Abstract
Stress response is associated with increased activity in the hypothalamic–pituitary–adrenocortical axis. Chronic stress-induced elevation in cortisol may alter its own negative regulation with multiple long-term consequences for physical and psychological health. One of the most reliable physical traits associated with mental, apparent physical health, and competitiveness is the degree of facial fluctuating asymmetry. However, to our knowledge there are no studies regarding the relationship between cortisol levels, facial symmetry and male competitiveness, and how cortisol changes after a stressful test depending on these traits. Here, a group of 100 college men were photographed to obtain their facial asymmetry levels. They then, answered the perceived stress scale and the intrasexual competition test and donated two saliva samples (pre-and post-test sample) to measure the change in their cortisol levels after a stressful test. We found that basal cortisol levels were positively correlated with both perceived stress and competitiveness, but not with facial fluctuating asymmetry. Cortisol levels increased in most symmetrical men after a short stressful test, but it decreased in most asymmetrical men. The results suggest differences in endocrine responses according to facial fluctuating asymmetry in men and how these responses could be related to the maintenance of social status.
Introduction
The stress response refers to the activation of a neuroendocrine cascade of events after confronting a stressful situation (acute stressor). This response is mediated by the hypothalamic–pituitary–adrenal (HPA) axis and the release of cortisol from the adrenal gland helps to maintain body homeostasis (McEwen, Citation1998, Citation2008). Chronic stress-induced cortisol elevation alters the HPA axis feedback regulation leading to long-term damages in physical and psychological health (Cohen & Herbert, Citation1996; Kurina, Schneider, & Waite, Citation2004; Leor, Poole, & Kloner, Citation1996; McEwen, Citation1998, Citation2008; Munck, Guyre, & Holbrook, Citation1984; Reul et al., Citation2015). One of the more popular tools for measuring psychological stress is a self-reported questionnaire that was designed to measure “the degree to which individuals appraise situations in their lives as stressful” (Cohen, Kamarak, & Mermelstein, Citation1983). The literature has shown that, in humans, one of the most reliable physical traits associated with mental and apparent physical health is the facial fluctuating asymmetry (FA) degree (Jones et al., Citation2001; Thornhill & Gangestad, Citation2006). FA is defined as small random deviations from perfect symmetry in normal bilateral traits in a population (van Valen, Citation1962). This trait may increase from the inability of individuals to cope with genetic and environmental stress, and a low FA has been used as a putative indicator of developmental stability (Hume & Montgomeri, Citation2001; Møller, Citation1997; Polak, Citation2003).
Fluctuating asymmetry even been shown to increase with stress in humans before birth. Genetic perturbations and prenatal cortisol exposure due to maternal stress can negatively affect fetal development. This in turn affects infant neuroendocrine stress axis regulation (Levendosky et al., Citation2016; Singh & Rosen, Citation2001; Zadzinska, Koziel, Kurek, & Spinek, Citation2013). As adults, high levels of stress might be visually perceived through higher facial FA scores. FA has also been associated with male fighting ability and competitiveness in adolescents. For example, symmetrical men are more likely to use direct competition tactics than asymmetrical men (Simpson, Gangestad, Christensen, & Leck, Citation1999). Thus, high symmetry is related to better performance in acquiring resources and coping with challenging situations (Muñoz-Reyes, Gil-Burmann, Fink, & Turiegano, Citation2012; Thornhill, Citation1992).
Acute hormonal responses to a competitive situation mobilize the energetic demands necessary to confront a challenge. Mehta, Jones, and Josephs (Citation2008) studied competitions and found that the winning men showed decreased cortisol levels compared to an increase in the losers. The same authors suggested that higher cortisol levels after a defeat could signal psychological stress and anxiety. However, to our knowledge, there are no studies of how cortisol levels are directly associated with facial FA scores and competitiveness. Nor studies on how cortisol levels change after a short stressful test as a function of male FA.
This study had two main goals. First, to investigate whether facial FA is related to basal cortisol levels, competitiveness scores, and perceived stress scores [according to Cohen et al. (Citation1983) the degree to which life events are appraised as stressful]. Second, to investigate whether cortisol responses vary according to facial FA, perceived stress, and competitiveness scores in men. We expected that men with lower facial FA scores (symmetrical men) would have an increase in cortisol levels after a short stressful test while men with higher facial FA scores (asymmetrical men) would have no change or even a decrease in cortisol levels after the test.
