Abstract
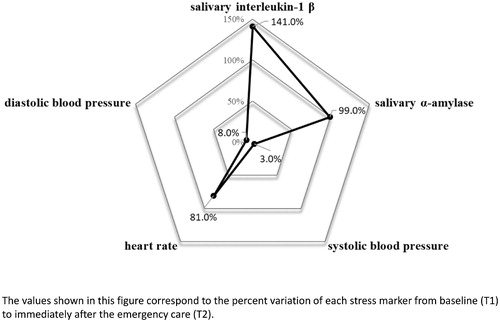
Providing care for simulated emergency patients may induce considerable acute stress in physicians. However, the acute stress provoked in a real-life emergency room (ER) is not well known. Our aim was to assess acute stress responses in residents during real emergency care and investigate the related personal and situational factors. A cross-sectional observational study was carried out at an emergency department of a tertiary teaching hospital. All second-year internal medicine residents were invited to voluntarily participate in this study. Acute stress markers were assessed at baseline (T1), before residents started their ER shift, and immediately after an emergency situation (T2), using heart rate, systolic, and diastolic blood pressure, salivary α-amylase activity, salivary interleukin-1 β, and the State-Trait Anxiety Inventory (STAI-s and STAI-t). Twenty-four residents were assessed during 40 emergency situations. All stress markers presented a statistically significant increase between T1 and T2. IL-1 β presented the highest percent increase (141.0%, p < .001), followed by AA (99.0%, p = .002), HR (81.0%, p < .001), DBP (8.0%, p < .001), and SBP (3.0%, p < .001). In the multivariable analysis, time of residency had a negative correlation with HR during the emergency (adjusted R-square = .168; F = 8.69; p = .006), SBP response (adjusted R-square = .210; F = 6.19; p = .005) and DBP response (adjusted R-square = .293; F = 9.09; p = .001). Trait anxiety (STAI-t) was positively correlated with STAI-s (adjusted R-square = .326; F = 19.9; p < .001), and number of procedures performed during emergency care had a positive association with HR response (adjusted R-square = .241; F = 5.02; p = .005). In the present study, emergency care provoked substantial acute stress in residents. Resident experience, trait anxiety, and number of emergency procedures were independently associated with acute stress response.
Introduction
Despite the wide variety of definitions and interpretations related to the term stress throughout the last two centuries, Hans Selye was the one who first coined the concept of stress as we known nowadays (Goldstein & Kopin, Citation2007). In fact, since his first publication in 1936, describing the “Syndrome Produced by Diverse Nocuous Agents,” thousands of studies based on the concept of “Generalized Adaptation Syndrome” have been published across diverse knowledge fields (Szabo, Tache, & Somogyi, Citation2012). According to this definition, stress response develops in three stages: the first stage is an expression of the general alarm reaction of the organism when suddenly confronted with a critical situation; the second stage, called resistance, reflects the organism’s attempt to adapt and cope with the stressor; the third phase occurs when, during the adaptation process, the resources are eventually depleted in the face of sustained demand, and the organism suffers exhaustion (Selye, Citation1936).
Although these last two phases, related to chronic stress, have been largely investigated in the past decades, the alarm phase, also denominated acute stress, has not received the same attention. In the emergency medicine field, for instance, which is well recognized as an intense and constant source of stressors to healthcare professionals (Levey, Citation2001; Phipps, Citation1988), the majority of studies are focused on the prevalence and effects of chronic stress (Çalişkan Tür, Toker, Şaşmaz, Hacar, & Türe, Citation2016; Johnston et al., Citation2016). A scarce number of studies have assessed acute stress response during emergency care (Clarke, Horeczko, Cotton, & Bair, Citation2014; Keitel et al., Citation2011). Some of these studies sought to investigate the factors associated with acute stress in emergency physicians, measuring heart rate (HR) parameters as biomarkers (Dutheil et al., Citation2012, Citation2013, Citationin press).
