Abstract
The glucocorticoid hypothesis suggests that overexposure to stress may cause permanent upregulation of cortisol. Stress in youth may therefore influence cortisol levels even in older age. Using data from the 6-Day Sample, we investigated the effects of high stress in childhood, adolescence and early adulthood – as well as individual variables contributing to these measures; parental loss, social deprivation, school and home moves, illness, divorce and job instability – upon cortisol levels at age 77 years. Waking, waking +45 min (peak) and evening salivary cortisol samples were collected from 159 participants, and the 150 who were not using steroid medications were included in this study. After correcting for multiple comparisons, the only significant association was between early-adulthood job instability and later-life peak cortisol levels. After excluding participants with dementia or possible mild cognitive impairment, early-adulthood high stress showed significant associations with lower evening and mean cortisol levels, suggesting downregulation by stress, but these results did not survive correction for multiple comparisons. Overall, our results do not provide strong evidence of a relationship between stress in youth and later-life cortisol levels, but do suggest that some more long-term stressors, such as job instability, may indeed produce lasting upregulation of cortisol, persisting into the mid-to-late seventies.
Stress in childhood and early adulthood, and cortisol levels in older age
Cortisol is produced by the hypothalamic–pituitary–adrenal (HPA) axis as an adaptive response to environmental stressors. However, glucocorticoids can also be toxic, and excessive levels can cause neurodegeneration (Abraham et al., Citation2001; Sapolsky, Citation1996). Sapolsky et al.’s (Citation1986) glucocorticoid cascade hypothesis suggests high levels of glucocorticoids damage neurons throughout the brain, including those providing feedback to the HPA axis. The resulting negative feedback impairs downregulation of glucocorticoids, increasing the impact of corticosteroid toxicity throughout the brain. Sapolsky et al. argued that this was particularly relevant in older age, as neurodegeneration and hypersecretion of glucocorticoids continually exacerbate one another, leading to cognitive impairment and contributing too many of the serious health problems associated with older age. Landfield et al. (Citation2007) later argued that the relationship between glucocorticoids and ageing was more complex, but that chronic overexposure still contributed to ageing-related neurodegeneration.
Chronic overexposure to cortisol may be instigated well in advance of older age, as stress earlier in life may have a lasting impact on the HPA axis. Prior studies have observed higher basal or mean cortisol levels in later childhood and adulthood among individuals who were abused as children (Luecken & Appelhans, Citation2006 [N = 88]; Schalinski et al., Citation2015 [43]; Schreier et al., Citation2015 [180]; van der Vegt et al., Citation2009 [623]). Childhood parental loss (Luecken & Appelhans, Citation2006; Nicolson, Citation2004 [57]) and socioeconomic disadvantage (Desantis et al., Citation2015 [1490]; Franz et al., Citation2013 [727]) have also been associated with higher cortisol levels in early, middle and late adulthood. Other factors with similar effects may include chronic illness, associated with higher daytime cortisol levels in childhood (Cardas et al., Citation2013 [89]), and residential mobility, associated with poor health and psychological distress in adulthood (Brown et al., Citation2012 [850]). However, others have observed lower basal or total cortisol in those who suffered greater childhood stress (Desantis et al., Citation2015; Flory et al., Citation2009 [63]; Trickett et al., Citation2010 [173]; Vedhara et al., Citation2007 [566]). Stress in youth has also been associated with both higher (Goldman-Mellor et al., Citation2012 [543]) and lower (Carpenter et al., Citation2009 [230]) cortisol reactivity in later life.
We assessed relationships between childhood, adolescent and early-adulthood stressors, and older-age cortisol levels. As above, not all previous work on stress and the HPA axis is in agreement, and few previous studies have investigated the relationship between stress in youth and cortisol levels in older age. Some work suggests that the HPA axis is highly adaptive, and that cortisol regulation may recover relatively quickly from any effects of stress (Dallman, Citation1993; Spencer & McEwen, Citation1990). However, we tested the hypothesis that exposure to stress leads to upregulation of cortisol, and that this upregulation is long-lasting. Consistent with this hypothesis, we expected that measures of high childhood, adolescent and early-adulthood stress, combining a number of different stressor variables, would be associated with higher older-age cortisol levels, at specific times of day or overall. We also explored whether each of the individual indicators predicted cortisol measures in later life.
