate or inhibit efficient cortisol stress responses.
Research in adults between the age of 54 and 76, showed positive associations between total daily cortisol concentrations and the magnitude of the cortisol stress response. However, no associations were found between diurnal cortisol decline and the magnitude of the cortisol stress response (Kidd et al., Citation2014) or, in 27- to 57-year-olds, between (basal) diurnal cortisol concentrations and the cortisol stress response (van Eck et al., Citation1996).
Research in 1.5- to 5-year-old (predominantly adopted) children showed that both lower morning cortisol concentrations and blunted diurnal change were associated with blunted cortisol stress responses (Koss et al., Citation2016). However, research on the dynamics of HPA-axis functioning in normally developing children at the beginning of elementary school is still scarce.
Hence, we investigated associations between the cortisol circadian rhythm and cortisol stress responses in normally developing 6-year-olds. An age-appropriate social evaluative stressor (de Weerth et al., Citation2013a), was used to provoke cortisol stress responses. The cortisol circadian rhythm was operationalized as total diurnal cortisol and diurnal cortisol decline (e.g. Nater et al., Citation2013; Saridjan et al., Citation2010; Simons et al., Citation2015; Watamura et al., Citation2004). Associations of these two circadian indices with two indices of cortisol stress responses (total stress cortisol and cortisol stress reactivity) were investigated. Directions of these associations, as well as the interactive effect of both circadian indices, were explored.
Methods
Participants
This study was a part of an ongoing longitudinal project focusing on psychobiological factors in child development (BIBO project; Radboud University). The original project and the 6-year data collection were approved by the Institutional Review Board, which adheres to the Helsinki Declaration (ECG 300107 and ECG 22111/130112, respectively). Originally, a total of 220 pregnant mothers enrolled in the project, and 193 mother-child dyads were still participating when the child was 3 months old (for details, see Beijers et al., Citation2011, Citation2013a,Citationb). Around the 6th birthday of the child, the 188 mother–child dyads still in the project were invited to take part in the current data collection. Parents who accepted the invitation were asked to sign an informed consent form. Of the invited group, 149 children participated in a school visit containing a social evaluative stress test (Mage = 6.09; SD = 0.14; Min = 5.87, Max = 6.85; 70 girls). Reasons for nonparticipation were: the school or the child chose not to participate (n = 4), the family had moved abroad (n = 3), or other reasons (e.g. parents perceived the procedure as too challenging for their child, parents perceived the study as too intensive, or personal reasons, n = 32). The group of children that did not participate in the current data collection after the invitation (n = 39) did not differ significantly from the participating group in maternal educational level during pregnancy, maternal age at delivery, gender of the child, and child age 4 temperament (Children’s Behavior Questionnaire short form; CBQ short form; Putnam & Rothbart, Citation2006), all p’s > .050.
Of the 149 children, five were excluded from the current study because of irregularities in the six cortisol saliva samples collected during the school visit to determine the cortisol stress response. Specific reasons were: the use of medication that potentially affects cortisol concentrations (n = 3), large time deviations from the saliva sampling protocol (n = 1), and all six samples were missing because the child refused to participate in saliva sampling (n = 1). This yielded a final sample of 144 children (Mage = 6.09; SD = 0.14; Min = 5.87, Max = 6.85; 68 girls).
Procedure
Cortisol circadian rhythm
To determine children’s cortisol circadian rhythm, mothers were asked to collect eight saliva samples of their child during two weekend days at four predefined time points: immediately after the child woke up (T1), at 11:00 h (T2), at 15:00 h (T3), and at 19:00 h (T4) (Simons et al., Citation2015). Of the 144 mother–child dyads in the final sample, 138 participated in collecting circadian saliva samples.
