Abstract
The interplay between children’s cortisol reactivity to challenge and cumulative cortisol exposure is not well understood. Examining the role of cortisol reactivity in early childhood may elucidate biological mechanisms that contribute to children’s chronic physiological stress and behavioral dysregulation. In a sample of 65 preschool-aged children, we examined the relation between children’s salivary cortisol reactivity to challenging tasks and their hair cortisol concentration (HCC). While both are biomarkers of the hypothalamic-pituitary-adrenal (HPA) axis, salivary cortisol reactivity reflects an acute cortisol response to a stressor and HCC reflects cumulative cortisol exposure. In addition, we examined the relations of these stress biomarkers with internalizing and externalizing problems. Salivary cortisol reactivity was associated with higher HCC and with increased externalizing behaviors. Child HCC also was positively correlated with parent HCC. Results highlight the contributions of salivary cortisol reactivity to children’s cumulative cortisol exposure, which may add to their biological risk for health problems later. The observed association between externalizing problems and salivary cortisol reactivity indicates concordances between dysregulated behavioral reactions and dysregulated cortisol responses to challenges. The finding that salivary cortisol reactivity to challenge in early childhood plays a role in children’s cumulative cortisol exposure and behavioral development suggests pathways through which cortisol reactivity may influence long-term physical and mental health.
Introduction
Cortisol dysregulation is a key biological mechanism through which early life stress leads to detrimental long-term health outcomes (McEwen, Citation2006). Cortisol, an indicator of biological stress and the end product of the hypothalamic–pituitary–adrenal (HPA) axis, can be measured in response to a stressor (McEwen, Citation1998) or as cumulative exposure across a period of time (Davenport, Tiefenbacher, Lutz, Novak, & Meyer, Citation2006). Children who experience repeated psychosocial stressors often show an atypical cortisol response (Lupien, King, Meaney, & McEwen, Citation2000) and individual differences in reactivity threshold, and efficiency of recovery from stressors may contribute to overall cumulative cortisol exposure.
Stress reactivity is typically assessed with salivary cortisol, indexing acute HPA axis activity (Kirschbaum & Hellhammer, Citation1994). Acute HPA axis activation allows children to mobilize physiological resources to face environmental challenges, but repeated elevations may lead to physiological dysregulation and increased risk for poor health outcomes (Gunnar & Quevedo, Citation2007). Hair cortisol concentration (HCC) is a biological index of cumulative cortisol exposure and is a reliable marker of overall HPA activity (see for review Staufenbiel, Penninx, Spijker, Elzinga, & van Rossum, Citation2013). Cortisol is deposited in the hair shaft as hair grows and one sample provides a timeline of long-term physiological responses to stress (Meyer & Novak, Citation2012). Salivary cortisol reactivity and HCC thus provide different information on HPA axis activation. However, little is known about the interplay of these indices in early childhood. Prior studies have found associations between parent and child HCC in infants and older children (Flom, St John, Meyer, & Tarullo, Citation2017; Ouellette et al., Citation2015), but the relationship between parent and child HCC in a preschool population is unexplored.
Examining how cortisol reactivity and cumulative cortisol exposure relate to behavioral outcomes, such as externalizing and internalizing problems, could help inform the use of biomarkers to identify at-risk children. A meta-analysis showed children who experience high environmental stress during development show more externalizing behaviors and elevated basal cortisol (Alink et al., Citation2008). For internalizing problems, higher cortisol reactivity related to more social withdrawal and anxiety (Granger, Weisz, McCracken, Ikeda, & Douglas, Citation1996). To our knowledge, no study has examined how children’s HCC relates to internalizing or externalizing problems. Including measures of both salivary cortisol reactivity and HCC would further give an understanding of how different stress biomarkers relate to children’s socio-emotional well-being.
Examining cortisol reactivity and HCC in young children can also help elucidate how repeated or prolonged cortisol responses to stressors contribute to long-term HPA axis dysregulation and to behavioral dysregulation. The current study focuses on the preschool period when the HPA axis is rapidly developing and behavioral problems may emerge. The first goal was to examine the association between children’s salivary cortisol reactivity and HCC. We expected that higher salivary cortisol reactivity would relate to increased HCC. Additionally, we wanted to explore the relationship between child and parent HCC in a preschool-aged sample as we expected them to be related. The second goal was to investigate associations of these two HPA biomarkers with behavioral problems. We expected higher salivary cortisol reactivity and HCC would relate to more internalizing and externalizing problems.