Recognizing and measuring acute stress response in physicians during actual emergency situations is the first step to understand the intensity of this alarm reaction phase and its possible effects on physicians’ mental and physical health, as well as its relation with performance and quality of patient care. In this context, the primary aim of the present study was to assess the acute stress response in second-year internal medicine residents during emergency situations in a real-life ER. The secondary aim was to investigate the personal and situational factors associated with acute stress in this setting.
Methods
Study design and setting
This is a cross-sectional (pre-test/post-test) study, carried out between February 2011 and July 2013, at the Emergency Department (ED) of the Hospital das Clínicas of the University of São Paulo Medical School, in Brazil. This study was approved by the Ethics and Research Committee of University of São Paulo (CAPPesq. Project number: 0629/10). The ED is part of a public tertiary teaching hospital and has approximately 55,000 annual visits, of which 14,000 result in hospital admissions, being 4500 in intensive care units. In the ED, there are two 4-bed ERs: one surgical ER for trauma/surgical emergencies and one medical ER for clinical emergencies. In the medical ER, a team formed by one attending physician, one second-year internal medicine resident, and two nurses is responsible for providing care for patients presenting clinical emergencies.
Participants
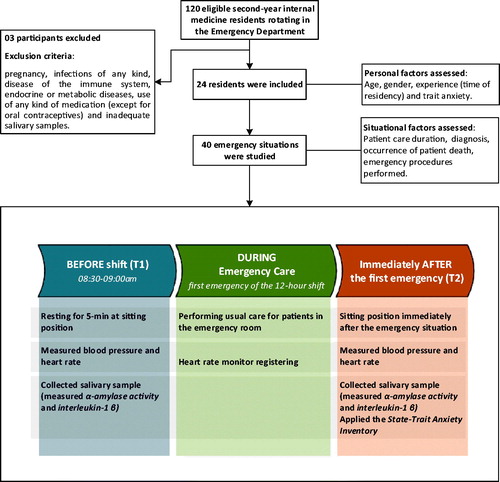
We invited all second-year internal medicine residents rotating in the ED during the study period to voluntarily participate in this study. All residents who accepted to participate were oriented regarding the written informed consent and provided a signature. In our hospital, the internal medicine residency has a duration of 2 years and 48 residents are admitted each year. Exclusion criteria were: pregnancy, infections of any kind, the disease of the immune system, endocrine or metabolic diseases, use of any kind of medication (except for oral contraceptives), and inadequate salivary samples. Of the 40 emergency situations included in the present research, 16 measures were reported in a previously published study, in which the aim was to compare acute stress response in a real-life setting to simulated scenarios (Daglius-Dias & Scalabrini-Neto, Citation2016).
Procedures
The ED rotation has a total duration of 45 d. Residents work in 12-h daytime shifts, performing a maximum of FIVE shifts (60 h) per week. The study protocol was applied only during workdays and participation of the same resident more than once was allowed, as long as in different days.
In order to assess acute stress response during emergency care, data collection was carried out before residents started their ER shift, between 08:30 am and 09:00 am. The resident (only one per day) was placed sitting at rest for 5 min and their baseline (T1) parameters were measured. Immediately after they completed the first emergency care situation of the 12-h shift (T2), the parameters were measured again and the State-Trait Anxiety Inventory (STAI) was applied. During the period between T1 and T2, the participant remained with a HR monitor. The study design and procedures are summarized in .
For this study, we considered the following clinical emergency care situations: a) shock (any kind); b) acute respiratory failure requiring invasive ventilation or noninvasive ventilation with positive pressure; c) cardiac arrest; and d) arrhythmias with hemodynamic instability. The end of the emergency care situation (T2) was considered when: a) patient’s mean arterial pressure was greater than 65 mmHg; b) oxygen saturation by pulse oximetry was greater than 90% after confirmed endotracheal tube position; c) there was return of spontaneous circulation maintained for at least 5 min or when terminated efforts and death was confirmed; or d) patient was hemodynamically stable after therapeutic measures. The researcher only approached the resident after the attending physician indicated that the resident’s presence was no longer needed for patient’s clinical stabilization.
Personal and situational factors
The following personal factors were investigated regarding their association with acute stress: age, gender, experience (time of residency), and trait anxiety (trait component of STAI).