Materials and methods
Participants
A group of 100 college men were recruited. All were heterosexual and had an age range of 18–30 years (mean ± SD =21.44 ± 2.44). The sample size was calculated with a confidence level of 95% according to Daniel (Citation1982). The participants were recruited via posters, e-mail advertisements, or in person. All volunteers received $10 USD for their participation, and all signed an informed consent letter in which the procedure and objective were clearly explained to them.
Facial asymmetry (FA) measurements
The participants were asked to sit in a chair so that a photograph of their face could be taken. We used a digital camera (Sony DSC H5) and a tripod located at a constant distance of 1.5 m from the chair. No flash was used, and all the photographs had the same natural light conditions. The participants were instructed to present their faces with a neutral and relaxed expression and to keep their mouth closed. The shoulders of each subject were aligned on a horizontal axis. Each photograph was digitized using a PC at a resolution of 300 dpi. The facial FA measurements (in mm) were made using Adobe Photoshop (CS3 Extended version 10.0).
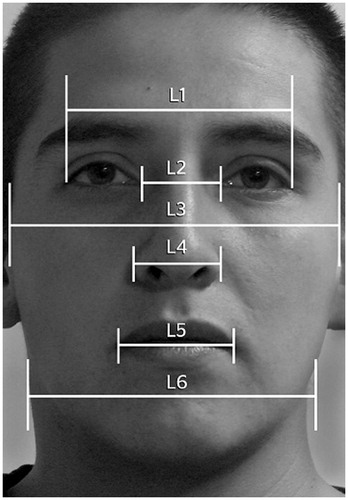
The FA was calculated following a procedure similar to that used by Grammer and Thonrhill (Citation1994) and Scheib, Gangestad, and Thornhill (Citation1999). We drew six horizontal points () corresponding to the outer and inner parts of the corners of the eyes (L1 and L2), the cheekbones (L3), the outer edges of the lower nose region (L4), the corners of the mouth (L5), and the bones of the jaw (L6). These delimited the outer part of the face along the horizontal axis of the mouth. The midpoints of each line were calculated using the following formula: [(left point – right point/2) + right point]. In a perfectly symmetrical face, all midpoints are positioned on the same vertical line and the sum of all the differences is zero. A face becomes less symmetrical as these points become farther from zero. The 100 faces showed the following FA values: mean ± SD =0.52 ± 0.45; maximum and minimum: 2.50–0.05. To ensure that the measurements were reliable, two researchers made the FA measurements independently in 20 images and calculated the intraclass correlation coefficient (r = 0.92, p < .001). We used the average of the two measures from two of the researchers.
Measuring perceived stress and competitiveness
After the photography, the volunteers completed the 14-item perceived stress scale (PSS), which is a measure of the perceptions on negative emotions of the last month (Cohen et al., Citation1983) (e.g. “in the last month, how often have you been upset because of something that happened unexpectedly?”, “in the last month, how often have you been able to control irritations in your life?”). This was previously used by González and Landero (Citation2007) in a Mexican population, and 12-item intrasexual competition test (ICT) which assesses the degree of male–male competitiveness (Buunk & Fisher, Citation2009) (e.g. “I want to be just a little better than other men”, “I always want to beat other men”, “I tend to look for negative characteristics in attractive men”) previously used by Borráz-León, Cerda-Molina, and Mayagoitia-Novales (Citation2016) in a Mexican population. The central tendency and dispersion measurements from the results of these questionnaires were as follows: PSS: mean ± SD =21.75 ± 5.94, maximum and minimum: 39–5; ICT: mean ± SD =36.00 ± 11.85, maximum and minimum: 65–12.
Stress response test
In individual sessions, each volunteer came to the laboratory and relaxed for approximately 5 min (Sumioka, Nakae, Kanai, & Ishiguro, Citation2013; Yamazaki et al., Citation2016). They then proceeded to donate a first saliva sample that was 6 ml in a sterile polypropylene tube (pretest sample). The exclusion criteria for the test were: (1) exercising, (2) eating, (3) drinking coffee, soda, or sugary beverages, (4) smoking, all of them one hour before the test. The inclusion criteria were: (1) brushing their teeth before coming to the lab, (2) drinking water. Volunteers answered a questionnaire on a personal computer about general data (e.g. age, weight, height, marital status, etc.). After collecting the pretest sample and answering the general data questionnaire, volunteers were briefly interviewed in a closed room face-to-face by a male researcher about personal and intimate data (e.g. number of sexual partners, drug and alcohol use, etc.) to create a stressful situation. Being interviewed face-to-face is known to increase stress and possibly cortisol levels because personal information could be judged by the interviewer putting the interviewed person in a situation of vulnerability or in a social-evaluative threat (Schmidt-Reinwald et al., Citation1999; Seta & Seta, Citation1995; see Dickerson & Kemeny, Citation2004 for a review). Finally, volunteers donated the second saliva sample 15 min after finishing the interview (post-test sample). The samples were taken between 10 am and 2 pm to minimize the effects of circadian hormonal fluctuations (Touitou & Haus, Citation2000).