Cortisol stress responses
To assess cortisol stress responses children were exposed to a social evaluative stress paradigm, the Children’s Reactions to Evaluation Stress Test (CREST; de Weerth et al., Citation2013a; Simons et al., Citation2016). The CREST contains elements of unpredictability and uncontrollability, and is carried out in front of a judge that evaluates the child’s performance (social-evaluation). The test consists of three forced-failure tasks with a total duration of 15 min, followed by a 5-minute period in which the child is anticipating a final performance evaluation by the judge. Specifically, the child is asked to perform three tasks in front of a judge who evaluates the child’s performance and rewards good performance with a present chosen in advance by the child. In the first task, the child is asked to stand as still as possible with an alarm clicked onto his/her clothing. The child is told that the alarm will go off with movement. However, independent of actual movement the alarm goes off twice within the total task duration of 1 min. In the second task, the child is played a tape of a story in which eight animals are mentioned, each followed by a pause. In this task (3 min), the child is asked to provide the sound made by each animal in the subsequent pause, and is told that the judge will show a green card upon good performance. However, independently of the child’s actual performance the judge only shows the green card in three out of eight sounds. In the third task (3 min) the child is asked to build a pyramidal tower of horizontally lying cans, imitating a tower shown by the researcher. The child is told that this task is considered easy by peers, whereas in reality it is almost impossible. After these three tasks the judge leaves for 5 min to decide on the child’s performance. Upon return, the judge rewards the child for good performance with the chosen present (for more details, see de Weerth et al., Citation2013a). Subsequentely, children are debriefed and assured again that they performed well. This procedure was found to be stressful in an earlier independent study on 5- to 6-year-old children (n = 42), as indicated by a significant increase in cortisol concentrations in response to the test (de Weerth et al., Citation2013a). Following the test, children were allowed to draw and watch movies during a 25-minute recovery phase, followed by 25 min in which they participated in tasks unrelated to the present study. This procedure took place in the afternoon (start of visits between 12:15 and 15:15 h; M = 13:34 h, SD = 0:21) of a regular school day in a mobile laboratory parked near the child’s school (or home, n = 7 of 144).
During this procedure, six saliva samples (C1–C6) were collected from each child. C1 was collected just before the CREST started. C2–C6 were collected 15, 25, 35, 50 and 58 min after the start of the CREST, respectively. As in the original CREST paradigm, C1 and C2 represent baseline cortisol concentrations (de Weerth et al., Citation2013a). Children were asked to refrain from eating, drinking, or being physically active in the 30 min prior to the school visit. Cortisol increases in saliva as a response to a stressor can best be measured from 21–30 min after stressor onset on (Dickerson & Kemeny, Citation2004). Hence, C3 and C4 represent cortisol concentrations in response to the stressor. C5 and C6 represent recovery cortisol concentrations. To control for potential effects of illnesses on cortisol concentrations, the procedure was rescheduled if a child was ill. Children with a cold on the testing day (n = 7 of 144) did not differ significantly in their cortisol concentrations from the rest of the group (all p’s > .050).
Measures
Cortisol analyses
Cortisol saliva samples for both the cortisol circadian rhythm and cortisol stress responses were collected using eye sponges (BD Visispeare, Waltham, MA; de Weerth et al., Citation2007). At each saliva sampling moment, participants were asked to put two eye sponges in their mouth and saturate them with saliva. Back at the lab, eye sponges were centrifuged to obtain the saliva which was stored in a freezer (−25 °C). Cortisol analyses were carried out at the Laboratory of Endocrinology of the University Medical Center Utrecht (for details, see Simons et al., Citation2015, Citation2016).
Cortisol circadian rhythm
Of the potential 1104 circadian saliva samples of the 138 children, 1023 analyzable samples were obtained. Of the analyzable samples 69 were eliminated (6.7%) because of large deviations from standard sampling times (for details, see Simons et al., Citation2015), illnesses, the use of medication that potentially affects cortisol concentrations, or biologically extreme concentrations. presents the average diurnal cortisol concentrations (nmol/L) as well as standard errors for each of the four diurnal cortisol saliva sampling moments.
Figure 1. Diurnal cortisol concentrations (nmol/L) for each of the four sampling moments (based on average scores over both sampling days). Error bars stand for one standard error above and one beneath the mean of each of the four diurnal sampling moments.
