Abstract
Maternal care is essential for an adequate pup development, as well as for the health of the dam. Exposure to stress in early stages of life can disrupt this dam–pup relationship promoting altered neurobiological and behavioral phenotypes. However, there is a lack of consensus regarding the effects of daily maternal separation (MS) on the pattern of maternal behavior. The aim of this study is to compare the patterns of maternal behavior between mice exposed to MS and controls. BALB/c mice were subjected to MS for a period of 180 min/day from postnatal day 2–7 (n = 17) or designated to be standard animal facility reared (AFR) controls (n = 19). Maternal behaviors were computed as frequency of nursing, licking pups and contact with pups, and nonmaternal behaviors were computed as frequency of actions without interaction with pups and eating/drinking. A total of 18 daily observations of maternal behavior were conducted during these six days, and considering the proportion of maternal and nonmaternal behaviors, an index was calculated. There was no difference when comparing the global index of maternal behavior between the AFR and MS animals by the end of the observed period. However, the pattern of maternal behavior between groups was significantly different. While MS dams presented low frequency of maternal behavior within the first couple days of the stress protocol, but increasing over time, AFR dams showed higher maternal behavior at the beginning, reducing over time. Together, our results indicate that MS alters the maternal behavior of the dams toward pups throughout the first week of the stress protocol and provoked some anxiety-related traits in the dams. The inversion of maternal behavior pattern could possibly be an attempt to compensate the low levels of maternal care observed in the first days of MS.
Introduction
The relationship between mother and litter is directly involved in the neurobiological and behavioral development of the pups, as well as for the health of the mother (Pena & Champagne, Citation2013; Liu, Diorio, Day, Francis, & Meaney, Citation2000; Nephew & Murgatroyd, Citation2013). The postnatal period is considered a time of major importance in the development, in which multiple processes (e.g. neurogenesis, migration, synaptogenesis and pruning) are still occurring and leading to a susceptibility of the brain toward environmental challenges (Aisa, Tordera, Lasheras, Rio, & Ramirez, Citation2007; Andersen, Citation2003). It is well known that early life adversities during this period could affect structural and functional patterns of brain development (Andersen & Teicher, Citation2009). The postnatal period is considered as a “vulnerability window,” in which exposure to a chronic stressor early in life can promote significant cognitive, neurobiological and behavioral alterations (Adriani & Laviola, Citation2004; Aisa et al., Citation2009; Andersen, Citation2003; Andersen & Teicher, Citation2009; Brenhouse & Andersen, Citation2011). Studies have shown that rodents exposed to daily periods of separation in the beginning of life presented increased anxiety (Daniels, Pietersen, Carstens, & Stein, Citation2004), depressive-like behavior (Bhansali, Dunning, Singer, David, & Schmauss, Citation2007) and altered dam-pup relationship (Bailoo, Jordan, Garza, & Tyler, Citation2014).
Maternal care is crucial for an adequate development of the pups, and this type of behavior can be classified as any action taken by the mother in order to nourish, soothe and protect the pups (Kristal, Citation2009; Pires, Tufik, & Andersen, Citation2015). In addition to provide nutrition and safety, it is essential to ensure the necessary stimuli for development (Caldji et al., Citation1998; Meaney, Citation2001), since it was already been observed that variations in maternal behavior (MB) can modulate neuroendocrine and psychological development, as well as stress responses (Francis, Diorio, Liu, & Meaney, Citation1999; Nephew & Murgatroyd, Citation2013; Weaver et al., Citation2004). There is evidence that dams of pups exposed to stressful situations demonstrate a decrease in the frequency of maternal behavior when compared to non-stressed animals (Ivy, Brunson, Sandman, & Baram, Citation2008). However, there are studies that point to an increase in maternal behaviors, such as licking, nursing or staying with the pups, after exposing the pups to Maternal Separation (MS) (Kosten & Kehoe, Citation2010; Own & Patel, Citation2013). This increased interaction between mother and pups could be an attempt to hyper-compensate the maternal care, influenced by stressful periods of separation and because of the remoteness of the pups (George, Bordner, Elwafi, & Simen, Citation2010).
Since there are no conclusive data regarding the impact of maternal separation protocol and maternal behavior, the aim of this study is to investigate the maternal behavior pattern across the first week of newborn BALB/c mice development in animals exposed to daily maternal separation and controls, as well as the impact of the MS in anxiety-related phenotypes of the dams.
Material and methods
Animals
Male and female BALB/c mice were obtained from the Center for Laboratory Animals at our university (CeMBE, PUCRS), Brazil. All animals were housed in standard plastic mouse cages (22 cm x 16 cm x 14 cm) and kept under constant room temperature (21 ± 1 °C), humidity (55 ± 5%) and ventilation. Furthermore, mice were maintained in a 12-h light/dark cycle (lights on 7 a.m. to 7 p.m.) with food and water ad libitum.