Methods
Participants
The sample for these analyses included 63 children aged 3.5 to 4.5 years old (M = 4.19 years, SD = 3.10 months) who participated in two separate laboratory visits. All were full term singletons who had no known auditory, visual, neurological, or developmental disorders (see for demographics).
Table 1. Demographic Information.
The primary goal was to examine how preschoolers salivary cortisol reactivity, assessed in the first visit is related to long-term cortisol exposure and behavioral problems, assessed in the second visit.
Procedure
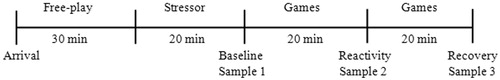
Participants were recruited from a department-maintained database of families who had expressed interest in participating in the research, from online advertising, and from community recruitment events. Two laboratory visits were scheduled within a few months of each other (M = 1.46 months, SD = 1.35 months). At the first visit, children arrived at the laboratory in the afternoon (between 1:00 and 5:00 pm) and were not allowed to eat or drink during the visit. The parent and child first completed a quiet free play for 20 min to make sure the child was comfortable in the laboratory. Next, the child completed a set of stress-inducing tasks while an experimenter collected three saliva samples. First, the child completed a stress paradigm that took 20 min and the first salivary sample was collected (between 1:30–3:30 pm), indicating children’s salivary cortisol baseline levels before the stressor and after the free play. Next, the child was again engaged in behavioral tasks (not included in current analyses) with the experimenter for 20 min and the second saliva sample was collected, indicating children’s salivary cortisol reactivity to the stressor. Finally, after another 20 minutes of quiet play, a third salivary sample was collected, indicating children’s salivary cortisol recovery levels after the stressor (see for collection timeline). During a separate visit, hair cortisol samples were collected from both the child and parent (M = 40.81 days between visits, SD = 41.06 days) and parents filled out questionnaires on hair care habits and children’s behavioral problems.
Figure 1. Upon arrival, the mother and child engaged in a free play to familiarize the child with the lab before the stressor. The first saliva sample was collected after the stress paradigm which took 20 min, reflecting salivary cortisol baseline levels before the stressor. The second sample was collected 20 min after the first collection, indicating peak salivary cortisol levels immediately following the stressor. The third sample was collected 40 min after the first collection, indicating children’s salivary cortisol levels after the stressor.
Measures
Stress paradigm
Children were asked to sit in a room with an unfamiliar, stern stranger while listening to a three minute audio recording of an argument between two adults. This procedure has demonstrated effectiveness in evoking emotional and behavioral distress responses in children in previous studies (e.g. El-Sheikh, Citation1994). Children also engaged in the Behavior Indicator of Resiliency to Distress (BIRD; Lejuez, Daughters, Danielson, & Ruggiero, Citation2006) task, a psychological stress task which has demonstrated effectiveness in increasing stress and negative affect (Daughters et al., Citation2009). In the BIRD task, children were told to select correctly numbered boxes at an increasingly difficult speed in order to free a bird from its cage. When the child successfully clicked on the correct box, indicated by a dot, a bird was released from its cage and they earn one point. If the child was unsuccessful (i.e. too slow or clicked the wrong box), they did not earn a point, the bird remained in its cage, and they heard an aversive loud noise. The BIRD became increasingly more difficult and the average latency between dot presentations was reduced beyond the child’s individual skill level, which resulted in constant forced failure and aversive noise (Daughters, Gorka, Matusiewicz, & Anderson, Citation2013). After the stress paradigm, children listened to a 20 s resolution to the adult argument (e.g. El-Sheikh, Cummings, & Reiter, Citation1996), were told that the BIRD game was broken, and received a prize for doing well.
Salivary cortisol collection
Trained research assistants placed a synthetic cotton swab (Salimetrics, State College, PA) in the child’s mouth for 60 s to collect saliva samples. Samples were kept frozen at −20° C until they were sent to Trier Laboratories in Germany to be assayed for cortisol. Salivary cortisol concentrations were determined by employing a competitive solid phase time-resolved fluorescence immunoassay with fluorometric end point detection (DELFIA; Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, Citation1992). The intra- and inter-assay variation coefficients computed for the mean of average duplicates was less than 6%.