Measuring cortisol
Immediately after collection, the samples were frozen with acetone and dry ice and stored at −20 °C. Samples were subjected to three subsequent freeze-thaw cycles to free them from mucopolysaccharides and proteins. Upon thawing, the samples were centrifuged at 3000 rpm for 30 min at 4 °C; the supernatants were collected, and the samples were frozen again and stored at −20 °C until processing (Schultheiss, Dargel, & Rohde, Citation2003). Cortisol measurements were carried out with commercial ELISA kits (Cortisol (Saliva) 11-CORHU-E01-SLV, ALPCO). The inter-assay and intra-assay coefficients for cortisol levels were 8.33% and 8.23%, respectively. The concentrations of cortisol were reported in ng/ml.
Statistical analysis
The sample size was found to be statistically significant using the following formula: n = ((t2) p (1–p)/m2), where n = required sample size, t = 95% confidence level (standard value of 1.96), p = estimated prevalence of volunteers who can participate (value 0.95), m = 5% of error range (standard value of 0.05). The Kolmogorov–Smirnov test shows that cortisol levels, competitiveness, and perceived stress scores were normally distributed (pretest cortisol: Z = 0.915, p = .372; post-test cortisol: Z = 1.190, p = .118; competitiveness: Z = 0.920, p = .365; perceived stress: Z = 0.757, p = .615), while facial FA scores were not (Z = 1.870, p = .002). The facial FA scores were transformed to logarithmic values (Log10) to normalize them (Z = 0.622, p = .834). To address the first goal, we used Pearson correlations to explore possible relationships between these variables.
To analyze cortisol response, participants were divided into three groups according to their observed change in cortisol levels: (1) “no-change group” (n = 20), (2) “decrease group” (n = 42), and (3) “increase group” (n = 38). Participants were assigned to a respective group if the change in cortisol levels was ≥10% versus baseline (Bair-Merritt et al., Citation2015). We then used the generalized linear model (GLM) including the grade of facial FA as dependent variable. Categories of cortisol responses were introduced as a factor, and perceived stress and competitiveness scores were covariates. Bonferroni’s post hoc contrast was used to examine significant differences in cortisol responses among the groups according to their facial FA. The data were analyzed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). All tests were two-tailed, and the significance was p ≤ .05.
Ethical note
This study adheres to the Declaration of Helsinki and the Mexican Official Norm for Research with Human Beings (NOM-012-SSA3-2012. http://dof.gob.mx/nota_detalle.php?codigo=5284148&fecha=04/01/2013). It was approved by the Bioethics Committee of the INPRFM.
Results
Correlations
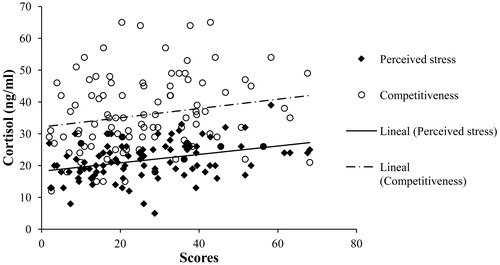
We found a positive correlation between basal cortisol levels and perceived stress (r = 0.353, p < .001) and a positive correlation between basal cortisol levels and competitiveness scores (r = 0.195, p = .050). In summary, men with higher levels of basal cortisol also have higher scores of perceived stress and competitiveness (). There were no significant results for facial FA scores and basal cortisol levels, for facial FA scores and perceived stress, nor facial FA scores and competitiveness (p > .05 in all cases).
Cortisol responses and facial FA
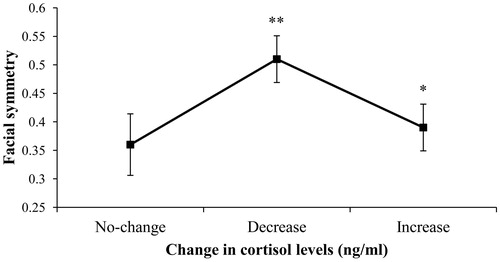
Cortisol levels increased in symmetrical men (lower FA scores), and it decreased in asymmetrical men (higher FA scores) after a short stressful test (B = 0.788, Wald =61.421, p < .001). We found a significant difference in cortisol responses between the “decrease group” and the “increase group” according to their facial FA (Bonferroni’s p = .046), and between the “no-change group” and the “decrease group” (Bonferroni’s p = .025), (). There was a tendency for perceived stress (B = 0.019, Wald =3.773, p = .052) and for competitiveness (B = 0.007, Wald =3.716, p = .054).