Methods
Participants
On 4th June 1947, the Scottish Council for Research in Education (SCRE) assessed the intelligence of almost all Scottish schoolchildren born in 1936 in the second Scottish Mental Survey (SMS1947; SCRE, Citation1949). A representative sample was also selected from this population for further assessment. Having been selected according to their birth date being one of 6 days throughout the year (the first of the even-numbered months), these participants were known as the 6-Day Sample (MacPherson, Citation1958). These 1208 original 6-Day Sample members were assessed more thoroughly through a series of almost annual home visits from age 11 to age 27, during which data on a wide range of environmental factors – including information on parents, home environment, schools attended, and, later on, employment and marriage – were collected (Deary et al., Citation2009; Maxwell, Citation1969).
In 2012, we traced as many of the original 6-Day Sample members as possible through the United Kingdom National Health Service Central Register. We invited the 634 who were found to be still alive and resident in Scotland, England or Wales (and one other, who had emigrated) to participate in a follow-up study (Brett & Deary, Citation2014; Deary & Brett, Citation2015). In 2013, 174 individuals (92 females) who had agreed to take part completed a detailed questionnaire booklet, as well as a physical testing kit. All provided informed consent in writing before participating. Surviving through to older age and agreeing to participate in the follow-up study was not random (Johnson et al., Citation2016). Those 174 who completed the questionnaire, on average, had higher cognitive ability scores as children, and were rated by their teachers as more dependable as adolescents, than the population as a whole (Deary & Brett, Citation2015). The present study focused on the 150 (77 females) participants who provided useable cortisol samples at follow-up. Descriptive statistics for the original 6-Day Sample, those who participated in the follow-up study, and those who were included in the present study are provided in .
Table 1. Descriptive statistics, summarizing key youth stress, older-age cortisol and control variables, for 6-Day Sample participants included at each stage of the study.
Youth stress measures
Parental loss
Information on parents’ deaths, divorces and separations was recorded systematically as part of the questionnaire booklet completed in 2013. The number of these events occurring by the age of 11 years was counted as parental loss in childhood. Parents’ deaths, divorces and separations between the ages of 12 and 17 years served as our measure of adolescent parental loss. Parents’ deaths (but not divorces or separations, representing, arguably, a far greater loss when participants were still living with and dependent upon their parents) between the ages of 18 and 30 years were counted as early-adulthood parental loss.
Deprivation
The occupation of each participant’s father was recorded in 1947 and 1954 (MacPherson, Citation1958). We derived father’s social class from his occupation in 1947, initially scored from I (highest) to V (lowest), according to the UK’s 1951 Classification of Occupations (General Register Office, Citation1956; Knight, Citation1967), and also counted recorded periods of unemployment. The numbers of rooms and residents in each participant’s household were also recorded annually from 1950 to 1954 (MacPherson, Citation1958). Home occupancy rate was calculated for each year, and then averaged across years. We gave participants a score of 1 if their fathers were of low social class (IV) or two if they were of the lowest social class (V), and increased this score by 1 if they were unemployed in 1947, producing a simple measure of childhood deprivation at that time, scored from 0 to 3. Father’s social class (being assessed towards the end of childhood/beginning of adolescence) was incorporated in the same way into a simple measure of adolescent deprivation, along with unemployment in 1954 and a particularly high (higher than 95% of the sample) mean home occupancy rate. Adolescent deprivation thus spanned ages 11–18 years (a slightly wider range than most other measures), and was scored from 0 to 4.
Home and school moves
The number of previous schools was recorded in 1947, changes of school since 1947 was recorded in 1950, and any changes of home address were then recorded annually up until 1963 (MacPherson, Citation1958; Maxwell, Citation1969; SCRE, Citation1949). School moves before 1947 was used as a measure of childhood mobility from starting school age to adolescence, that is from around 5 to 11 years of age. Subsequent school moves and home moves from 12 to 17 years were summed as a measure of adolescent mobility. Home moves from 18 to 27 years served as a measure of early-adulthood mobility. Although some moves may have been associated with positive changes in life, we expected that a particularly high number of moves would be disruptive and stressful.
Illness
Participants underwent a medical examination in 1950, and notes on physical health were taken during home visits in 1951, 1953 and 1954 (MacPherson, Citation1958). A count of serious illnesses (deemed serious enough to pose a long-term risk to health) at these four times, spanning ages 14–18 years (a slightly narrower range than most other measures), was used as a measure of adolescent illness.