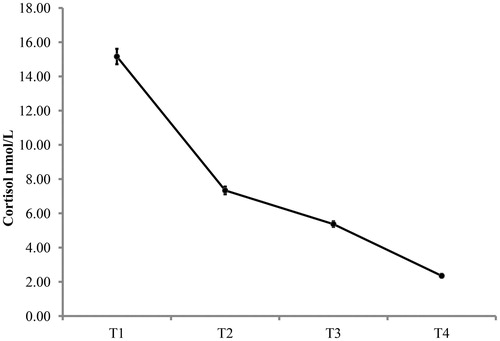
Two often-used indices of the cortisol circadian rhythm were calculated: total diurnal cortisol and diurnal cortisol decline (e.g. Nater et al., Citation2013; Saridjan et al., Citation2010; Simons et al., Citation2015; Watamura et al., Citation2004). Total diurnal cortisol was calculated as the area under the curve as follows: AUCdiurnal = ((T2 + T1) × time between sample T1 and T2/2 + (T3 + T2) × time between sample T2 and T3/2 + (T4 + T3) × time between sample T3 and T4/2). Diurnal cortisol decline was calculated as sample T1 minus T4. These measures were averaged across days (Simons et al., Citation2015; Watamura et al., Citation2004). Spearman’s rho correlations across days were rho = .62, p < .001, and rho = .29, p = .004, for total diurnal cortisol and diurnal cortisol decline, respectively. These correlations are in line with previous research in childhood and adolescence describing that total diurnal cortisol concentrations (AUC measures) are moderately stable across two days whereas the stability of diurnal decline scores is lower (Rotenberg et al., Citation2012).
In addition, since both indices of the cortisol circadian rhythm were significantly correlated with the length of the day (time distances between samples T1 and T4 in minutes; Spearman’s rho = .23, p = .016 and Spearman’s rho = .27, p = .002, for total diurnal cortisol and diurnal cortisol decline, respectively), both total diurnal cortisol and diurnal cortisol decline were also calculated corrected for the length of the day. This was done by saving the standardized residuals of regression analyses predicting the cortisol circadian measures based on the length of the day (based on de Veld et al., Citation2012; Schuetze et al., Citation2008; Simons et al., Citation2016). Using these corrected indices of the cortisol circadian rhythm in the main regression analyses resulted in comparable results to those with uncorrected indices. To facilitate interpretation of the results, findings of analyses using the uncorrected indices will be reported in the results section.
Cortisol stress responses
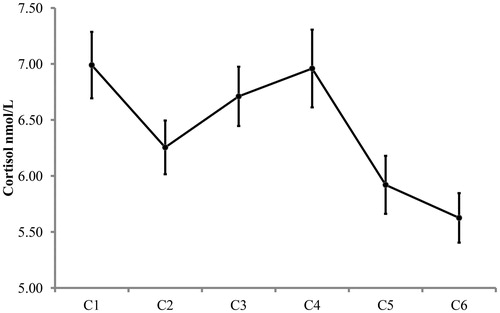
Of the potential 864 cortisol stress response saliva samples of the 144 children, 843 analyzable samples were collected. In , mean cortisol concentrations (nmol/L) and standard errors for each of the six cortisol sampling moments can be found. A paired samples t-test indicated that the CREST induced a significant increase in cortisol concentrations from baseline (lowest of C1 and C2; M = 6.06, SD = 2.70) to peak response concentrations (highest of C3 and C4; M = 7.12, SD = 3.79), t(141) = −4.41, p < .001, Cohen’s d = 0.37.
Figure 2. Stress response cortisol concentrations (nmol/L) per sampling moment. Error bars stand for one standard error above and beneath the mean of each of the six stress response sampling moments. Note that this is an adapted figure from Simons et al. (Citation2016). Data are reprinted with permission of the publisher: John Wiley and Sons. License number: 4002560272811.
Two indices of the cortisol stress response were calculated: total stress cortisol and cortisol stress reactivity. Total stress cortisol was calculated as the area under the curve over the six saliva samples as follows: AUCstress = ((C2 + C1) × 15/2 + (C3 + C2) × 10/2 + (C4 + C3) × 10/2 + (C5 + C4) × 15/2 + (C6 + C5) × 8/2). Cortisol stress reactivity was calculated as the standardized residuals of a regression predicting peak response cortisol from the baseline (cf. de Veld et al., Citation2012; Schuetze et al., Citation2008; Simons et al., Citation2016). The correlation between baseline and peak response cortisol concentrations was Spearman’s rho = .69, p < .001. Both indices of the cortisol stress response (total stress cortisol and cortisol stress reactivity) were log transformed before they were used in the analyses.
Confounders
Child gender (i.e. biological sex) and maternal educational level at child age 6 were determined as potential confounders. Child gender was scored as 0 (girl) or 1 (boy), and maternal educational level was scored as the mother’s highest educational level from 1 (primary) to 8 (university).
Results
Preliminary analyses
Children who participated in the CREST in the mobile lab parked at their home (n = 7 of 144) did not differ significantly on the outcome or predictor variables from children that participated with the lab parked at their school (all p’s > .050) and were therefore included in the analyses. presents descriptive statistics of the untransformed study variables.