The breeding procedures consisted in housing two females and one male in the same cage for a 24 h mating period. After this period, the male was removed and both females remained together for 15 days, when they were inspected daily for pregnancy. The pregnant females were individually housed and from at gestation-day 18 parturition was inspected once a day (9 a.m. to 10 a.m.) to verify the presence of pups. The first day pups were found was defined as postnatal day (PND) 1. During the first 24 h after birth, cross-fostering was conducted with half of the litter, followed by litter control of 5 to 7 pups per litter. Animals were randomly assigned to undergo two experimental conditions, MS or standard animal facility reared controls (AFR). Litters were not sexed at birth to minimize the handling of pups. For this study 36 dams were utilized, in which 17 were designated for the AFR group and 19 for the MS group. The dams used for breeding were primiparous and did not had any other experiences of breeding or manipulation, except for the weekly cage cleaning performed by the colony technicians. To investigate the effects of MS in anxiety-related traits the dams were tested after weaning at PND21 to PND23 in the Open Field test, Social Interaction test and Light/Dark test, respectively. All the dams were tested in the same order and sacrificed immediately after the light/dark test for brain dissections.
All experiments followed the guidelines of the International Council for Laboratory Animal Science (ICLAS) and were approved by the Ethics Committee on Animal Use (CEUA) from PUCRS, Brazil under the registration #14/00421.
Maternal separation (MS)
Maternal Separation procedures were followed as described by previous studies with BALB/c strain (Bhansali et al., Citation2007; Wang, Jiao, & Dulawa, Citation2011). In this protocol, MS pups were separated daily from their dams for a period of 180 min, during the end of the light/dark cycle (4–7 p.m.), from PND2 to PND7. To begin the MS protocol, dams were removed from home cage and allocated in a new cage containing standard clean bedding. Dams were also transferred to another colony room to avoid ultrasonic vocalization between dams and pups. Pups were then removed from home cage and allocated in new cage with clean bedding. The cage was on top of a digital-regulated heating pad (around 32 °C ± 3 °C) in order to prevent stress by hypothermia. At the end of the MS period, litters were returned to their home cage followed by the dam. AFR group was left undisturbed in their home-cage during the separations experiments, except for routine cleaning and housing changes (once a week).
Maternal behavior
Observations of maternal behavior during dam–pups interaction were conducted from PND2 to PND7. The observations were conducted six times over a period of 15 min (0, 3rd, 6th, 9th, 12th and 15th min) and were performed during three shifts of the day – 9 a.m., 3:30 p.m. (pre-MS) and 7:15 p.m. (post-MS) – totalizing 18 daily observations. The frequency of behaviors evaluated were nursing (N) [dams were nursing in any position], licking pups (L) [each time dams were liking their pups], contact with pups (C) [dams were in the nest in contact with pups but not feeding or licking them], retrieving the pups (R) [when the dams retrieved the pups to the net], no interaction with pups (X) [any behavior without contact with pups excepted eating or drinking] and eating/drinking (E) (Brown, Mathieson, Stapleton, & Neumann, Citation1999; Champagne, Curley, Keverne, & Bateson, Citation2007). The behaviors N, L, C and R were categorized as maternal behaviors because they impact directly on the pup and can provide physical and emotional care (Pires et al., Citation2015). Furthermore, no interaction with the pups (X) and eating/drinking (E) were considered as behaviors not related with maternal care. We computed total maternal (MB) and total non-maternal behavior (NMB) by using the sum of all specific behaviors [MB = N + L + C] and [NMB = X + E]. The behavior of R was not used in the sum of maternal behaviors due to its low frequency in all groups. Moreover, an index of maternal behavior was computed by using the formula [MB/(MB + NMB)] to evaluate the relation between maternal and nonmaternal behaviors (Own & Patel, Citation2013). The observations were performed silently in the colony room by direct visual observations in front of the cages and the animals were not handled or perturbed during this procedure.
Open field test
Spontaneous locomotor activity and measures of anxiety-like behavior were evaluated with the open field test. The animal was placed in the center of a Plexiglas Square Box (33 × 33 cm) and allowed to explore for 5 min. Video was recorded using a professional camera and then analyzed in AnyMaze Software (Stoelting CO, Wood Dale, IL). To evaluate the time in the center and periphery of the apparatus, the box was divided into 16 squares, where four squares were defined as the central zone, and 12 squares were defined as the peripheral zone. Furthermore, total distance covered (in meters) and frequency of rearing and self-grooming were measured.
Social interaction test
The dams were tested in the social interaction test as described by (Iñiguez et al., Citation2014) Briefly, the task consisted of two 150 s phases under red light conditions performed in the same open field arena containing a stainless steel wired enclosure. In the first phase, the tested animal was placed in the arena and allowed to explore freely without any mice in the enclosure. Then, in the second phase, immediately after the first, one stranger mouse was placed in the enclosure and the tested mice was allowed to freely explore in the presence of a stranger C57 (paired with sex and age). The time that the animal spent exploring the empty enclosure, as well as the time spent interacting with the stranger mouse was used as a measure of social interaction. Interaction was considered as direct touching the nose in the enclosure or when the head was towards the enclosure at a distance lower than 2 cm.
Light/dark test
The apparatus used for the light/dark test consisted of a plexiglas box (21 × 42 × 20 cm) divided by two chambers of equal size where one was completely dark, with just one small transition partition and the other chamber was brightly illuminated at 400 lux. Mice were placed in the bright side of the box and allowed to freely explore the chamber for 600 s. The latency to enter the dark side, the number of transitions and the time in the bright compartment were used as dependent variables.