Divorce
Information on participants’ divorces was recorded retrospectively when records became available in 1969. Divorces between the ages of 18 and 30 years were used as another early-adulthood stress measure.
Job instability
Participants’ occupations were recorded annually from leaving school (usually at around age 15 or 16) up until age 27. We considered whether participants were ever unemployed, whether they were unemployed more than once, and whether they changed occupation a high number of times (higher than 90% of the sample) or a very high number of times (higher than 95% of the sample). These four variables were summed to produce a simple measure of job instability, scored from 0 to 4. Although some of participants’ earliest positions of employment would have been held during later adolescence, all were included in this early-adulthood measure.
High stress
The above measures were also combined, producing measures representing high stress in childhood (up to around 11 years), adolescence (around 12–17 years) and early adulthood (around 18–30 years). In childhood, participants were considered as being exposed to high levels of stress if they lost a parent, scored highest on deprivation, or moved school more often than 95% of the sample. By these criteria, around 11% of participants included in this study were exposed to high levels of stress in childhood. Adolescent high stress was composed in the same way, but also included participants for whom serious illness was recorded on three or more occasions throughout adolescence. The proportion of participants included was therefore slightly higher, at around 15%. High stress in early adulthood also incorporated parents’ deaths and a particularly high number of moves (higher than 95% of the sample), but also divorce and at least two indicators of job instability. Due primarily to a higher number of parental losses throughout the 12 years of early adulthood assessed than in childhood or adolescence, early-adulthood high stress included a greater proportion of participants: around 30%. The purpose of including these measures was to assess the hypothesis that only particularly high levels of stress produces lasting effects on the glucocorticoid system. This was also the reason why they were dichotomous, although very few participants experienced high stress in relation to more than one contributing variable.
Associations among childhood, adolescent and early-adulthood stress measures are reported in Table S1.
Cortisol samples
As part of the home physical testing kit, follow-up participants were provided with three Salivette sample tubes (Sarstedt, Rommelsdorf, Germany). Participants were asked to provide three samples of saliva for cortisol analysis – immediately after waking, 45 min later and at 22:00 the same day. The second measurement is subsequently referred to as “peak” cortisol level, although cortisol levels could have been higher earlier or later in the day. Samples were returned to us by post, and upon receipt were centrifuged for 5 min at 3000 rpm before being frozen at −80 °C. Cortisol levels were then measured at the University of Dresden, using a commercial immunoassay kit with chemiluminescence detection (IBL-Hamburg, Hamburg, Germany). Any extreme values (above 70 μg/mlFootnote1) were deemed to be outliers and were removed from subsequent analyses. Participants who reported use of any glucocorticoid or other steroid medications were also excluded from all analyses. The resulting distributions were close to normal, but negatively skewed, so we adjusted raw values using natural log transformation. Cortisol awakening response (CAR) was calculated as the difference between adjusted peak and waking cortisol levels, diurnal cortisol slope was calculated as the difference between adjusted evening and waking levels, and mean cortisol level was calculated as the mean of adjusted waking, peak and evening levels. Participants also recorded the times at which they provided each sample, and for those who reported a delay of over one hour between the first two samples (N = 5), peak and CAR measures were excluded.
Control variables
We included sex, recorded at the time of the SMS1947 (SCRE, 1949), as a covariate in all analyses, as older-age cortisol levels (Zhao et al., Citation2003) and associations between stress and later cortisol levels (Goldman-Mellor et al., Citation2012) have both previously been shown to depend on sex. All participants in the SMS1947 completed the Moray House Test No. 12, a group-administered test, primarily of verbal reasoning, described and reproduced in full by SCRE (Citation1933). We converted this to an IQ-type score by residualizing over age at the time of the SMS1947, and standardizing with a mean of 100 and SD of 15. This measure of childhood IQ was also included as a covariate in all analyses, as well as years of education, recorded during the original 6-Day Sample study between 1947 and 1963 (MacPherson, Citation1958; Maxwell, Citation1969), and age at follow-up, calculated from participants’ dates of birth and the dates on which they returned study materials to us by post (Brett & Deary, Citation2014). Three participants reported a diagnosis of dementia (as part of a section in the questionnaire booklet on current and previous health conditions), including two who also provided cortisol samples and were therefore part of this study sample. Those who participated in the telephone interview completed an adapted Mini Mental State Examination (MMSE; Folstein et al., Citation1975; Newkirk et al., Citation2004; Roccaforte et al., Citation1992). The maximum possible score was 26; a score of 20 or less was taken as indication of mild cognitive impairment (MCI). This cutoff was not prescribed by Newkirk et al., but is comparable (proportionally) to criteria used to detect MCI with other versions of the MMSE. Analyses were repeated after excluding the two participants who reported a diagnosis of dementia and five others who scored 20 or below on the MMSE.