Table 1. Descriptive statistics of all study variables.
In , Spearman correlations between all study variables are presented. Total diurnal cortisol (AUCdiurnal) was positively associated with total stress cortisol (AUCstress; Spearman’s rho = .24, p = .013). Higher total diurnal cortisol concentrations were associated with higher total stress cortisol concentrations. Potential confounders (child gender and maternal educational level) were not significantly correlated with an outcome variable (all p’s > .050; total stress cortisol or cortisol stress reactivity) and were hence left out of the main regression analyses (Tabachnick & Fidell, Citation2007). Note that main regression analyses including child gender (as confounder or predictor variable) resulted in similar findings to those reported in the main analyses section.
Table 2. Spearman correlations between all study variables.
Of the 144 children, 33 dropped out of both main regression analyses because of missing predictor (diurnal decline n = 18, AUCdiurnal n = 33) and outcome variables (AUCstress n = 10, cortisol stress reactivity n = 2). These 33 children did not differ significantly from the 111 children remaining in the main analyses on maternal educational level, maternal age, child gender, or age 4 temperament (CBQ short form; Putnam & Rothbart, Citation2006), all p’s > .050.
Main analyses
Two hierarchical linear regression analyses were conducted. In the first analysis, total stress cortisol (AUCstress) was predicted from the two indices of the cortisol circadian rhythm (AUCdiurnal and diurnal cortisol decline) in Step 1, and the interaction of the two indices of the cortisol circadian rhythm in Step 2. The model of Step 1 was significant, F(2, 102) = 13.83, p < .001, R2 = .21. Both total diurnal cortisol (β = .59, p < .001) and diurnal cortisol decline (β = −.25, p = .030) significantly predicted total stress cortisol (see ). Higher total diurnal cortisol and a smaller diurnal cortisol decline were both uniquely associated with higher total stress cortisol. Step 2 did not significantly improve the model (p > .050), and the total model remained significant, F(3, 101) = 9.26, p < .001, R2 = .22 (see ).
Table 3. Results from regressions predicting total stress cortisol and cortisol stress reactivity from the indices of the cortisol circadian rhythm.
In the second hierarchical linear regression analysis, cortisol stress reactivity was predicted from the two indices of the cortisol circadian rhythm (AUCdiurnal and diurnal cortisol decline) in Step 1, and their interaction in Step 2. No significant effects were found, all p’s > .050 (see ).
Discussion
This study investigated associations between the cortisol circadian rhythm and cortisol stress responses to a social evaluative stress test in normally developing 6-year-olds. The laboratory stress test was effective in producing a significant rise in cortisol in the children. Higher total diurnal cortisol and a smaller diurnal cortisol decline were both uniquely associated with higher total stress cortisol. Together, these indices of the cortisol circadian rhythm explained 21% of the variance of total stress cortisol. No associations between the cortisol circadian rhythm indices and the cortisol stress reactivity index were found.
The positive association between total diurnal cortisol (AUCdiurnal) and total stress cortisol (AUCstress) suggests that a generally higher circadian baseline cortisol production is associated with secreting more cortisol in stressful situations. The HPA-axis may be generally more active and/or both aspects of cortisol production may facilitate each other. As the total AUCstress measure represents the cortisol concentration of basal/anticipatory, response, and recovery periods together, this might mean that children with generally higher total diurnal cortisol concentrations are more physiologically stressed by the entire laboratory procedure, instead of only by the stress-inducing components. That no associations between the cortisol circadian indices and the cortisol stress reactivity index were found may support this idea. Alternatively, given that the CREST was carried out during a school day, children with higher total diurnal cortisol may generally have elevated cortisol concentrations at school, or even more generally in daily life and not in response to the stress test procedure per se.
A potential underlying mechanism explaining the above described cortisol production patterns may be self-regulation. Higher self-regulatory capacities allow children to control their emotions, behavior and the stressfulness of events, and may result in lower cortisol stress concentrations and faster recovery during stressful situations, as well as during the day in general. Support for this idea comes from a study by Watamura et al. (Citation2004) who found a negative association between effortful control (which is supported by self-regulatory capacities) and overall diurnal cortisol concentrations in 12- to 36-month-olds.