Analysis of glucocorticoid receptor mRNA expression
Immediately after the last behavioral test, animals were sacrificed by cervical dislocation. The brains were rapidly removed for whole hippocampus dissection, and the tissue was stored at −80 °C until further analysis. Total RNA was extracted using Qiazol protocol by Qiagen. The concentration of RNA was measured using a NanoDrop, and 500 ng from each sample was reverse transcribed using the miScript II RT kit (Qiagen, Redwood City, CA). cDNA was then used for real-time-qPCR performed in the RotorGene using the miScriptSybr Green PCR kit (Qiagen).
To perform the RT-qPCR, the GAPDH gene was used as reference, and relative expression of the glucocorticoid receptor (Nr3c1) was performed in duplicates using the following primers: Nr3c1: (forward: 5′-GGACCACCTCCCAAACTCTG-3′; reverse: 5′-ATTGTGCTGTCCTTCCACTG-3′). GAPDH: (forward: 5′-GTCATATTTCTCGTGGTTCACACC-3′; reverse: 5′- CTGAGTAT-GTCGTGGAGTCTACTGG-3′). The fold change relative expression was calculated using the ΔΔCt method.
Statistical analysis
All data are presented as group mean ± standard error of the mean (SEM). Normality of the data distribution was analyzed for all variables. Repeated measure ANOVA was used with a within-subjects factor of days (PND2, PND3, PND4, PND5, PND6, PND7) and a between-subject factor of group (MS, AFR). Mauchly’s test indicated that the assumption of sphericity had been violated (χ2(14) = 28.71, p = .012); therefore, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = 0.76). Student’s t-test was used for parametric variables and Mann–Whitney U-test for nonparametric variables. To analyze the between group effects on maternal behavior in each day, we compared both groups using multiple Student’s t-tests. A p value <.05 was considered statistically significant. Statistical analyses were performed using SPSS software v.21.0 (SPSS, Chicago, IL) and the graphs were designed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA).
Results
Total maternal behavior
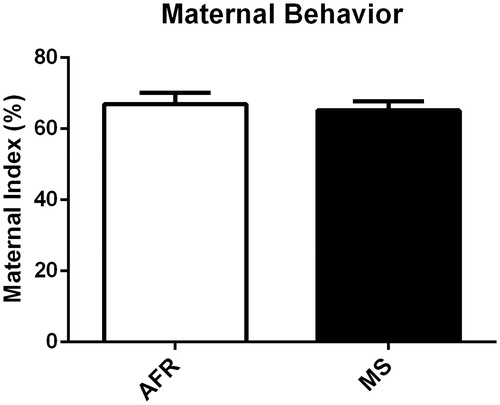
The first analysis was performed to compare the index of maternal behavior considering the observations from PND2 to PND7. Overall, the results revealed that there was no group difference when comparing the index of maternal behavior of AFR and MS animals (t(34) = 0.437, p = .66) (). Moreover, we compared the index of maternal behavior of AFR and MS animals pre-MS (9 a.m. and 3:30 p.m.) and post-MS (7:15 p.m.), but there was no significant difference between the groups (t(34) = 0.148, p = .88); t(25.44) = −1.32, p = .19, respectively).
Figure 1. Effects of MS on the index of maternal behavior. No significant difference was observed when the index of maternal behavior from the AFR and MS animals was compared. Data presented as mean ± standard error of the mean (SEM), as well as estimated marginal means and confidence interval; AFR: n = 17 e MS: n = 19. Student’s t-test for independent samples.
Since some MS protocols use separation from P2 to P15, we ran a separate experiment in order to verify the pattern of maternal behavior from P8 to P15; however, we did not find any quantitative or qualitative differences between groups during this period (F(7,56) = 0.42, p = .88) (data not shown).
Frequency of maternal behavior
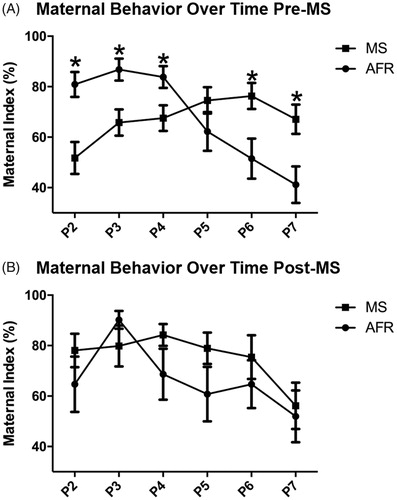
Our second analysis sought to investigate the index of maternal behavior pre-MS and post-MS in each day from PND2 to PND7. When the index of maternal behavior was analyzed pre-MS in a daily basis, we found a moderate-to-large effect of interaction between days and group, F(5, 30) = 9.03, p < .001, ηp2 = 0.21 (). Regarding post-MS analysis, no interaction was identified F(5, 30) = 0.88, p = .471, ηp2 = 0.25 (). During the individual pre-MS analysis, we observed that on PND2 (t(34) = 3.556, p ≤ .001), PND3 (t(34) = 3.049, p = .004) and PND4 t(34) = 2.408, p = .022), there was a decreased number of maternal behaviors in the MS group when compared to AFR. On PND6 (t(34) = −2.675, p = .011) and PND7 (t(34) = −2.818, p = .08), the pattern was altered, and the animals in the MS group presented an increased number of maternal behaviors when compared to the AFR group. This is interesting since mice are altruistic as they nurse and feed their young pups, possibly preparing the pups to survive in a hostile environment (Dutta & Sengupta, Citation2016). Daily post-MS analysis demonstrated that there is no difference in the frequency of maternal behavior in the animals exposed to maternal separation and control group: PND2 (t(34) = −1.068, p = .293), PND3 (t(34) = 1.125, p = .268), PND4 t(34) = −1474, p = .150), PND5 (t(34) = −1.497, p = .144), PND6 (t(34) = −.837, p = .408) and PND7 (t(34) = −0.304, p = .763).