Analysis
Following the construction of youth stress variables and preprocessing of older-age cortisol data, as above, we analyzed the longitudinal associations between all stressors and all cortisol measures using Matlab R2013a (The MathWorks, Natick, MA). Generalized linear modeling was used for all analyses, in order to incorporate covariates and to accommodate non-normally distributed variables. After excluding outliers and participants on steroid medications, our sample had 80% power to detect a standardized β coefficient of .2. First, we assessed the effects of a number of key study variables – those stressors that were recorded for the whole of the original sample, as well as all control variables – on survival to older age and on agreeing to participate at follow-up. We then assessed the relation between each youth stress measure and each older-age cortisol measure using generalized linear modeling, including sex, education and age at follow-up as covariates in each model, as well as childhood IQ, due to previously reported associations between intelligence in youth and cortisol levels later in adulthood (Franz et al., Citation2011). All p values were corrected for multiple comparisons using false discovery rate (FDR; Benjamini & Hochberg, Citation1995); resulting q values were still compared to an α of .05.
Results
The effects of youth stress measures available for the whole 6-Day Sample and control variables on survival to older age and on participation at follow-up are reported in . Women (β = .16, p < .001), those of higher intelligence (β = .18, p < .001; also assessed by Johnson et al., Citation2016), those who spent longer in full-time education (β = .17, p < .001) and also those who changed home address more often throughout early adulthood (β = .08, p = .01) were significantly more likely to survive to age 76 years. On the other hand, childhood deprivation (β = −.15, p < .001), adolescent deprivation (β = −.16, p < .001) and early-adulthood job instability (β = −.14, p < .001) were significantly associated with mortality by this age. Higher childhood intelligence (β = .31, p < .001) and greater time spent in full-time education (β = .23, p < .001) also significantly predicted participation in the follow-up study among those who survived to age 76 years, whereas deprivation in childhood (β = −.09, p = .02) and adolescence (β = −.10, p = .01) were negatively associated with follow-up study participation. All of these results remained significant after FDR correction for multiple comparisons. Childhood and adolescent moves were not significantly related to mortality or participation, and neither of the early-adulthood stressors significantly predicted participation in the follow-up study.
Table 2. Relations between key study variables, survival to older age, and follow-up study participation.
Associations among youth stress measures are reported in Table S1. The childhood, adolescence and early-adulthood high stress variables incorporated other measures, and therefore each showed several significant positive associations with their indicator variables. However, there were only two other significant associations, after correcting for multiple comparisons. First, childhood and adolescent deprivation showed a particularly high association (β = .97, p < .001), partly due to the use of the common indicator variable, father’s social class, but also perhaps due to stability of father’s status over several years. Second, and less expectedly, adolescent illness showed a moderate association with divorce in early adulthood (β = .40, p < .001).
Associations between childhood, adolescent and early-adulthood stress measures, and later-life cortisol measures are reported in . All associations were assessed while controlling sex, childhood IQ, years of education and age at follow-up. Considering childhood stressors first, there was no significant effect of parental loss during childhood on any measure of cortisol in older age, the largest being on mean cortisol level (β = −.11, p = .27). Nor were there any significant effects of childhood deprivation or school moves throughout childhood on later cortisol measures; the largest associations here were a modest association with a flatter diurnal cortisol slope (β = .15, p = .13) and a modest positive effect on evening cortisol (β = .14, p = .17), respectively.
Table 3. Relations between all youth stress measures and all older-age cortisol measures.
Results were similarly weak for individual measures of adolescent stress. Parental loss throughout adolescence did show a modest positive association with waking cortisol in older age (β = .16, p = .07), but this did not quite achieve significance at p < .05, even before correcting for multiple comparisons, and its associations with all other older-age cortisol measures were smaller. As for childhood deprivation, adolescent deprivation’s strongest association was with diurnal cortisol slope (β = .15, p = .12), which was not significant even before correcting for multiple comparisons. Adolescent illness also showed no significant associations with later-life cortisol, its strongest being with mean cortisol level (β = .13, p = .17). Total number of home and school moves throughout adolescence did show a modest negative association with mean cortisol in older age (β = −.17, p = .08), but again, this did not achieve significance even before correcting for multiple comparisons.