Child behavior checklist for ages 1.5–5 (CBCL)
The CBCL (Achenbach & Rescorla, Citation2000) is a widely-used 100-item questionnaire in which parents classify statements about their children’s behavioral and emotional problems. It includes two broad groupings, internalizing and externalizing problems. Internalizing problems are mainly within the self, including emotional reactivity, anxiety, and depression. Externalizing problems involve conflicts with others, including attention problems and aggression. The primary caregiver (57 mothers and six fathers) rated how well each item describes the child within the last two months, from 0 (not true at all) to 2 (very true/often true). The items of the CBCL had an internal consistency coefficient of 0.94. The CBCL yields standardized T scores for internalizing (M = 44.33; SD = 9.06; actual range = 29–66; α = 0.84) and externalizing scales (M = 45.19; SD = 10.32; actual range = 28–65; α = 0.92).
Hair cortisol
Hair cortisol measurement procedures followed our validated methods (Davenport et al., Citation2006; Meyer, Novak, Hamel, & Rosenberg, Citation2014). We collected hair samples, which were then assayed to determine HCC, from both the parent and child based on past work finding a positive correlation between maternal and child HCC (Flom et al., Citation2017; Ouellette et al., Citation2015). A small amount (15–30 mg) of hair from the posterior vertex of the head was collected representing the 3 cm closest to the scalp. Because washing, hair straightening or dying, and styling products may affect HCC (Hoffman, Karban, Benitez, Goodteacher, & Laudenslager, Citation2014), parents were asked about their own and their child’s hair histories including the frequency that the hair got wet. Human scalp hair grows at approximately 1 cm per month (LeBeau, Montgomery, & Brewer, Citation2011), so the 3 cm sample indexed cortisol output was of over the past three months. Hair samples were stored in plastic tubes labeled with subject ID and were frozen at −20° C until cortisol analysis. Hair samples were weighed, washed twice with isopropanol to remove contaminants, dried, and ground into a fine powder. Cortisol was extracted into methanol, which was then evaporated, and the residue was reconstituted in assay buffer. Reconstituted extracts were analyzed for cortisol using a sensitive and selective commercially available enzyme immunoassay (Salimetrics, LLC; Carlsbad, CA). Assay readout was converted to pg cortisol per mg of dry hair weight. Intra- and inter-assay CVs for children were less than 13% and for parents were less than 14 %, respectively.
Data management
Missing data
Of the 77 children who participated in both visits, 11 were excluded from the current analyses due to the misplacement of one or more saliva samples, two were excluded due to steroid use and one was excluded due to biologically implausible salivary cortisol values (raw baseline salivary cortisol sample = 7.06 µg/dl). This resulted in a final sample of 63 children (34 male).
Normalization of salivary cortisol and hair cortisol variables
Statistically extreme salivary cortisol values for area under the curve with respect to ground (AUCg) and area under the curve with respect to increase (AUCi) were winsorized to three standard deviations from the mean (Brummelte et al., Citation2011). Five children required winsorizing of AUCg, AUCi or both values (AUCg = 4; AUCi = 2) to restore normality of distribution. Salivary cortisol values were log-transformed as necessary to meet the assumptions of normality (Brummelte et al., Citation2015).
Raw HCC levels were log transformed because the data were not normally distributed. Two children’s HCC values were statistical outliers; these values were winsorized to within three SDs of the mean so that they would not have undue influence on the analyses. Of the 63 parent-child dyads in our sample, 10 hair samples (eight due to collection error, two due to medication) from children and 11 hair samples from parents (10 due to collection error, one due to medication) were excluded. This resulted in 53 children and 52 parents with usable hair samples. Parent’s HCC was unrelated to color treatment of hair and there was no evidence that more frequently washed hair was related to lower hair cortisol in either parent or child.
Determination of salivary cortisol biomarkers
Cortisol reactivity was measured by calculating two variables, using previously validated formulas (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, Citation2003). AUCg takes into account the distance from zero and reflects the total area under the curve of all cortisol measurements (baseline, 20-min post-stressor, and 40-min post-stressor). AUCg reflects both sensitivity (the difference between the single measurements from each other) and intensity (the distance of these measures from ground). AUCi is computed with reference to the baseline measurement and does not take into account the distance from zero for all measurements. While AUCg is often used as a measure more related to total hormonal output, AUCi emphasizes the changes over time and is more related to sensitivity of the system (Fekedulegn et al., Citation2007).