The situational factors analyzed were: time between baseline (T1) and care initiation; emergency care duration; emergency diagnosis (respiratory failure, cardiac arrest, shock or unstable arrhythmias); occurrence of death until end of care (T2); use of vasopressor agents (epinephrine, norepinephrine, dopamine, dobutamine or vasopressin); and emergency procedures performed during care (orotracheal intubation, chest compression, central venous catheterization, and electrical therapy). Electrical therapy involved one or a combination of the following: defibrillation, synchronized cardioversion, transcutaneous pacing or transvenous pacing. Since various emergency procedures may have been performed during the same emergency situation, we investigated if the total number of procedures performed by residents was associated with acute stress responses.
Acute stress markers
Heart rate (HR)
This parameter was continuously measured using a HR monitor (FT2 Model, Polar Electro Oy, Kempele, Finland). The maximum and average HR (during the stress situation) was recorded in the watch system. HR response was calculated as the difference between the maximum HR and the baseline in T1. For HR response, the maximum HR value was considered as T2.
Systolic blood pressure (SBP)
This measure was obtained using an aneroid sphygmomanometer (Model Shock Resistant, Welch Allyn Inc., Skaneateles Falls, NY, USA) and the mean of three measures was considered. SBP response was calculated as the SBP difference between T2 and T1.
Diastolic blood pressure (DBP)
This measure was obtained using an aneroid sphygmomanometer (Model Shock Resistant, Welch Allyn Inc.) and the mean of three measures was considered. DBP response was calculated as the DBP difference between T2 and T1.
Salivary analyses
The collection, storing, and analysis methods were carried out as described by us in a previous study (Daglius-Dias & Scalabrini-Neto, Citation2016). Acute stress response was calculated as the difference between T2 and T1. For the salivary samples, we measured:
Salivary α-amylase activity (AA): Measured by a kinetic colorimetric test (alpha-amylase Salivary Assay Kit, SALIMETRICS Inc., State College, PA). AA is a marker of acute stress in healthy adults (Takai et al., Citation2004), and commonly used in research involving clinical simulation (Valentin et al., Citation2015).
Salivary interleukin-1 β (IL-1β): Measured by an immune-enzymatic method (Salivary IL-1β Kit, SALIMETRICS Inc.). IL-1β is a recent marker used to measure acute stress (Daglius-Dias & Scalabrini-Neto, Citation2016; Slavish, Graham-Engeland, Smyth, & Engeland, Citation2015).
State anxiety scale (STAI-s): This is one of the two components of the STAI (Spielberger, Citation1970). It is a widely used self-report scale, reflecting state anxiety, translated and validated for Brazilian Portuguese (Gorenstein & Andrade, Citation1996). The STAI-s was applied to participants immediately after the end of emergency care (T2). The trait component (STAI-t), also applied in T2, was used to indicate trait anxiety.
Statistical analysis
A sample size calculation was performed based on the nomogram proposed by Altman (Citation1991) and the results of our previous study with simulated and real emergency situations (Daglius-Dias & Scalabrini-Neto, Citation2016). In this study, a standard deviation of 11.6 beats per minute (baseline) was found in the real-life group. At least 16 observations were required to detect an increase of 15% from baseline (T1), setting alpha error at .05 (two-sided) with 80% power. The Shapiro–Wilk test was used to assess normality for each variable. Data was expressed as median and interquartile ranges (IQR) for continuous variables or number and percentage for categorical variables. Continuous data was compared between T1 and T2 (related samples) with the Wilcoxon signed-rank test.
Pairwise association between each personal and situational factor and each acute stress marker, as well as among the different acute stress markers, was performed using univariate analysis. For continuous variables, Spearman’s rank order correlation test was used. For categorical variables, Mann–Whitney U test (two categories) and Kruskal–Wallis test (more than two categories) were adopted.