We assessed early-adulthood stressors in the same way. Early-adulthood parental loss showed no significant associations with cortisol measures, the strongest being with higher waking cortisol levels (β = .09, p = .31). Divorce showed a modest association with a reduced CAR in later life (β = −.16, p = .08), but this was not significant, and its associations with all other cortisol measures were smaller. Home moves throughout early adulthood showed several modest associations with later-life cortisol measures, including some that came close to achieving significance; the strongest were between a higher number of moves and lower evening (β = −.17, p = .06) and mean (β = −.18, p = .07) cortisol levels. However, early-adulthood job instability significantly predicted lower peak cortisol levels in later life (β = −.34, p < .001), and this effect remained significant after FDR correction for multiple comparisons.
Finally, we also assessed the high stress measures, representing high scores on any of the aforementioned measures of childhood, adolescent and early-adulthood stress. These measures also showed no significant associations with measures of older-age cortisol (). The strongest associations were between high stress in childhood and lower waking cortisol (β = −.08, p = .36), adolescent high stress and a steeper diurnal cortisol slope (β = −.08, p = .36), and early-adulthood high stress and lower mean cortisol levels (β = −.11, p = .23).
Figure 1. Older-age cortisol levels in participants exposed to high levels of stress in childhood, adolescence or early adulthood. Note: Bars represent mean salivary cortisol concentrations upon waking, around 45 min later (peak) and in the evening of the same day; in those exposed to high levels of stress at each stage of life and in remaining participants; error bars represent standard error.
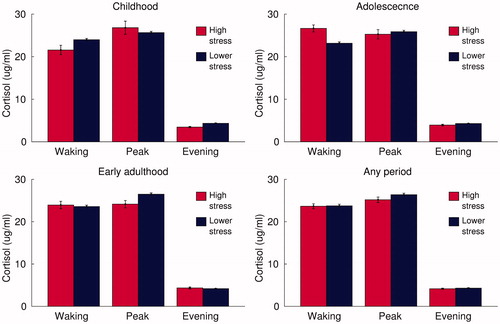
All of these associations between youth stress and older-age cortisol measures were tested again while excluding participants who either reported a diagnosis of dementia (N = 2) or scored below 22 on the telephone-administered MMSE (N = 5). As shown in Table S2, this made little difference to the results, except for high stress throughout early adulthood. After excluding participants with dementia or possible MCI, early-adulthood stress showed significant associations with lower evening cortisol (β = −.23, p = .01), steeper diurnal slope (β = −.19, p = .04) and lower mean cortisol levels (β = −.20, p = .04). However, none remained significant after FDR correction.
Discussion
We investigated longitudinal associations between stress in childhood (up to age 11 years), adolescence (12–17 years) and early adulthood (18–30 years), and measures of cortisol taken at age 77 years. We expected high stress in each of these periods of youth – as well as higher scores on indicator variables, parental loss, deprivation, home and school moves, illness, divorce and job instability – to predict higher cortisol levels in older age, whether basal, peak or overall. Although we tested longitudinal associations in a relatively small sample, and our methods also presented a number of other limitations (discussed below), we did assess a range of youth stress measures, and would still have been able to detect fairly modest effects. However, of the 84 associations we tested, only one was significant: early-adulthood job instability was associated with lower peak cortisol levels in older age. After excluding participants with dementia or possible MCI, high early-adulthood stress significantly predicted lower evening and mean cortisol levels, as well as a steeper diurnal cortisol slope. However, after correcting for multiple comparisons, the association between early-adulthood job instability and later peak cortisol was the only one that remained significant.
Parental loss in childhood has previously been associated with higher overall cortisol levels in early to middle adulthood (Luecken & Appelhans, Citation2006; Nicolson, Citation2004). Although we assessed associations over a slightly longer period, we expected to observe further evidence of the same positive association. However, we observed no significant effect of parental loss in childhood, adolescence or early adulthood on any cortisol measure. It may be that changes to HPA axis activity resulting from early parental loss, though lasting, do not persist into the mid-to-late seventies; perhaps more temporally proximal exposure to stress made a more substantial contribution to the salivary cortisol levels of our follow-up participants.