Figure 2. Effects of MS on the daily frequency of maternal care pre-MS and post-MS. Analysis of the maternal index pre-MS throughout the 6 days showed an interaction between days and group, but this effect was not observed post-MS. Pre-MS on PND2, PND3 and PND4 there was an increased number of maternal behaviors in the AFR group when compared to the animals exposed to MS. On PND6 and PND7, the pattern was altered, and the animals in the MS group presented an increased number of maternal behaviors when compared to the AFR group. Post-MS, there was no difference between animals exposed to stress and control group. Data are expressed as mean ± standard error of the mean (SEM), as well as estimated marginal means and confidence interval; AFR: n = 17 e MS: n = 19. Repeated measure ANOVA and Student’s t-test for independent samples *p < .05.
Individual analysis of maternal behaviors
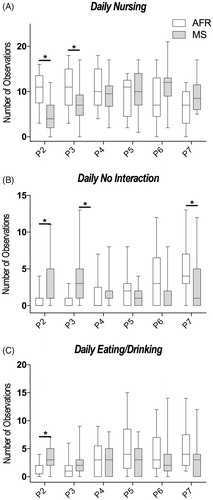
In subsequent analysis, the aim was to separate the different types of MB and NMB to allow for a better understanding of the contribution of every type of MB during the first week of the stress protocol. The results for individual maternal and nonmaternal behaviors analyzed are expressed in . On PND2 and PND3, maternal behavior of nursing was observed with more frequency in the AFR animals when compared to the MS, but in PND7, there was an inversion on this pattern, observed by an increased frequency of nursing in the MS animals, while AFR exhibited a reduction on this frequency (PND2: U(36) = 55.50, p = .001; PND3: U(36) = 92.50, p =.029; PND7: U(36) = 87.00, p = .029, ). On PND2 and PND3, the nonmaternal behavior specified as no interaction was observed with more frequency in the MS animals when compared to AFR animals, but in PND7 the opposite occurred and no interaction was observed more frequently in the AFR group (PND2: U(36) = 85.00, p = .012; PND3: U(36) = 58.00, p = .001; PND7 U(36) = 94.00, p = .049, ). Furthermore, on PND2 MS dams presented higher eating/drinking behavior when compared to the AFR group (PND2: U(36) = 79.00, p = .009, ). The behavior of licking did not present any statically significant difference (PND2: U(36) = 169.5, p = .802; PND3: U(36) = 158.5, p = .925; PND4: U(36) = 119.5, p = .186; PND5: U(36) = 169.0, p = .827; PND6: U(36) = 179.0, p = .594; PND7: U(36) = 193.0, p = .195). Contact with pups also did not present statistically significant difference during any of the days (PND2: U(36) = 156.0, p = .876; PND3: U(36) = 132.0, p = .363; PND4: U(36) = 127.5, p = .285; PND5: U(36) = 157.5, p = .900; PND6: U(36) = 197.0, p = .271; PND7: U(36) = 121.0, p = .303). Retrieving the pups was observed less than 1% of the total observations, so it was not considered for analysis.
Figure 3. Analysis of different maternal behaviors throughout the days. (A) Maternal behavior of nursing was more frequent on PND2 and PND3. There was an inversion on the frequency of nursing on PND7; AFR: n = 17 and MS: n = 19 (B) The nonmaternal behavior of no interaction was more frequent on PND2 and PND3. There was a decrease in the MS group regarding no interaction behavior on PND7 when compared to control; AFR: n = 17, MS: n = 19. (C) On PND2, MS dams presented higher eating/drinking behavior when compared to the control group. AFR: n = 17, MS: n = 19. Data are presented as the mean of the observed behaviors ± SEM, as well as estimated marginal means and confidence interval. Mann–Whitney U-test *p < .05.
Anxiety-related traits in the dams
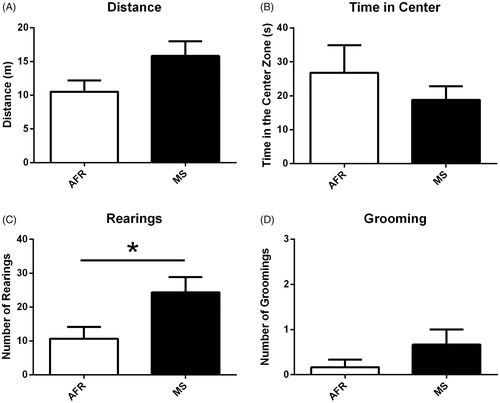
To investigate the impact of MS in the dams, we used three different anxiety-related tests. First, in the open field test, we observed that dams exposed to MS did not differ from the AFR dams in spontaneous locomotor activity (t(10) = 1.933, p ≥ 0.05, ) as well as regarding the time spent in the center of the apparatus (t(10) = 0.399, p = .39, ). However, when we look at the rearing behavior in the open field, the MS dams showed a significant increase compared to AFR dams (t(10) = 2.393, p = .03, ), but there was no difference in the number of self-grooming (t(10) = 1.342, p = .20, ).