The use of univariate analyses to screen independent variables for inclusion in multivariable analysis has been considered inappropriate (Sun, Shook, & Kay, Citation1996). Since our study has several factors potentially associated with acute stress, we performed a multiple regression analysis using best subset selection method in order to assess variables that may be independently associated with each acute stress marker. Therefore, all independent variables were included in this regression analysis in order to identify the best-fitting regression model. The corrected Akaike information criterion was set as the goodness of fit to assess subset. Adjusted R-square, F value and 95% confidence intervals (CI) were reported for all multivariable comparisons. The level of statistical significance was set at .05 (two-side). All analyses and graph creation were conducted using the software Excel 2016 (Microsoft Inc., Redmond, WA) and SPSS Statistics (version 22.0, IBM Inc., Armonk, NY).
Results
During the study period, 120 eligible residents rotated in the ED. Of these, 27 (22.5%) accepted to participate in the study and three were excluded: one due to use of beta-blocker medication and two due to inadequate salivary samples. Therefore, a total of 24 participants had their acute stress response assessed during 40 emergency situations in the ER. The demographic characteristics of the participants are shown in .
Table 1. Demographic characteristics.
Patients with cardiac arrest initially presented ventricular fibrillation (two cases), pulseless electrical activity (seven cases), and asystole (three cases). Patients with shock presented septic shock (10 cases) and cardiogenic shock (one case). Four patients received defibrillation, two received synchronized cardioversion, one received transcutaneous pacing and one received transcutaneous pacing followed by transvenous pacing. During emergency situations, medical procedures were performed by either the resident or other team members. Residents performed eight (40.0%) orotracheal intubations; five (38.5%) central venous catheterizations; seven (100.0%) electrical therapies; and performed thoracic compressions in seven (58.3%) patients.
The HR during the stress situation presented a median of 94.0 (IQR: 84.0–101.0) beats/min. The median STAI-t score was 35.5 (IQR: 33.0–41.5) points. Parameters at baseline (T1) and immediately after the emergency care (T2) are shown in . All physiological markers increased significantly from baseline (T1) to T2. Percentage of variation from baseline (T1) of each acute stress marker was calculated. shows the comparison between the six acute stress markers in relation to the median variation among all residents.
Table 2. Acute stress markers between T1 and T2.
Univariate analysis demonstrated that time of residency presented a negative correlation with HR during the emergency situation (coef. correlation = −.38, p = .018), SBP response (coef. correlation = −.49, p = .001), and DBP response (coef. correlation = −.51, p = .001). Trait anxiety presented a positive correlation with STAI-e (coef. correlation = .57, p < .001). The remaining univariate analysis did not present statistical significance ( and ).
Table 3. Univariate analysis between personal and situational factors (continuous variables) and acute stress markers.
Table 4. Univariate analysis between personal and situational factors (categorical variables) and acute stress markers.
Multivariable best subset analysis, including all independent variables, evidenced that time of residency, trait anxiety, and number of emergency procedures were factors independently associated with acute stress response, as shown in . Correlation analyses among the six different parameters measuring acute stress response demonstrated, overall, a poor correlation ().
Table 5. Independent factors associated with acute stress assessed by multivariable best subset regression model.
Table 6. Correlation analysis among the different acute stress markers.
Discussion
In the present study, we assessed physiological and psychological markers related to acute stress response in residents during real-life emergency care. The main findings were that time of residency and number of emergency procedures presented an independent association with acute stress response. Furthermore, the presence of trait anxiety among residents was associated with STAI-s. This last finding is in accordance with a previous study involving medical residents (Peterlini, Tibério, Saadeh, Pereira, & Martins, Citation2002) and is also supported by Jezova, Makatsori, Duncko, Moncek, & Jakubek (Citation2004), who reported an inadequate neuroendocrine response associated with high trait anxiety in healthy subjects.
Acute stress markers
In accordance with a previous study carried out by our group comparing simulated and real-life settings (Daglius-Dias & Scalabrini-Neto, Citation2016), a significant increase of salivary IL1-β was observed in residents during emergency care. The effect of stress on the immune response of emergency physicians was also assessed by Dutheil et al. (Citation2013). The authors reported an increase of urinary interleukin-8 after ED shifts associated with chronic stress. To our knowledge, we are the first to demonstrate the effects of acute stress in salivary IL-1 β in the emergency setting. Since residents provide care for several emergency patients during ER shifts, future studies may investigate the effects of repeated inflammatory responses provoked by acute stress. Some studies have suggested an association between inflammatory response caused by chronic stress and cardiovascular diseases (Cohen, Edmondson, & Kronish, Citation2015), but little is known regarding the effects of repeated acute stress responses.