Similarly, previous studies have shown that low socioeconomic status and other measures of childhood disadvantage may be associated with increased cortisol levels in early to late adulthood (Desantis et al., Citation2015; Franz et al., Citation2013), yet we found no evidence of the same relationships among our sample. Again, this may be attributable to the age of our sample, if other factors are more important than childhood social deprivation in predicting older-age cortisol levels. Alternatively, our simple measures of social deprivation may not have incorporated the environmental aspects that are most stressful, or most disruptive to the glucocorticoid system.
Although we are unaware of any studies of the long-term effects of illness in youth on cortisol levels, a contemporaneous association between chronic illness and daytime cortisol levels in childhood has been reported previously (Cardas et al., Citation2013). We hypothesized that serious illness in adolescence would act as a stressor and therefore predict higher cortisol levels in older age. Again, we found no evidence to support this hypothesis. It is feasible that a more detailed assessment of illness throughout youth would have been necessary to discern a significant effect.
Similarly, we do not know of any previous studies assessing the effects of divorce on cortisol, but divorce is generally a stressful experience (Booth & Amato, Citation1991), which we therefore expected may affect later cortisol levels. We found no evidence to support this, but there was one modest association of above 0.1 – that with a lower CAR – suggesting that divorce should perhaps still be considered in future studies of influences on later-life cortisol levels.
Previous work has not assessed the longitudinal or even contemporaneous relation of repeated moving to cortisol, but greater residential mobility has been shown to predict poor health and psychological distress (Brown et al., Citation2012). Number of home and school moves throughout youth was therefore another factor that we supposed may act as a stressor and predict higher cortisol levels in older age. Contrary to our hypothesis, the strongest associations were with lower cortisol levels in older age, but these results were not significant.
Finally, we also assessed early-adulthood job instability – another factor whose relations to cortisol measures has not previously been assessed, but that would be expected to induce stress. This measure showed almost no association with waking or evening levels, nor with diurnal slope, but a moderate, significant negative association with peak cortisol and, consequently, modest non-significant negative associations with CAR and mean cortisol level. This one significant result is the only evidence this study provides for an effect from early to late adulthood of stress on cortisol. It was also in the opposite direction to that which was expected, although this is consistent with other studies suggesting stress leads to cortisol downregulation (Desantis et al., Citation2015; Flory et al., Citation2009; Trickett et al., Citation2010; Vedhara et al., Citation2007). It is surprising that this measure appeared to influence later-life cortisol more than other stressors that may be regarded as more severe. However, while the loss of a parent, for example, may be more traumatic, it represents a stressful event, with associated high level of stress likely declining relatively quickly over the following weeks. On the other hand, job instability may represent chronic stress over a period of many years. Further research into the long-term effects of chronic stress related to job instability is needed to support this hypothesis, but our results suggest that this research may be warranted.
Although the individual variables discussed above do represent potential stressors, they do not necessarily represent particularly high levels of stress. We therefore also used them together to create three measures of high stress – during childhood, adolescence and early adulthood. However, these were generally not associated with later-life cortisol measures either.
Limitations
Only one of the measures of stress in youth assessed in this study was related to cortisol in older age. However, it may be that other stressors throughout youth that were not measured in the 6-Day Sample would have shown a significant relation to HPA axis function in older age, or that other cortisol measures, such as responsivity to stress, would have been more strongly associated with those stressors that were assessed. Additionally, some of the variables that we assessed (for example social deprivation) have been measured in very different ways in previous studies, and using these other measures may have produced different results. Another limitation due to the availability of data was that the age ranges used for childhood, adolescence and early adulthood were not consistent across measures, which may have affected results. This study was also limited by the follow-up sample, which, being dependent on surviving to age 77 and volunteering to participate, was not as representative as the original 6-Day Sample (Johnson et al., Citation2016).
In fact, we observed that some of our youth stress measures, primarily childhood and adolescent deprivation, were associated with lower chances of both surviving to age 76 and participating in the follow-up study. Our results may therefore have been influenced by dropout of the 6-Day Sample members who were exposed to most stress in youth (and/or with the highest cortisol levels in later life, perhaps due to associated health problems), although other studies of cortisol in older age would be similarly affected. Survival to older age and participation in the follow-up study were also dependent on higher intelligence and education, which may help individuals to manage the effects of youth stress, thereby reducing any associations with later cortisol measures.