Figure 3. Mean (±SE) of facial FA according to cortisol responses (no-change, decrease, and increase groups). Cortisol levels increase in symmetrical men after a short stressor compared to the decrease group *p = 0.046; cortisol levels decreased in the most asymmetrical men compared to the no change group **p = 0.025.
Discussion
The first goal was to investigate whether facial FA was related to cortisol levels, competitiveness scores, and perceived stress scores in men. We found no significant correlations for these variables. However, we found that basal cortisol levels were positively related to perceived stress and competitiveness scores. The literature has consistently related cortisol levels to stress in humans (e.g. Kurina et al., Citation2004; see Dickerson & Kemeny, Citation2004 for a review), and our results support these findings. However, the literature regarding the relationship between cortisol levels and competitiveness is scarce. For example, it has been shown that acute stress increases the level of testosterone (a steroid hormone related to the expression of competitive behaviors) (Wingfield, Hegner, Dufty, & Ball, Citation1990; Wingfield & Sapolsky, Citation2003), but chronic stress inhibit testosterone levels in blood of several animal species such as mice, rats, non-human primates, and human beings (Dong et al., Citation2004; Elman, Goldstein, Adler, Shoaf, and Breier, Citation2001; Razzoli et al., Citation2006; Sapolsky, Citation1986). According to this previous literature, we suggest that the positive relationship between cortisol levels and competitiveness could reflect the optimum functioning of endocrine systems such as the HPA and the hypothalamic–pituitary–gonads axis that allow individuals to use positive coping skills in conflict situations to reduce costs, and that the ability to resolve these conflicts could depend on acute increases of cortisol.
Although a relationship between basal cortisol levels and facial FA was not found, this relationship was different depending on the cortisol response. Regarding our second goal, we found that cortisol levels increase in symmetrical men but they decreases in asymmetrical men. Thus acute hormone responses, rather than baseline samples, might better explain the relationship between psychological and physiological traits (Carré, McCormick, & Hariri, Citation2011; McGlothlin, Jawor, & Ketterson, Citation2007).
The literature has shown the effects of perceiving psychological or physical stressors on increased cortisol (Dickerson & Kemeny, Citation2004). The main function of acute cortisol increase is to send energy to muscles, increase the mobilization of glucose, and suppresses unessential functions such as the immune and digestive systems, as well as growth and reproduction to be more efficient during conflict situations (Sapolsky, Citation1998; Sapolsky, Romero, & Munck, Citation2000). The acute cortisol levels in symmetrical men could be related to the willingness to respond effectively to stressful situations. It could be also related to the disposition to compete and maintain social status. While the acute decrease in cortisol levels in asymmetrical men could be related to an inefficient function of the HPA axis for responding to a stressful situation, the literature has shown that people who suffered from violence or trauma during early childhood have a blunted or decreased HPA axis response. Consequently, they have a physiological habituation to stress (Worthman & Costello, Citation2009).
We did not ask our male subjects if they suffered from violence or trauma in their childhood, but the literature has shown that asymmetrical men could suffer from diverse previous social experiences and stress because asymmetrical people has been perceived as less intelligent (Banks, Batchelor, & McDaniel, Citation2010), less healthy (Jones et al., Citation2001), less attractive (Fink, Neave, Manning, & Grammer, Citation2006; Baudouin & Tinbergen, Citation2004), and less assertive (Borráz-León & Cerda-Molina Citation2015); see Little, Jones, and DeBruine (Citation2011) for a review. Thus, it is reasonable to think that asymmetrical men may have suffered from previous bad experiences and that they have a dysregulation in their HPA axis. Another possible explanation for this dysregulation could be because the association between abnormal intrauterine development as deformational plagiocephaly, abnormal neurodevelopment such as craniosynostosis, and greater facial asymmetry (Rekate, Citation1998; St John, Mulliken, Kaban, & Padwa, Citation2002) possibly leading to bad social and health experiences and therefore to altered cortisol response during exposure to stress.