The present study demonstrates a wide variability and poor correlation among the different acute stress markers. These results are corroborated by recent stress theories that, using the concept of allostasis, explain stress and coping strategies as an integrative state determined by genetic, developmental, environmental, and previous experiential factors (Goldstein & Kopin, Citation2007). According to the allostatic concept of stress, adaptive activities of effector systems are coordinated in specific patterns, including cardiovascular, neuroendocrine, immune, and behavioral. These patterns serve different needs and, therefore, the response of each system may vary depending on a myriad of factors (Goldstein & McEwen, Citation2002). Specific patterns of the stress response, allied to sharing of effectors between multiple systems, make stress research an intriguing challenge. Aiming to maintain organism stability through repeated changes during life, in other words, to adapt, organisms use allostatic mechanisms to cope with stress. This consists in setting homeostats for each system in order to control the level of afferent information (demand and resource appraisals) that will trigger efferent information to effectors (cardiovascular, neuroendocrinological, immune, and psychological stress responses) (Goldstein, Citation1995). According to the allostatic adaptation model, continuous or prolonged repeated stress events lead to homeostat resetting in order to redefine the conditions required to maintain homeostasis. In fact, studies involving human and animal stress have reported lessening of physiological stress response occurring with repetition (Kelsey et al., Citation1999). This habituation to stressors may explain our results, which evidenced a negative association between resident experience and HR and blood pressure responses. Similarly, other studies also have reported smaller acute stress response among senior residents compared to junior residents (Tendulkar et al., Citation2005). Nevertheless, this habituation mechanism may represent a homeostat oversetting only in the cardiovascular system, and not necessarily indicates acute stress attenuation throughout the residency training. Indeed, only the cardiovascular response was negatively associated with the time of residency in our study and the other acute stress markers (psychological and immunological) did not present such correlation. These findings indicate that future studies involving acute stress should use several different measurements, since the different systems may present diverse allostatic regulation.
Personal and situational factors associated withacute stress
In our study, the number of procedures performed by residents in the same emergency situation presented a positive correlation with acute stress, when it was assessed by HR response. In contrast, Wrenn, Lorenzen, Jones, Zhou, & Aronsky (Citation2010) did not find an association between number of procedures reported by residents at the end of an ER shift and acute stress assessed by self-reported instruments. However, the authors used a subjective measure of stress and the residents reported all activities performed during an entire shift.
Our findings suggest that a high workload generated from either inadequate task management or human resources scarcity, may be related to acute stress in residents, and this aspect requires the attention of residency program coordinators.
Future directions
Allostatic responses are usually adaptive in the short term. However, prolonged continuous or intermittent activation of effector systems may lead to a state known as allostatic load. In this state, intense and/or prolonged homeostat oversetting may induce instability toward maladaptive behavioral and deleterious effects (McEwenn & Stellar, Citation1993). Future studies should be designed in order to determine the impact of acute stress on physician’s mental and physical health, as well as in their clinical performance related to the quality of patient care. We suggest assessing the intensity of this alarm reaction phase and the impact of repeated critical events using several markers, once acute stress response presents an intricate complexity. Since critical, challenging, and sometimes threating situations are inherent to residency training, the identification of negative effects of acute stress may play an important role in the resident well-being and quality of patient care in the ED.