Further, the associations between the assessed variables and later cortisol levels could have been too weak to be reliably detected in the relatively small follow-up sample – which did not have sufficient power to detect a standardized β coefficient of less than 0.2 – but significant associations have previously been observed in smaller samples (Flory et al., Citation2009 [63]; Luecken & Appelhans, Citation2006 [88]; Nicolson, Citation2004 [N = 57]; Schalinski et al., Citation2015 [43]). By combining several stressors, our high stress measures may have improved chances of identifying significant effects. However, these variables were not perfect measures of high stress, as they may have included participants who did experience what we refer to as stressful events, such as the loss of a parent, but did not experience high levels of stress as a result. They may also have excluded participants who were exposed to high levels of stress due to factors on which data were not available to us. A more comprehensive assessment of stressors and stress in youth may be required to identify those who actually experienced high levels of stress.
Prior studies have also assessed the long-term effects of stress on cortisol reactivity (Carpenter et al., Citation2009; Goldman-Mellor et al., Citation2012), but this was not possible with the current sample. As participants were spread throughout the country, they collected their own cortisol samples, which raises issues of reliability (Kudielka et al., Citation2003). Finally, our sample represents a specific population – those born in Scotland in 1936 – so cultural and cohort differences must be considered when generalizing to other populations.
Conclusions
Overall, we found little evidence of a relationship between stress in childhood and early adulthood, and cortisol measures in older age. Our study was limited by small sample size, incomplete availability of data on youth stress, and the influence of youth stress on follow-up study participation, among other things. However, we did assess a range of stressors assessed in youth and their relation to a number of cortisol measures provided up to 66 years later, which few other studies are able to do. Still, after correcting for multiple comparisons, we observed only one significant longitudinal stress-cortisol association. These results, generally, do not support the scenario in which youth stressors are related to HPA-axis functioning in older age, as might be predicted by the glucocorticoid hypothesis. Instead, the majority of our results indicate, at least, that the upregulation of cortisol by stress in youth does not persist into the mid-to-late seventies, and may be consistent with greater adaptability of the HPA axis. However, we did observe one significant moderate effect suggesting downregulation of cortisol by early-adulthood job instability. We therefore call for further investigation into the relationship between stress in youth and cortisol levels in older age, using larger samples, more systematic assessment of stress throughout youth, and perhaps paying particular attention to the stress of job instability.
ISTS_1289168_SupplementalTables.pdf
Download PDF (126.5 KB)Disclosure statement
None of the authors has any financial, professional or personal conflicts of interest to declare. All authors were employed by the University of Edinburgh at the time the study was conducted; C.E.B. is now employed by Liverpool John Moores University.
Note
Additional information
Funding
Notes
1 Consistent with information provided by Clemens Kirschbaum (personal communication, September 2014).
References
- Abraham IM, Harkany T, Horvath KM, Luiten PG. (2001). Action of glucocorticoids on survival of nerve cells: promoting neurodegeneration or neuroprotection? J Neuroendocrinol 13:749–60.
- Benjamini Y, Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300.
- Booth A, Amato P. (1991). Divorce and psychological stress. J Health Soc Behav 32:396–407.
- Brett CE, Deary IJ. (2014). Realising health data linkage from a researcher’s perspective: following up the 6-Day Sample of the Scottish Mental Survey 1947. Longitud Life Course Stud 5:283–98.
- Brown D, Benzeval M, Gayle V, Macintyre S, O’Reilly D, Leyland AH. (2012). Childhood residential mobility and health in late adolescence and adulthood: findings from the West of Scotland Twenty-07 Study. J Epidemiol Community Health 66:942–50.
- Cardas J, Azpiroz A, Pascual-Sagastizabal E, Perez-Yarza EG, Etexebarria AE, Azuremendi A, Sanchez-Martin JR. (2013). Factors associated with cortisol levels and health in 5–6-year-old children. Am J Hum Biol 25:606–16.
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. (2009). Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry 66:69–75.
- Dallman MF. (1993). Stress update Adaptation of the hypothalamic–pituitary–adrenal axis to chronic stress. Trends Endocrinol Metab 4:62–9.
- Desantis AS, Kuzawa CW, Adam EK. (2015). Developmental origins of flatter cortisol rhythms: socioeconomic status and adult cortisol activity. Am J Hum Biol 27:458–67.
- Deary IJ, Brett CE. (2015). Predicting and retrodicting intelligence between childhood and old age in the 6-Day Sample of the Scottish Mental Survey 1947. Intelligence 50:1–9.
- Deary IJ, Johnson W, Houlihan LM. (2009). Genetic foundations of human intelligence. Hum Genet 126:215–32.