The negative association between diurnal cortisol decline and AUCstress indicates that children with a smaller diurnal decline had higher cortisol concentrations in the stressful situation. Previous research has linked a smaller diurnal decline in children to lower levels of maternal parenting quality (involvement and warmth; Pendry & Adam, Citation2007), and more parent–child conflict at home (auditory assessments; Slatcher & Robles, Citation2012). Moreover, diurnal cortisol of foster children following an intensive family-based therapeutic intervention became more normative over time whereas the diurnal declines of foster children not following this intervention became smaller over time (Fisher et al., Citation2007). Regarding cortisol stress responses, Bernard and Dozier (Citation2010) found that in 11- to 20-month-olds, a disorganized attachment style, often seen as a reflection of lower quality maternal care, was associated with a cortisol increase in response to a stressor. Children with an organized attachment style did not show a cortisol increase in response to this situation. Additionally, in adults, attachment anxiety was positively associated with the cortisol response to an acute stressor (Quirin et al., Citation2008). This may suggest that lower parenting quality may underlie a smaller diurnal decline and heightened total stress cortisol, potentially via mechanisms such as early life stress and/or reduced parental scaffolding during the development of self-regulation.
We found no associations between the cortisol circadian rhythm and the cortisol stress reactivity index in our study. In line with this, Kidd et al. (Citation2014) also found no association between diurnal cortisol decline and the magnitude of the cortisol response to a stressor in older adults (between the ages of 54 and 76). Moreover, van Eck et al. (Citation1996) found no association between (basal) diurnal cortisol concentrations and the cortisol stress response to a laboratory stressor in 27- to 57-year-olds. Although this is speculative and more research is needed, our results may add to this that also in 6-year-olds diurnal HPA-axis activity may not be associated with cortisol stress reactivity. This may suggest that in young children responding with increased cortisol to an acute stressor, with the goal of mobilizing energy to cope with the stressor (e.g. Nicolson, Citation2007), is independent of diurnal HPA-axis activity. Cortisol stress reactivity may be predicted (more strongly) by other factors, such as the early life environment, genetic factors, or the type of stressful situation (e.g. de Weerth et al., Citation2013b; Dickerson & Kemeny, Citation2004; Gunnar et al., Citation2009; Kudielka et al., Citation2004; Tollenaar et al., Citation2011).
However, Kidd et al. (Citation2014) did find a positive association between total diurnal cortisol concentrations and the magnitude of the cortisol stress response. Additionally, Koss et al. (Citation2016) found that blunted diurnal decline was associated with a blunted cortisol response toward a laboratory challenge in a group of predominantly adopted children. Differences between the study populations may at least in part underlie the differences in results. Koss et al. (Citation2016) studied children that may be assumed to have had harsh early life environments (i.e. post-institutionalized and foster children), while our children came from a normal middle class sample background. The severe early life stress that is associated with harsh environments is known to affect the development of the HPA-axis (e.g. Loman & Gunnar, Citation2010; Lupien et al., Citation2009; Kudielka et al., Citation2009), potentially also affecting the dynamics of HPA-axis functioning. Differences between our findings and those of Kidd et al. (Citation2014) might be explained by the age of the study populations: older adults vs. children in whom the cortisol circadian rhythm still appears to develop (Shirtcliff et al., Citation2012; Simons et al., Citation2015). However, also other study characteristics, such as morning-afternoon differences, may explain our results. Supporting this idea, Kudielka et al. (Citation2004) found that higher basal saliva cortisol concentrations were associated with lower stress-related net increases in saliva cortisol in the morning but not in the afternoon, when analyzing both separately. Contrary to our study, Kidd et al. (Citation2014) collected cortisol stress response data both in morning and afternoon hours, possibly explaining the differences in findings. Examining HPA-axis functioning at various time points, at different ages, and in normative as well as clinically relevant populations, will help to further understand the associations between the cortisol circadian rhythm and cortisol stress responses.
Finally, we did not find support for associations of the interaction between the two indices of the cortisol circadian rhythm on cortisol stress responses. This may suggest that the indices of the cortisol circadian rhythm do not further facilitate each other’s individual effects, but more research is needed to further explore this.