Figure 4. Open field test comparing MS-exposed dams to AFR. (A) Dams exposed to MS presented the spontaneous locomotor activity as the controls; (B) Dams exposed to MS did not differ from the AFR dams in time spent in the center of the apparatus; (C) MS dams showed a significant increase in the number of rearing compared to AFR dams; (D) There was no difference in the number of self-grooming in the open field test; Data presented as mean ± standard error of the mean (SEM); AFR: n = 6e MS: n= 6. Student’s t-test for independent samples. *p < .05.
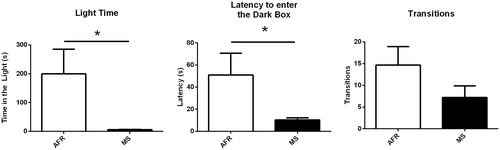
Secondly, in the light/dark test, MS dams spent significantly less time in the bright compartment compared to AFR dams (t(10) = 2.258, p < .05, ). Also, the latency of the first entry in the dark compartment was significantly shorter in the MS dams (t(10) = 2.287, p < .05, ). The MS dams did not differ from the AFR dams in the number of transitions between the dark and the bright side of the apparatus (t(10) = 1.412, p = .19, ).
Figure 5. The light/dark box test comparing MS-exposed dams to AFR. (A) MS dams spent significantly less time in the bright compartment compared to the AFR dams; (B) latency to first enter in the dark box was significantly higher in the MS dams; (C) MS dams did not differ from the AFR dams in the number of transitions between the dark and the bright side of the apparatus. Data presented as mean ± standard error of the mean (SEM); AFR: n = 6e MS: n = 6. Student’s t-test for independent samples. *p < .05.
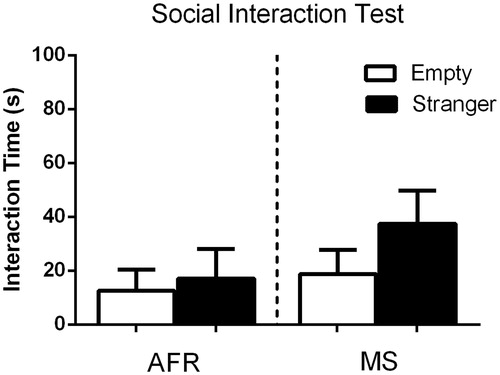
Finally, when looking at the social interaction test, we found no differences between the MS and AFR dams in the time spent exploring the empty enclosure, as well as time spent interacting with the stranger mouse (U(10) = 16.00, p = 0.74 and U(10) = 12.00, p = 0.35, respectively, ).
GR mRNA expression
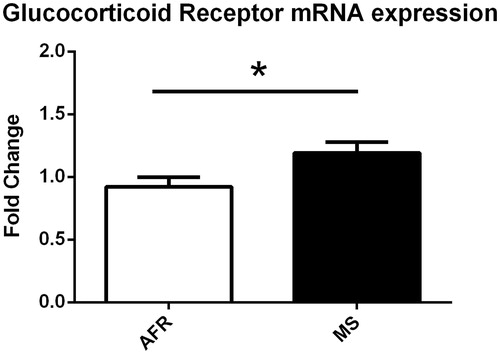
To investigate the long-lasting consequences of MS exposure in the brain, we performed a RT-qPCR of the glucocorticoid receptor gene (Nr3c1) in the hippocampus of the dams. Previous studies showed that a different pattern of expression of this gene in the hippocampus could represent vulnerability to potential injuries following chronic stress (Conrad, Citation2008). We found that dams exposed to MS showed increased expression of Nr3c1 in the hippocampus when compared to AFR dams (t(10) = 2.304, p = .03, ), suggesting that they could be susceptible to potential damage following a chronic stress protocol.
Discussion
The main findings of the present study are (1) overall when all behaviors across all days of observation are grouped there is no difference in total frequency of maternal behavior between controls and MS animals, but (2) there is a variation in the pattern of maternal behavior during the first week of newborn development, but with opposite patterns between groups. The control group presents increased maternal behavior in the first two days, but it was possible to identify an inversion on the pattern in the following days in the MS group. Also, (3) we found that the dams exposed to MS protocol presented some anxiety-related traits, specifically in the light/dark test as well as an increased expression of GR in the hippocampus.
In line with our results, previous studies reported that total maternal behavior is not altered during early life stress period (Macri, Mason, & Wurbel, Citation2004; Rice, Sandman, Lenjavi, & Baram, Citation2008). However, there are studies reporting an increased (Wei, David, Duman, Anisman, & Kaffman, Citation2010) and decreased (Franklin et al., Citation2010) maternal care following early life stress. A possible reason for these conflicting results regarding maternal behavior after exposure to early life stress is that the authors compared the overall scores of maternal behaviors. Our observations suggest that a daily analysis instead of one overall score could provide more detailed information on how the pattern of maternal behavior can vary throughout the postnatal period.