Therefore, the observed acute increase in cortisol levels seen in symmetrical men could be interpreted as a motivator to express behaviors or desirable personality traits to deal with stressful situations, or to maintain their social acceptance in comparison with the observed decrease in cortisol of asymmetrical men. In this regard, Borráz-León, Cerda-Molina, Hernández-López, Chavira-Ramírez, and de la O-Rodríguez (Citation2014) showed that symmetrical men rated the faces of more assertive men as more attractive to women and as the most likely represent potential rivals. The effectiveness of this evaluation could increase the opportunities of symmetrical men to maintain their acceptance among women. Moreover, van der Meij, Buunk, and Salvador (Citation2010) showed that the cortisol levels of men increased when they had contact with an attractive woman. While the authors in that study did not measure facial FA scores, their study supports the hypothesis that acute increases in cortisol levels could be related to the expression of behaviors that allow men to get better results during a potential courtship situation. Thus, the observed acute increase in cortisol levels of the more symmetrical men observed here may be focused on the strategies that these individuals could use to maintain their social status and deal with stressful situations. In summary, having acute normal cortisol responses could allow men to show more efficient strategies related to intrasexual competition. Acute fluctuations in cortisol levels vary according to facial FA in human males.
On the other hand, it is reasonable to think that chronic cortisol exposure and chronic stress through life could be more closely associated with the expression of higher levels of facial FA more than just acute cortisol responses. This could explain why we did not find an association between cortisol levels and facial FA. Chronically high levels of cortisol are related to pathologies and disturbances in the development of organisms and people’s well-being. For example, chronically elevated cortisol levels are associated with a variety of diseases and disorders, e.g. depression (Gold & Chrousos, Citation2013; Tsigos & Chrousos, Citation1994), myopathy, and adult-onset diabetes (McEwen, Citation2008), etc. Similarly, Toussaint, Shields, Dorn, and Slavich (Citation2016) recently showed that greater lifetime stress predict worse mental and physical health. Thus, higher facial FA could be an indicator of chronic high stress levels and possibly cortisol levels in men. FA is the principal physical trait related to high levels of genetic and environmental stress in nature (Møller, Citation1997). It is also related to higher amounts of parasites and malnutrition (Hume & Montgomeri, Citation2001; Møller, Citation1997; Polak, Citation2003) as well as premature birth, psychosis, perceived health, death from degenerative diseases, and mental retardation in newborns (Fink et al., Citation2006; Jones et al., Citation2001; Livshits & Kobylianski, Citation1991; Shackelford & Larsen, Citation1997; Weisensee, Citation2013). Further studies need to test this hypothesis.
Although PSS is one of the principal measures of an individual’s psychological response to a stressor, it may not accurately represent an individual’s chronic stress burden, as psychological stress may primarily reflect current and not chronic exposure (Worthman & Costello, Citation2009). Finally, we highlight the need to continue investigating the relationship between cortisol levels, psychological traits and physical indicators of stress. In fact, the molecular mechanisms by which cortisol and stress are related to the development of diseases and physical perturbations are widely unknown. McEwen (Citation1998) has suggested that the chronic activation of the HPA axis could contribute to their onset. Our results provide evidence for the differences in endocrine responses according to facial FA and how this could be related to indicators of perceived stress and competitiveness.
Limitations of this study
One limitation of our study is that it only included men. We propose a similar experiment in women to explore whether cortisol responses are similar to those of men or whether they vary depending on steroid hormones related to menstrual cycle phases. On the other hand, we propose that in future studies, it could be useful to include the subjective units of distress scale (SUDS) to evaluate the subjective distress level that participants experience after a stressful test. Likewise, we suggest using more than two saliva samples that allow more robust calculations of stress response looking at baseline and return. This would strengthen the results. We hope that this paper can provide new directions in the study of stress and its relationship with biological and social factors.
Acknowledgements
We thank the facilities and support of the National Institute of Psychiatry “RFM”, the National Autonomous University of Mexico and the volunteers who participated in the study. We would also like to acknowledge the contributions of three anonymous referees for improving the presentation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bair-Merritt, M.H., Voegtline, K., Ghazarian, S.R., Granger, D.A., Blair, C., & Johnson, S.B. (2015). Maternal intimate partner violence exposure, child cortisol reactivity and child asthma. Child Abuse & Neglect, 48, 50–57. http://dx.doi.org/10.1016/j.chiabu.2014.11.003
- Banks, G.C., Batchelor, J.H., & McDaniel, M.A. (2010). Smarter people are (a bit) more symmetrical: A meta-analysis of the relationship between intelligence and fluctuating asymmetry. Intelligence, 38, 393–401. http://dx.doi.org/10.1016/j.intell.2010.04.003
- Baudouin, J.Y., & Tinbergen, G. (2004). Symmetrym averageness, and features size in the facial attractiveness in women. Acta Psychologica, 117, 313–332. http://dx.doi.org/10.1016/j.actpsy.2004.07.002
- Borráz-León, J.I., Cerda-Molina, A.L., Hernández-López, L., Chavira-Ramírez, R., & de la O-Rodríguez, C. (2014). Steroid hormones and facial traits in the recognition of a potential rival in men. Ethology, 120, 103–1023. http://dx.doi.org/10.1111/eth.12274
- Borráz-León, J.I., Cerda-Molina, A.L., & Mayagoitia-Novales, L. (2016). Resource holding potential in human males: Testosterone, personality and benefits. Paper presented at the 16th Congress of the International Society for Behavioral Ecology, Exeter, UK.