Strengths, limitations and future research
Strengths of the current study are the relatively large sample size and early age of the participants. Another strength is the use of an innovative and effective social evaluative stress test that was especially designed to induce cortisol stress responses at this specific age (de Weerth et al., Citation2013a). Moreover, the combination of several indices of both the cortisol circadian rhythm as well as the cortisol stress response provides insight in the dynamics of HPA-axis functioning. A limitation of the current study is the fact that the cortisol stress responses were measured on a week/school day, whereas the cortisol circadian rhythm was measured during weekend days. This may have decreased associations between the two aspects of HPA-axis functioning making our findings an underestimation of the real effects. Additionally, we did not assess the cortisol awakening response (CAR), another index of the cortisol circadian rhythm (e.g. Nicolson, Citation2007), because mothers of 6-year-olds may often miss their child’s exact awakening time, resulting in unreliable CAR assessments. However, including a reliable measure of this index (e.g. by using actigraphy to determine time of awakening) may potentially have resulted in the ability to explain more variance of the cortisol stress responses. Finally, in this study causality cannot be inferred from the results, since inverse relations between cortisol stress responses and circadian cortisol cannot be excluded.
Since in childhood and adolescence alterations in both the cortisol circadian rhythm and the cortisol stress response are associated with physical and psychological health (e.g. Buske-Kirschbaum et al., Citation2003; Carrion et al., Citation2002; Hankin et al., Citation2010; Luby et al., Citation2003; Ruttle et al., Citation2013; Shirtcliff & Essex, Citation2008; Watamura et al., Citation2010; White et al., Citation2000), a question for future research is whether alterations in the associations between the two aspects of cortisol production are linked to behavioral functioning, syndromes and illnesses. Moreover, since the cortisol circadian rhythm continues to develop during childhood and adolescence (e.g. Shirtcliff et al., Citation2012; Simons et al., Citation2015) a future question is how associations between these two patterns of HPA-axis functioning change with age. Finally, in this sample large inter-individual differences were found in cortisol responses to the stress test (e.g. 54.9% of the children showed an increase in cortisol from basal to peak response concentrations while the rest showed no change or a decrease). In future studies it would be interesting to further explore these inter-individual differences, for example, by determining whether they are explained by additional correlates such as stress early in life and/or genetic factors.
Conclusions
In this study, higher total diurnal cortisol and a smaller diurnal cortisol decline were both uniquely associated with higher total stress cortisol concentrations in normally developing 6-year-olds. Possible explanations for the patterns found are links with children’s self-regulatory capacities and parenting quality.
Acknowledgements
We thank the families who kindly participated in the BIBO study. We also thank all research assistants, students, and PhD students, for their assistance with data collection.
Disclosure statement
None of the authors had conflicts of interest, nor was scientific writing assistance used. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Beijers R, Jansen J, Riksen-Walraven JMA, de Weerth C. (2011). Attachment and infant night waking: a longitudinal study from birth through the first year of life. J Dev Behav Pediatr 32:635–43.
- Beijers R, Riksen-Walraven JMA, de Weerth C. (2013a). Cortisol regulation in 12-month-old human infants: associations with the infants' early history of breastfeeding and co-sleeping. Stress 16:267–77.
- Beijers R, Riksen-Walraven JMA, Putnam S, de Jong M, de Weerth C. (2013b). Early non-parental care and toddler behaviour problems: links with temperamental negative affectivity and inhibitory control. Early Child Res Q 28:714–22.
- Bernard K, Dozier M. (2010). Examining infants' cortisol responses to laboratory tasks among children varying in attachment disorganization: stress reactivity or return to baseline? Dev Psychol 46:1771–8.
- Buske-Kirschbaum A, von Auer K, Krieger SMA, Weis SMD, Rauh WMD, Hellhammer DH. (2003). Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosom Med 65:806–10.
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. (2002). Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry 51:575–82.
- de Veld DM, Riksen-Walraven JMA, de Weerth C. (2012). The relation between emotion regulation strategies and physiological stress responses in middle childhood. Psychoneuroendocrinology 37:1309–19.
- de Weerth C, Buitelaar JK, Beijers R. (2013b). Infant cortisol and behavioral habituation to weekly maternal separations: links with maternal prenatal cortisol and psychosocial stress. Psychoneuroendocrinology 38:2863–74.
- de Weerth C, Jansen J, Vos MH, Maitimu I, Lentjes EGWM. (2007). A new device for collecting saliva for cortisol determination. Psychoneuroendocrinology 32:1144–8.
- de Weerth C, Zijlmans MAC, Mack S, Beijers R. (2013a). Cortisol reactions to a social evaluative paradigm in 5- and 6-year-old children. Stress 16:65–72.
- Dickerson SS, Kemeny ME. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–91.