Limitations
First, less than one-third of all eligible residents accepted to participate in this study, favoring the occurrence of selection bias. Moreover, acute stress was assessed only during daytime period and in the first emergency situation of the resident’s shift. Futures studies should assess acute stress at different times of the day in order to investigate other potential associated factors. Due to the impossibility of programing real-life situations, the post-emergency time (T2) presented a wide variation. This limitation cannot be resolved because emergencies happen by chance. Additionally, due to the limited sample size, we evaluated only some personal and situational factors to allow a multivariable analysis. Future investigations, using a larger sample, may evaluate other factors, such as the occupational characteristics of the ED, sleep condition, and physical activity. Regarding the stress markers, we applied the STAI-s instrument only at the end of the emergency care. However, this parameter should ideally be used before and after the stress situation, in order to measure the changes in the anxiety level (Jezova et al., Citation2004). Salivary α-amylase activity presents a circadian rhythm of production (Rantonen & Meurman, Citation2000) and this may have influenced the variation between T1 and T2 in our study. Future studies, using a control group without stressors, can address this limitation. Finally, since this study was carried out in a single center and included only second-year internal medicine residents, the findings may not necessarily generalize to other settings.
Conclusions
In summary, this study demonstrates that real-life emergency care provokes substantial acute stress response in residents, as assessed by physiological, psychological, and immune markers. In addition, the findings suggest that resident experience, trait anxiety, and number of emergency procedures are associated with acute stress. Additional research is necessary to determine the effects of prolonged repeated acute stress on physician’s health, performance, and quality of care.
Acknowledgements
This research was funded by FAPESP (São Paulo Research Foundation).
Disclosure statement
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported. The sponsors had no role in the design or conduct of the present research; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Additional information
Funding
References
- Altman, G.G. (1991). Practical statistics for medical research. London: Chapman & Hall.
- Çalişkan Tür, F., Toker, İ., Şaşmaz, C.T., Hacar, S., & Türe, B. (2016). Occupational stress experienced by residents and faculty physicians on night shifts. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 24, 34. doi:10.1186/s13049-016-0225-4
- Clarke, S., Horeczko, T., Cotton, D., & Bair, A. (2014). Heart rate, anxiety and performance of residents during a simulated critical clinical encounter: A pilot study. BMC Medical Education, 14, 153. doi:10.1186/1472-6920-14-153
- Cohen, B.E., Edmondson, D., & Kronish, I.M. (2015). State of the art review: Depression, stress, anxiety, and cardiovascular disease. American Journal of Hypertension, 28, 1295–1302. doi:10.1093/ajh/hpv047
- Daglius-Dias, R., & Scalabrini-Neto, A. (2016). Stress levels during emergency care: A comparison between reality and simulated scenarios. Journal of Critical Care, 33, 8–13. doi:10.1016/j.jcrc.2016.02.010
- Dutheil, F., Boudet, G., Perrier, C., Lac, G., Ouchchane, L., Chamoux, A., … Schmidt, J. (2012). JOBSTRESS study: Comparison of heart rate variability in emergency physicians working a 24-hour shift or a 14-hour night shift-a randomized trial. International Journal of Cardiology, 158, 322–325. doi:10.1016/j.ijcard.2012.04.141
- Dutheil, F., Marhar, F., Boudet, G., Perrier, C., Naughton, G., Chamoux, A., … Schmidt, J. (in press). Maximal tachycardia and high cardiac strain during night shifts of emergency physicians. International Archives of Occupational and Environmental Health. doi:10.1007/s00420-017-1211-5
- Dutheil, F., Trousselard, M., Perrier, C., Lac, G., Chamoux, A., Duclos, M., … Schmidt, J. (2013). Urinary interleukin-8 is a biomarker of stress in emergency physicians, especially with advancing age – the JOBSTRESS* randomized trial. PLoS One, 8, e71658. doi:10.1371/journal.pone.0071658
- Goldstein, D.S. (1995). Stress as a scientific idea: A homeostatic theory of stress and distress. Homeostasis, 36, 177–215.
- Goldstein, D.S., & Kopin, I.J. (2007). Evolution of concepts of stress. Stress (Amsterdam, Netherlands), 10, 109–120. doi:10.1080/10253890701288935
- Goldstein, D.S., & McEwen, B. (2002). Allostasis, homeostats, and the nature of stress. Stress (Amsterdam, Netherlands), 5, 55–58. doi:10.1080/102538902900012345
- Gorenstein, C., & Andrade, L. (1996). Validation of a Portuguese version of the Beck Depression Inventory and the State-Trait Anxiety Inventory in Brazilian subjects. Brazilian Journal of Medical and Biological Research, 29, 453–457.