During the first two weeks of the postnatal period, the offspring depends on their dams to provide nutrition and maintain body temperature. After the second week, the pups still depend on their mother for milk. Although this is the period of maximum milk production (Knight, Maltz, & Docherty, Citation1986), the dams spend more time outside the nest doing different activities, such as eating and/or building the nest (König & Markl, Citation1987). To have a better understanding of the maternal behavior pattern throughout the first six days of MS stress, we analyzed the index of maternal behavior during different observation days. The first days of life are a critical period of development, where the pups are more vulnerable, and in full cortical development (Aisa et al., Citation2007; Andersen, Citation2003). When the animals were exposed to ELS paradigm, here via maternal separation, we observed a disruption in the maternal care during the initial post-natal days. This observation is in line with the findings of Ivy et al (Citation2008) who used a model of restricted bedding material and observed an abnormal pattern of maternal behavior in the dams. After exposure to early life stress, they significantly reduced the frequency of licking and grooming. Interestingly, in our study, during PND6 and PND7 of observation, there was an inversion on the pattern. Dams from the MS group started to increase the maternal behavior frequency in relation to the control group. Own and Patel (Citation2013) used a similar maternal separation protocol and observed that the percentage of maternal care pre-MS during the first two weeks does no differ between the control and stressed animals, but after analyzing the maternal care postreunion, they observed a significant increase in maternal care in the animals exposed to maternal separation. This increase could possibly be explained by an attempt of the dam to compensate the lack of maternal care in the first couple of days caused by the stress protocol, and provide a better preparation of the pups for possible environmental challenges (Francis et al., Citation1999; Meaney, Citation2001).
Regarding the different behaviors analyzed, the nursing behavior is considered critical for the development of the pup (Caldji et al., Citation1998). A previous study showed that offspring from dams with high arched-back nursing, licking and grooming presented lower response to stress as well as less fear behavior during adulthood (Weaver et al., Citation2004). Moreover, disruption on maternal behaviors induces an increased stress reactivity and anxiety-like behavior in the offspring (Gudsnuk & Champagne, Citation2012). Our data suggest that the pattern of maternal behavior observed was driven by alterations on the nursing frequency, which is essential for the adequate development of the pups (Shoji & Kato, Citation2009). Furthermore, the lack of interaction with pups during this critical period of life is represented by the opposite pattern when compared to nursing. As expected, dams with increased nursing behavior, demonstrate a decreased no interaction with pups behavior.
Previous studies showed that chronic stress exposure could lead to an anxiety-related phenotype. In a recent systematic review, Tractenberg et al. (Citation2016) found that BALB/c mice are the more vulnerable mice strain to the effects of MS, and numerous evidences highlighted in that study supports an increased anxiety-like behavior following exposure to stress (Andersen, Citation2015; Andersen & Teicher, Citation2009). These data are consistent with our findings and although we did not followed the pups to investigate some possible long-lasting effects of MS, it is plausible hypothesize that they would be more vulnerable to stress and present increased anxiety-like phenotype.
The hippocampus is an important limbic region that regulates some behavioral measures of anxiety. Following chronic stress, glucocorticoids receptors and mineralocorticoid receptors (MR) are key players mediating the possible deleterious effects of chronic stress exposure. In the brain, the densities of these receptors are increased in the hippocampus (Herman, Citation1993). Taking into account the role of GR and MR in regulating the stress response via the hypothalamic–pituitary–adrenal (HPA) axis and the abundance of these receptors in the hippocampus, it has been suggested that alteration in the expression pattern of these genes is associated with the pathophysiology of stress-related disorders, such as anxiety and depressive-like behaviors (Chourbaji & Gass, Citation2008; Müller, Holsboer, & Keck, Citation2002). Furthermore, disrupted mother–infant interaction could promote altered HPA axis function, since the HPA axis of rodents is sensitized during pregnancy and postpartum (Brummelte & Galea, Citation2010; Brunton & Russell, Citation2008). It is important to emphasize that dysregulation on stress-induced behaviors, such as anxiety-like behavior could be associated to the abnormal pattern of maternal behavior presented by the dams involved in the MS protocol. The dams were increasing the amount of maternal behavior instead of decreasing over time. Interestingly, we only observed this irregular pattern pre-MS, possibly an attempt to prepare the pups for the period off-nest.
There were a few limitations during the assessment of maternal behavior. Firstly, the number of observations could be higher because according to Franks (2011) a minimum of 480 observations should be recorded regarding the interaction between dam and pups. Secondly, maternal behaviors could be evaluated more deeply, for example, arched-back nursing, blanket nursing and supine nursing as subgroups of the nursing behavior and anogenital licking and body licking as subgroups of the licking behavior (Shoji & Kato, Citation2006). Unfortunately we did not assess maternal aggression, despite this could be really interesting. Although GR and MR are recognized as biomarkers of stress-related behavioral alterations, we understand that protein levels of corticosterone and ACTH could provide a more clear picture regarding the impact of maternal separation on the levels of stress hormones. Unfortunately, we do not have tissue or plasma of the animals to perform those analysis. Finally, we only used BALB/c mice strain because it is a strain known to be more vulnerable to the effects of stress when compared to other strains but is also important to evaluate and compare different mice strains because they may have altered patterns of maternal care (Shoji & Kato, Citation2006; Tractenberg et al., Citation2016).