- Borráz-León, J.I., & Cerda-Molina, A.L. (2015). Facial asymmetry is negatively related to assertive personality but unrelated to dominant personality in men. Persnality and Individual Differences, 75, 94–96. http://dx.doi.org/10.1016/j.paid.2014.11.019
- Buunk, A.P., & Fisher, M. (2009). Individual differences in intrasexual competition. Journal of Evolutionary Psycholigy, 1, 37–48. http://dx.doi.org/10.1556/JEP.7.2009.1.5
- Carré, J.M., McCormick, C.M., & Hariri, A.R. (2011). The social neuroendocrinology of human aggression. Psychoneuroendocrinology, 36, 935–944. http://dx.doi.org/10.1016/j.psyneuen.2011.02.001
- Cohen, S., & Herbert, T.B. (1996). Health psychology: Psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annual Review of Psychology, 47, 113–142. http://dx.doi.org/10.1146/annurev.psych.47.1.113
- Cohen, S., Kamarak, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. http://dx.doi.org/10.2307/2136404
- Daniel, W. (1982). Biostatistics (pp. 91–95). México: Limusa.
- Dickerson, S.S., & Kemeny, M.E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–391. http://dx.doi.org/10.1037/0033-2909.130.3.355
- Dong, Q., Salva, A., Sottas, C.M., Niu, E., Holmes, M., & Hardy, M.P. (2004). Rapid glucocorticoid mediation of suppressed testosterone biosythesis in male mice subjected to immobilization stress. Journal of Andrology, 25, 973–981. http://dx.doi.org/10.1002/j.1939-4640.2004.tb03170.x
- Elman, I., Goldstein, D.S., Adler, C.M., Shoaf, S.E., & Breier, A. (2001). Inverse relationship between plasma epinephrine and testosterone levels during acute glucoprivation in healthy men. Life Sciences, 68, 1889–1898. https://doi.org/10.1016/S0024-3205(01)00982-1
- Fink, B., Neave, N., Manning, J.T., & Grammer, K. (2006). Facial symmetry and judgments of attractiveness, health and personality. Persnality and Individual Differences, 41, 491–499. http://dx.doi.org/10.1016/j.paid.2006.01.017
- Gold, P.W., & Chrousos, G.P. (2013). Melancholic and atypical subtypes of depression represent distinct pathophysiological entitie: CRH, neural circuits, and the diathesis for anxiety and depression. Molecular Psychiatry, 18, 632–634. http:dx.doi.org/10.1038/mp.2013.5
- González, R.M.T., & Landero, H.R. (2007). Factor structure of the perceived stress scale (PSS) in a sample from Mexico. Spanish Journal of Psychology, 10, 199–206. https://doi.org/10.1017/S1138741600006466
- Grammer, K., & Thonrhill, R. (1994). Human (Homo sapiens) facial attractiveness and sexual selection: The role of symmetry and averageness. Journal of Comparative Psychology (Washington, D.C. : 1983), 108, 233–242. http://dx.doi.org/10.1037/0735-7036.108.3.233
- Hume, D.K., & Montgomeri, R. (2001). Facial attractiveness signals different aspects of “quality” in women and men. Evolution and Human Behavior, 22, 93–112. https://doi.org/10.1016/S1090-5138(00)00065-9
- Jones, B.C., Little, A.C., Penton-Voak, I.S., Tiddeman, B.P., Burt, D.M., & Perret, D.I. (2001). Facial symmetry and judgments of apparent health. Support for a “good genes” explanation of the attractiveness-symmetry relationship. Evolution and Human Behaviour, 22, 417–429. https://doi.org/10.1016/S1090-5138(01)00083-6
- Kurina, L.M., Schneider, B., & Waite, L.J. (2004). Stress, symptoms of depression and anxiety, and cortisol patterns in working parents. Stress Health, 20, 53–63. http://dx.doi.org/10.1002/smi.998
- Leor, J., Poole, W.K., & Kloner, R.A. (1996). Sudden cardiac death triggered by an earthquake. New England Journal of Medicine, 334, 413–419. http://dx.doi.org/10.1056/NEJM199602153340701
- Levendosky, A.A., Bogat, G.A., Lonstein, J.S., Martinez-Torteya, C., Muzik, M., Granger, D.A., & von Eye, A. (2016). Infant adrenocortical reactivity and behavioural functioning: Relation to early exposure to maternal intimate partner violence. Stress, 19, 37–44. http://dx.doi.org/10.3109/10253890.2015.1108303
- Little, A.C., Jones, B.C., & DeBruine, L.M. (2011). Facial attractiveness: Evolutionary based research. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 366, 1638–1659. http://dx.doi.org/10.1098/rstb.2010.0404
- Livshits, G., & Kobylianski, E. (1991). Fluctuating asymmetry as a possible measure of developmental homeostasis in humans: A review. Human Biology, 63, 441–466.