- Edwards S, Clow A, Evans P, Hucklebridge F. (2001). Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci 68:2093–103.
- Engelmann JM, Herrmann E, Tomasello M. (2012). Five-year olds, but not chimpanzees, attempt to manage their reputations. PLoS One 7:1–7.
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. (2007). Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology 32:892–905.
- Gunnar MR, Talge NM, Herrera A. (2009). Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology 34:953–67.
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. (2010). Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biol Psychiatry 68:484–90.
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. (2013). Daytime trajectories of cortisol: demographic and socioeconomic differences – findings from the national study of daily experiences. Psychoneuroendocrinology 38:2585–97.
- Kidd T, Carvalho LA, Steptoe A. (2014). The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biol Psychol 99:34–40.
- Kirschbaum C, Hellhammer DH. (1989). Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22:150–69.
- Koss KJ, Mliner SB, Donzella B, Gunnar MR. (2016). Early adversity, hypocortisolism, and behavior problems at school entry: a study of internationally adopted children. Psychoneuroendocrinology 66:31–8.
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. (2004). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 29:983–92.
- Kudielka BM, Hellhammer DH, Wüst S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34:2–18.
- Loman MM, Gunnar MR. (2010). Early experience and the development of stress reactivity and regulation in children. Neurosci Biobehav Rev 34:867–76.
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. (2003). Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry 60:1248–55.
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–45.
- Nater UM, Hoppmann CA, Scott SB. (2013). Diurnal profiles of salivary cortisol and alpha-amylase change across the adult lifespan: evidence from repeated daily life assessments. Psychoneuroendocrinology 38:3167–71.
- Nicolson NA. (2007). Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Thousand Oaks, CA: Sage Publications, Inc. p. 37–74.
- Pendry P, Adam EK. (2007). Associations between parents' marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int J Behav Dev 31:218–31.
- Putnam SP, Rothbart MK. (2006). Development of short and very short forms of the children's behavior questionnaire. J Pers Assess 87:102–12.
- Quirin M, Pruessner JC, Kuhl J. (2008). HPA system regulation and adult attachment anxiety: individual differences in reactive and awakening cortisol. Psychoneuroendocrinology 33:581–90.
- Rotenberg S, McGrath JJ, Roy-Gagnon MH, Tu MT. (2012). Stability of the diurnal cortisol profile in children and adolescents. Psychoneuroendocrinology 37:1981–9.
- Ruttle PL, Javaras KN, Klein MH, Armstrong JM, Burk LR, Essex MJ. (2013). Concurrent and longitudinal associations between diurnal cortisol and body mass index across adolescence. J Adolesc Health 52:731–7.
- Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, Kirschbaum C, et al. (2010). Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? the generation R study. Horm Behav 57:247–54.
- Schuetze P, Lopez FA, Granger DA, Eiden RD. (2008). The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7-month-old infants. Dev Psychobiol 50:819–34.
- Shirtcliff EA, Essex MJ. (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol 50:690–703.
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. (2012). Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol 54:493–502.
- Simons SSH, Beijers R, Cillessen AHN, de Weerth C. (2015). Development of the cortisol circadian rhythm in the light of stress early in life. Psychoneuroendocrinology 62:292–300.
- Simons SSH, Cillessen AHN, de Weerth C. (2016). Cortisol stress responses and children’s behavioral functioning at school. Dev Psychobiol 9999:1–8.
- Slatcher RB, Robles TF. (2012). Preschoolers' everyday conflict at home and diurnal cortisol patterns. Health Psychol 31:834–8.
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston, MA: Alyn & Bacon; 2007. p. 980.
- Tollenaar MS, Beijers R, Jansen J, Riksen-Walraven JMA, de Weerth C. (2011). Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress 14:53–65.
- van Eck MM, Nicolson NA, Berkhof H, Sulon J. (1996). Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biol Psychol 43:69–84.
- Watamura SE, Coe CL, Laudenslager ML, Robertson SS. (2010). Child care setting affects salivary cortisol and antibody secretion in young children. Psychoneuroendocrinology 35:1156–66.
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. (2004). Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol 45:125–33.
- Weisberg DP, Beck SR. (2012). The development of children's regret and relief. Cogn Emot 26:820–35.
- White BP, Gunnar MR, Larson MC, Donzella B, Barr RG. (2000). Behavioral and physiological responsivity, sleep, and patterns of daily cortisol production in infants with and without colic. Child Dev 71:862–77.