We observed that maternal behavior could be manipulated using one model of early life stress, which is in line with previous studies successfully showing that this manipulation could impact how the dams take care of the offspring (Heun-Johnson & Levitt, Citation2016). What is intriguing is the fact that maternal behavior increases in the end of the first week of stress protocol. This could be possibly one attempt of the dam to compensate for the time away from the litter provoked by maternal separation (Francis et al., Citation1999). Unfortunately, we do not have observations from all the first three weeks after birth, which corresponds to the pre-weaning period. A future study addressing this question is guaranteed and could possibly provide more data to better understand how the variations of maternal behavior could be manipulated by a stress protocol. This is interesting because most of the widely used early life stress protocols rely on the variations of the maternal behavior to explain why the pups from stressed moms could be more vulnerable to the stress effects later in life.
In conclusion, the results indicate that MS alters the pattern of maternal behavior throughout the first week of the stress protocol. The lack of maternal care that occurs in the first couple of days is followed by an increase in maternal care in the MS dams during the subsequent days. Furthermore, the alterations on the frequency of nursing and no interaction with pups are the main responsible for the observed pattern. These alterations in the maternal care pattern are followed by anxiety-related traits in the dams following MS, as well as increased GR mRNA expression. To fully comprehend the pattern and alterations in the maternal behavior, new studies could look at the specific variations during the pre-weaning period, considering that in our observation the maternal care pattern was altered throughout this period.
The findings of this study pinpointed that the overall amount of maternal care is not the only factor necessary for an adequate pup development, but the care quality and pattern can prominently affect litter neurodevelopment. Furthermore, disrupted maternal care does not only affect the litter, but also the dam, since altered GR expression in the hippocampus and anxiety-related traits following MS exposure were observed in stressed dams.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adriani, W., & Laviola, G. (2004). Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behavioural Pharmacology, 15, 341–352.
- Aisa, B., Gil-Bea, F.J., Marcos, B., Tordera, R., Lasheras, B., Del Rio, J., & Ramirez, M.J. (2009). Neonatal stress affects vulnerability of cholinergic neurons and cognition in the rat: Involvement of the HPA axis. Psychoneuroendocrinology, 34, 1495–1505. doi:10.1016/j.psyneuen.2009.05.003
- Aisa, B., Tordera, R., Lasheras, B., Rio, J.D., & Ramirez, M.J. (2007). Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology, 32, 256–266. doi:10.1016/j.psyneuen.2006.12.013
- Andersen, S.L. (2003). Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews, 27, 3–18.
- Andersen, S.L. (2015). Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Development and Psychopathology, 27, 477–491. doi:10.1017/S0954579415000103
- Andersen, S.L., & Teicher, M.H. (2009). Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neuroscience and Biobehavioural Reviews, 33, 516–524. doi:10.1016/j.neubiorev.2008.09.009
- Bailoo, J.D., Jordan, R.L., Garza, X.J., & Tyler, A.N. (2014). Brief and long periods of maternal separation affect maternal behavior and offspring behavioral development in C57BL/6 mice. Developmental Psychobiology, 56, 674–685. doi:10.1002/dev.21135
- Bhansali, P., Dunning, J., Singer, S.E., David, L., & Schmauss, C. (2007). Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. Journal of Neuroscience, 27, 1467–1473. doi:10.1523/JNEUROSCI.4632-06.2007
- Brenhouse, H.C., & Andersen, S.L. (2011). Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neuroscience and Biobehavioural Reviews, 35, 1687–1703. doi:10.1016/j.neubiorev.2011.04.013
- Brown, R.E., Mathieson, W.B., Stapleton, J., & Neumann, P.E. (1999). Maternal behavior in female C57BL/6J and DBA/2J inbred mice. Physiology and Behavior, 67, 599–605.
- Brummelte, S., & Galea, L.A. (2010). Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Hormones and Behavior, 58, 769–779. doi:10.1016/j.yhbeh.2010.07.012
- Brunton, P.J., & Russell, J.A. (2008). The expectant brain: Adapting for motherhood. Nature Reviews. Neuroscience, 9, 11–25. doi:10.1038/nrn2280
- Caldji, C., Tannenbaum, B., Sharma, S., Francis, D., Plotsky, P.M., & Meaney, M.J. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy Science USA, 95, 5335–5340.
- Champagne, F.A., Curley, J.P., Keverne, E.B., & Bateson, P.P. (2007). Natural variations in postpartum maternal care in inbred and outbred mice. Physiology and Behavior, 91, 325–334. doi:10.1016/j.physbeh.2007.03.014
- Chourbaji, S., & Gass, P. (2008). Glucocorticoid receptor transgenic mice as models for depression. Brain Research Reviews, 57, 554–560. doi:10.1016/j.brainresrev.2007.04.008
- Conrad, C.D. (2008). Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Reviews in the Neuroscience, 19, 395–411.
- Daniels, W.M., Pietersen, C.Y., Carstens, M.E., & Stein, D.J. (2004). Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metabolic Brain Disease, 19, 3–14.
- Dutta, S., & Sengupta, P. (2016). Men and mice: Relating their ages. Life Sciences, 152, 244–248. doi:10.1016/j.lfs.2015.10.025
- Francis, D., Diorio, J., Liu, D., & Meaney, M.J. (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science, 286, 1155–1158.