- McEwen, B.S. (1998). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338, 171–179. http://dx.doi.org/10.1056/NEJM199801153380307
- McEwen, B.S. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583, 174–185. http://dx.doi.org/10.1016/j.ejphar.2007.11.071
- McGlothlin, J.W., Jawor, J.M., & Ketterson, E.D. (2007). Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. American Naturalist, 170, 864–875. https://doi.org/10.1086/522838
- Mehta, P.H., Jones, A.C., & Josephs, R.A. (2008). The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. Journal of Personality and Social Psychology, 94, 1078–1093. http://dx.doi.org/10.1037/0022-3514.94.6.1078
- Møller, A.P. (1997). Developmental stability and fitness: A review. American Naturalist, 149, 916–942. https://doi.org/10.1086/286030
- Munck, A., Guyre, P.M., & Holbrook, N.J. (1984). Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews, 5, 25–44. http://dx.doi.org/10.1210/edrv-5-1-25
- Muñoz-Reyes, J.A., Gil-Burmann, C., Fink, B., & Turiegano, E. (2012). Facial asymmetry and aggression in Spanish adolescents. Personality and Individual Differences, 53, 857–861. http://dx.doi.org/10.1016/j.paid.2012.06.012
- Polak, M. (2003). Developmental instability: Causes and consequences. New York: Oxford University Press.
- Razzoli, M., Roncari, E., Guidi, A., Carboni, L., Arban, R., Gerrard, P., & Bacchi, F. (2006). Conditioning properties of social subordination in rats: Behavioral and biochemical correlates of anxiety. Hormones and Behavior, 50, 245–251. http://dx.doi.org/10.1016/j.yhbeh.2006.03.007
- Rekate, H.L. (1998). Occipital plagiocephaly: A critical review of the literature. Journal of Neurosurgery, 89, 24–30. http://dx.doi.org/10.3171/jns.1998.89.1.0024
- Reul, J.M., Collins, A., Saliba, R.S., Misfud, K.R., Carter, S.D., Gutierres-Mecinas, M., … Linthorst, A.C.E. (2015). Glucocorticoids, epigenetic control and stress resilience. Neurobiology Stress, 1, 44–59. http://dx.doi.org/10.1016/j.ynstr.2014.10.001
- Sapolsky, R.M., Romero, L.M., & Munck, A.U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21, 55–89. http://dx.doi.org/10.1210/edrv.21.1.0389
- Sapolsky, R.M. (1986). Stress-induced elevation of testosterone concentration in high ranking baboons: Role of catecholamines. Endocrinology, 118, 1630–1635. http://dx.doi.org/10.1210/endo-118-4-1630
- Sapolsky, R.M. (1998). Why Zebras don’t get ulcers: An updated guide to stress, stress-related disease and coping. New York: WH. Freeman and Co.