- Jezova, D., Makatsori, A., Duncko, R., Moncek, F., & Jakubek, M. (2004). High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28, 1331–1336. doi:10.1016/j.pnpbp.2004.08.005
- Johnston, A., Abraham, L., Greenslade, J., Thom, O., Carlstrom, E., Wallis, M., Crilly, J. (2016). Review article: Staff perception of the emergency department working environment: Integrative review of the literature. Emergency Medicine Australasia, 28, 7–26. doi:10.1111/1742-6723.12522
- Keitel, A., Ringleb, M., Schwartges, I., Weik, U., Picker, O., Stockhorst, U., Deinzer, R. (2011). Endocrine and psychological stress responses in a simulated emergency situation. Psychoneuroendocrinology, 36, 98–108. doi:10.1016/j.psyneuen.2010.06.011
- Kelsey, R.M., Blascovich, J., Tomaka, J., Leitten, C.L., Schneider, T.R., & Wiens, S. (1999). Cardiovascular reactivity and adaptation to recurrent psychological stress: Effects of prior task exposure. Psychophysiology, 36, 818–831.
- Levey, R.E. (2001). Sources of stress for residents and recommendations for programs to assist them. Academic Medicine, 76, 142–150.
- McEwenn, B.S., & Stellar, E. (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 28, 897–902.
- Peterlini, M., Tibério, I.F., Saadeh, A., Pereira, J.C., & Martins, M.A. (2002). Anxiety and depression in the first year of medical residency training. Medical Education, 36, 66–72.
- Phipps, L. (1988). Stress among doctors and nurses in the emergency department of a general hospital. CMAJ, 139, 375–376.
- Rantonen, P.J., & Meurman, J.H. (2000). Correlations between total protein, lysozyme, immunoglobulins, amylase, and albumin in stimulated whole saliva during daytime. Acta Odontologica Scandinavia, 58, 160–165.
- Selye, H. (1936). A syndrome produced by diverse nocuous agents. Nature, 138, 32.
- Slavish, D.C., Graham-Engeland, J.E., Smyth, J.M., & Engeland, C.G. (2015). Salivary markers of inflammation in response to acute stress. Brain, Behavior, and Immunity, 44, 253–269. doi:10.1016/j.bbi.2014.08.008
- Spielberger, C.D. (1970). Manual for state-trait anxiety inventory. Palo Alto (CA): Consulting Psychologists Press.
- Sun, G.W., Shook, T.L., & Kay, G.L. (1996). Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology, 49, 907–916.
- Szabo, S., Tache, Y., & Somogyi, A. (2012). The legacy of Hans Selye and the origins of stress research: A retrospective 75 years after his landmark brief “letter” to the editor# of nature. Stress, 15, 472–478. doi:10.3109/10253890.2012.710919
- Takai, N., Yamaguchi, M., Aragaki, T., Eto, K., Uchihashi, K., & Nishikawa, Y. (2004). Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Archives of Oral Biology, 49, 963–968. doi:10.1016/j.archoralbio.2004.06.007
- Tendulkar, A.P., Victorino, G.P., Chong, T.J., Bullard, M.K., Liu, T.H., & Harken, A.H. (2005). Quantification of surgical resident stress “on call”. Journal of the American College of Surgeons, 201, 560–564. doi:10.1016/j.jamcollsurg.2005.05.004
- Valentin, B., Grottke, O., Skorning, M., Bergrath, S., Fischermann, H., Rörtgen, D., et al. (2015). Cortisol and alpha-amylase as stress response indicators during pre-hospital emergency medicine training with repetitive high-fidelity simulation and scenarios with standardized patients. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 23, 31. doi:10.1186/s13049-015-0110-6
- Wrenn, K., Lorenzen, B., Jones, I., Zhou, C., & Aronsky, D. (2010). Factors affecting stress in emergency medicine residents while working in the ED. The American Journal of Emergency Medicine, 28, 897–902. doi:10.1016/j.ajem.2009.05.001