- Franklin, T.B., Russig, H., Weiss, I.C., Graff, J., Linder, N., Michalon, A., … Mansuy, I.M. (2010). Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry, 68, 408–415. doi:10.1016/j.biopsych.2010.05.036
- George, E.D., Bordner, K.A., Elwafi, H.M., & Simen, A.A. (2010). Maternal separation with early weaning: A novel mouse model of early life neglect. BMC Neuroscience, 11, 123. doi:10.1186/1471-2202-11-123
- Gudsnuk, K., & Champagne, F.A. (2012). Epigenetic influence of stress and the social environment. ILAR Journal, 53, 279–288. doi:10.1093/ilar.53.3-4.279
- Herman, J.P. (1993). Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cellular and Molecular Neurobiology, 13, 349–372.
- Heun-Johnson, H., & Levitt, P. (2016). Early-life stress paradigm transiently alters maternal behavior, dam-pup interactions, and offspring vocalizations in mice. Frontiers in Behavioral Neuroscience, 10, 142. doi:10.3389/fnbeh.2016.00142
- Ivy, A.S., Brunson, K.L., Sandman, C., & Baram, T.Z. (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience, 154, 1132–1142. doi:10.1016/j.neuroscience.2008.04.019
- Iñiguez, S.D., Riggs, L.M., Nieto, S.J., Dayrit, G., Zamora, N.N., Shawhan, K.L., … Warren, B.L. (2014). Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress (Amsterdam, Netherlands), 17, 247–255. doi:10.3109/10253890.2014.910650
- Pena, C.J., & Champagne, F.A. (2013). Implications of temporal variation in maternal care for the prediction of neurobiological and behavioral outcomes in offspring. Behavioral Neuroscience, 127, 33–46. doi:10.1037/a0031219
- Knight, C.H., Maltz, E., & Docherty, A.H. (1986). Milk yield and composition in mice: Effects of litter size and lactation number. Comparative Biochemistry and Physiology. A, Comparative Physiology, 84, 127–133.
- Kosten, T.A., & Kehoe, P. (2010). Immediate and enduring effects of neonatal isolation on maternal behavior in rats. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 28, 53–61. doi:10.1016/j.ijdevneu.2009.09.005
- Kristal, M.B. (2009). The biopsychology of maternal behavior in nonhuman mammals. ILAR Journal, 50, 51–63.
- König, B., & Markl, H. (1987). Maternal care in house mice. Behavioral Ecology and Sociobiology, 20, 1–9. doi:10.1007/BF00292161
- Liu, D., Diorio, J., Day, J.C., Francis, D.D., & Meaney, M.J. (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience, 3, 799–806. doi:10.1038/77702
- Macri, S., Mason, G.J., & Wurbel, H. (2004). Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. European Journal of Neuroscience, 20, 1017–1024. doi:10.1111/j.1460-9568.2004.03541.x
- Meaney, M.J. (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience, 24, 1161–1192. doi:10.1146/annurev.neuro.24.1.1161
- Müller, M., Holsboer, F., & Keck, M.E. (2002). Genetic modification of corticosteroid receptor signalling: Novel insights into pathophysiology and treatment strategies of human affective disorders. Neuropeptides, 36, 117–131.
- Nephew, B., & Murgatroyd, C. (2013). The role of maternal care in shaping CNS function. Neuropeptides, 47, 371–378. doi:10.1016/j.npep.2013.10.013
- Own, L.S., & Patel, P.D. (2013). Maternal behavior and offspring resiliency to maternal separation in C57Bl/6 mice. Hormones and Behavior, 63, 411–417. doi:10.1016/j.yhbeh.2012.11.010
- Pires, G.N., Tufik, S., & Andersen, M.L. (2015). Effects of REM sleep restriction during pregnancy on rodent maternal behavior. Revista Brasileira De Psiquiatria (Sao Paulo, Brazil: 1999), 37, 303–309. doi:10.1590/1516-4446-2014-1629
- Rice, C.J., Sandman, C.A., Lenjavi, M.R., & Baram, T.Z. (2008). A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology, 149, 4892–4900. doi:10.1210/en.2008-0633
- Shoji, H., & Kato, K. (2006). Maternal behavior of primiparous females in inbred strains of mice: A detailed descriptive analysis. Physiologuy and Behavior, 89, 320–328. doi:10.1016/j.physbeh.2006.06.012
- Shoji, H., & Kato, K. (2009). Maternal care affects the development of maternal behavior in inbred mice. Developmental Psychobiology, 51, 345–357. doi:10.1002/dev.20375
- Tractenberg, S.G., Levandowski, M.L., de Azeredo, L.A., Orso, R., Roithmann, L.G., Hoffmann, E.S., … Grassi-Oliveira, R. (2016). An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neuroscience and Biobehavioral Reviews, 68, 489–503. doi:10.1016/j.neubiorev.2016.06.021
- Wang, L., Jiao, J., & Dulawa, S.C. (2011). Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology (Berl), 216, 207–218. doi:10.1007/s00213-011-2209-4
- Weaver, I.C., Cervoni, N., Champagne, F.A., D'alessio, A.C., Sharma, S., Seckl, J.R., … Meaney, M.J. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7, 847–854. doi:10.1038/nn1276
- Wei, L., David, A., Duman, R.S., Anisman, H., & Kaffman, A. (2010). Early life stress increases anxiety-like behavior in Balb c mice despite a compensatory increase in levels of postnatal maternal care. Hormones and Behavior, 57, 396–404. doi:10.1016/j.yhbeh.2010.01.007