- Scheib, J.E., Gangestad, S.W., & Thornhill, R. (1999). Facial attractiveness, symmetry and cues of good genes. Proceedings of the Royal Society B: Biological Sciences, 266, 1913–1917. http://dx.doi.org/10.1098/rspb.1999.0866
- Schmidt-Reinwald, A., Pruessner, J.C., Hellhammer, D.H., Federenko, I., Rohleder, N., Schurmeyer, T.H., & Kirschbaum, C. (1999). The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Science, 64, 1653–1660. https://doi.org/10.1016/S0024-3205(99)00103-4
- Schultheiss, O.C., Dargel, A., & Rohde, W. (2003). Implicit motives and gonadal steroid hormones: effects of menstrual cycle phase, oral contraceptive use, and relationship status. Hormones and Behavior, 43, 293–301. http://dx.doi.org/10.1016/S0018-506X(03)00003-5
- Seta, C.E., & Seta, J.J. (1995). When audience presence is enjoyable. The influences of audience awareness of prior success on performance and task interest. Basic and Applied Social Psychology, 16, 95–108. http://dx.doi.org/10.1080/01973533.1995.9646103
- Shackelford, T.K., & Larsen, R.J. (1997). Facial asymmetry as an indicator of psychological, emotional, and physiological distress. Journal of Personality and Social Psychology, 72, 456–466. http://dx.doi.org/10.1037/0022-3514.72.2.456
- Simpson, J.A., Gangestad, S.W., Christensen, P.N., & Leck, K. (1999). Fluctuating asymmetry, sociosexuality, and intrasexual competitive tactics. Journal of Personality and Social Psychology, 76, 159–172. http://dx.doi.org/10.1037/0022-3514.76.1.159
- Singh, D., & Rosen, V.C. (2001). Effects of maternal body morphology, morning sickness, gestational diabetes and hypertension on fluctuating asymmetry in young women. Evolution and Human Behavior, 22, 373–384. http://dx.doi.org/10.1016/S1090-5138(01)00082-4
- St John, D., Mulliken, J.B., Kaban, L.B., & Padwa, B.L. (2002). Anthropometric analysis of mandibular asymmetry in infants with deformational posterior plagiocephaly. Journal of Oral and Maxillofacial Surgery, 60, 873–877. http://dx.doi.org/10.1053/joms.2002.33855
- Sumioka, H., Nakae, A., Kanai, R., & Ishiguro, H. (2013). Huggable communication medium decreases cortisol levels. Scientific Reports, 3, 3034. http://dx.doi.org/10.1038/srep03034
- Thornhill, R., & Gangestad, S.W. (2006). Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evolution and Human Behavior, 27, 131–144. http://dx.doi.org/10.1016/j.evolhumbehav.2005.06.001
- Thornhill, R. (1992). Fluctuating asymmetry and the mating system of the Japanese scorpionfly, Panorpa japonica. Animal Behavior, 44, 867–879. http://dx.doi.org/10.1016/S0003-3472(05)80583-4
- Touitou, Y., & Haus, E. (2000). Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiology International, 17, 369–390. http://dx.doi.org/10.1081/CBI-100101052
- Toussaint, L., Shields, G.S., Dorn, G., & Slavich, G.M. (2016). Effects of lifetime stress exposure on mental and physical health in young adulthood: How stress degrades and forgiveness protects health. Journal of Health Psychology, 21, 1004–1014. http://dx.doi.org/10.1177/1359105314544132
- Tsigos, C., & Chrousos, G.P. (1994). Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinology and Metabolism Clinics of North America, 23, 451–466.
- van der Meij, L., Buunk, A.P., & Salvador, A. (2010). Contact with attractive women affects the release of cortisol in men. Hormones and Behavior, 58, 501–505. http://dx.doi.org/10.1016/j.yhbeh.2010.04.009
- van Valen, L. (1962). A study of fluctuating asymmetry. Evolution, 16, 125–142. http://dx.doi.org/10.1111/j.1558-5646.1962.tb03206.x
- Weisensee, K.E. (2013). Assessing the relationship between fluctuating asymmetry and cause of death in skeletal remains: A test of the development origins of health and disease hypothesis. American Journal of Human Biology, 25, 411–417. http://dx.doi.org/10.1002/ajhb.22390
- Wingfield, J.C., Hegner, R.E., Dufty Jr A.M., & Ball, G.F. (1990). The ‘challenge hypothesis’: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist, 136, 829–846. https://doi.org/10.1086/285134
- Wingfield, J.C., & Sapolsky, R.M. (2003). Reproduction and resistance to stress: When and how. Journal of Neuroendocrinology, 15, 711–724. http://dx.doi.org/10.1046/j.1365-2826.2003.01033.x
- Worthman, C.M., & Costello, J. (2009). Tracking biocultural pathways in population health: The value of biomarkers. Annals of Human Biology, 36, 281–297. http://dx.doi.org/10.1080/03014460902832934
- Yamazaki, R., Christensen, L., Skov, K., Chang, C.-C., Damholdt, M.F., Sumioka, H., … Ishiguro, H. (2016). Intimacy in phone conversations: Anxiety reduction for Danish seniors with Hugvie. Frontiers in Psychology, 7, 537. http://dx.doi.org/10.3389/fpsyg.2016.00537
- Zadzinska, E., Koziel, S., Kurek, M., & Spinek, A. (2013). Mother's trauma during pregnancy affects fluctuating asymmetry in offspring’s face. Anthropologischer Anzeiger; Bericht Uber Die Biologisch-Anthropologische Literatur, 70, 427–437. https://doi.org/10.1127/0003-5548/2013/0383