- Flory JD, Yehuda R, Grossman R, New AS, Mitropoulou V, Siever LJ. (2009). Childhood trauma and basal cortisol in people with personality disorders. Compr Psychiatry 50:34–7.
- Folstein MF, Folstein S, McHugh PR. (1975). “Mini-mental status”: a practical method of grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–98.
- Franz CE, O’Brien RC, Hauger RL, Mendoza SP, Panizzon MS, Prom-Wormley E, Eaves LJ, et al. (2011). Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: the Vietnam Era Twin Study of Aging. Psychoneuroendocrinology 36:1040–52.
- Franz CE, Spoon K, Thompson W, Hauger RL, Hellhammer DH, Jacobson KC, Lupien S, et al. (2013). Adult cognitive ability and socioeconomic status as mediators of the effects of childhood disadvantage on salivary cortisol in aging adults. Psychoneuroendocrinology 38:2127–39.
- General Register Office. (1956). Census 1951: classification of occupations. London: Her Majesty’s Stationery Office.
- Goldman-Mellor S, Hamer M, Steptoe A. (2012). Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology 37:1755–68.
- Johnson W, Brett CE, Calvin C, Deary IJ. (2016). Childhood characteristics and participation in Scottish Mental Survey 1947 6-Day Sample follow-ups: implications for participation in aging studies. Intelligence 54:70–9.
- Kudielka BM, Broderick JE, Kirschbaum C. (2003). Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med 65:313–19.
- Knight R. (1967). Changes in the occupational structure of the working population. J R Stat Soc A 130:408–22.
- Landfield PW, Blalock EM, Chen K-C, Porter NM. (2007). A new glucocorticoid hypothesis of brain aging: implications for Alzheimer’s disease. Curr Alzheimer Res 4:205–12.
- Luecken LJ, Appelhans BM. (2006). Early parental loss and salivary cortisol in young adulthood: The moderating role of family environment. Dev Psychopathol 18:295–308.
- MacPherson JS. (1958). Eleven year olds grow up. London: University of London Press.
- Maxwell J. (1969). Sixteen years on: a follow-up of the 1947 Scottish Survey. London: University of London Press.
- Newkirk LA, Kim JM, Thompson JM, Tinklenberg JR, Yesavage JA, Taylor JL. (2004). Validation of a 26-point telephone version of the Mini-Mental State Examination. J Geriatr Psychiatry Neurol 17:81–7.
- Nicolson NA. (2004). Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology 29:1012–18.
- Roccaforte WH, Burke WJ, Bayer BL, Wengel SP. (1992). Validation of a telephone version of the mini-mental state examination. J Am Geriatr Soc 40:697–702.
- Sapolsky RM. (1996). Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1:1–19.
- Sapolsky RM, Krey LC, McEwen BS. (1986). The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 7:284–301.
- Schalinski I, Elbert T, Steude-Schmiedgen S, Kirschbaum C. (2015). The cortisol paradox of trauma-related disorders: lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS One 10:e0136921.
- Schreier HMC, Bosquet Enlow M, Ritz T, Gennings C, Wright RJ. (2015). Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. J Epidemiol Community Health 69:1169–74.
- Scottish Council for Research in Education. (1933). The intelligence of Scottish children: a national survey of an age group. London: University of London Press.
- Scottish Council for Research in Education. (1949). The trend of Scottish intelligence: a comparison of the 1947 and 1932 surveys of the intelligence of eleven-year-old pupils. London: University of London Press.
- Spencer RL, McEwen BS. (1990). Adaptation of the hypothalamic–pituitary–adrenal axis to chronic ethanol stress. Neuroendocrinology 52:481–9.
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. (2010). Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol 22:165–75.
- van der Vegt EJ, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. (2009). Early neglect and abuse predict diurnal cortisol patterns in adults A study of international adoptees. Psychoneuroendocrinology 34:660–9.
- Vedhara K, Miles J, Crown A, McCarthy A, Shanks N, Davies D, Lightman S, et al. (2007). Relationship of early childhood illness with adult cortisol in the Barry Caerphilly Growth (BCG) cohort. Psychoneuroendocrinology 32:865–73.
- Zhao ZY, Lu FH, Xie Y, Fu YR, Bogdan A, Touitou Y. (2003). Cortisol secretion in the elderly. Influence of age, sex and cardiovascular disease in a Chinese population. Steroids 68:551–5.