Analysis plan
In preliminary analyses, we first tested parent education, household income, age, and gender as potential covariates. Pearson correlations examined parent education, household income, and child age in relation to salivary cortisol variables, HCC, internalizing, and externalizing problems. Group differences between male and female children in HCC, salivary cortisol, or behavioral problems were assessed using independent samples t-test. Group differences between male and female parents in HCC were also assessed using independent samples t-tests. Any demographic variable that related to cortisol or behavioral variables of interest would be included as a covariate in subsequent analyses with that variable. Because some HCC differences by ethnicity have been reported (Rippe et al., Citation2016), perhaps reflecting ethnic variation in hair growth rates, we next examined whether there were ethnicity differences in HCC. Separate independent samples t-tests were conducted comparing HCC levels between Caucasian participants and non-Caucasian participants; African American participants and non-African-American participants; Asian participants and non-Asian participants.
In the main analyses, to assess whether children showed an HPA response to the stressor, we followed common practice in the field and used paired t tests (Allwood, Handwerger, Kivlighan, Granger, & Stroud, Citation2011; Blair, Granger, Willoughby, & Kivlighan, 2008; Blair et al., Citation2006; Epel et al., Citation2000; Quirin, Pruessner, & Kuhl, Citation2008) to examine changes in salivary cortisol over the course of the stressor task. Next, to examine how children’s salivary cortisol reactivity and total salivary cortisol output related to children’s long-term cortisol exposure, we used Pearson correlations to test the relationship between AUCg and child HCC, as well as AUCi and child HCC. In addition, to explore the relationship between parent and preschool children’s long-term cortisol exposure, we used Pearson correlations to test the association between parent and child HCC. Finally, in order to investigate how salivary cortisol reactivity, total salivary cortisol output, and long-term cortisol exposure are related to behavioral problems in children, we used Pearson correlations to test the relationships between salivary and hair cortisol indicators with internalizing and externalizing problems.
Results
Preliminary analyses
Log transformed values of AUCg, AUCi, and HCC were used in all analyses, but for ease of interpretation, untransformed means are presented in tables with descriptive statistics (see ). Household income, parental education, children’s age, gender, and ethnicity were not associated with any of the salivary cortisol, HCC, or behavioral problem variables, thus they were not included as covariates in further analyses. Parent’s gender was also not associated with their HCC levels and was not included as a covariate.
Table 2. Raw salivary cortisol and hair cortisol concentration values.
Children’s salivary cortisol reactivity
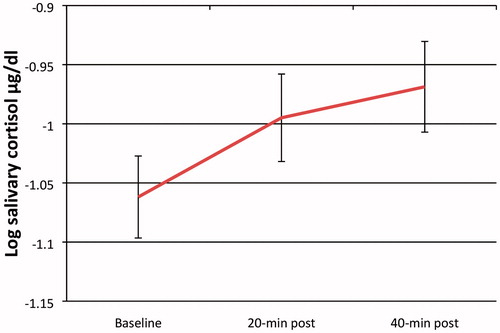
Across all participants, paired t tests indicated that children had significantly higher salivary cortisol levels 20-min post-stressor than at baseline, t(62) = −2.39, p = 0.020, d = 0.29 (see ). Consistent with previous findings (Blair et al., Citation2008; Glenn et al., Citation2015), our sample as a whole did not exhibit a significant decline 40-min post-stressor for salivary cortisol. This might be because children may still have been reacting to the stressor and we did not have a long time period to capture the full recovery in this particular age group.
Figure 2. Preschool children’s salivary cortisol reactivity in response to a challenging task. Salivary cortisol levels increased significantly from baseline to 20-min post- stressor (t = −2.39, p = 0.020), indicating reactivity to the paradigm. Salivary cortisol levels were not significantly different from 20-min to 40-min post-stressor, indicating children did not show recovery within the same time frame. Error bars represent ±1 SE.
Salivary cortisol and HCC
Correlations among all study variables can be found in . We examined how children’s salivary cortisol AUCg and AUCi related to their cumulative cortisol exposure, as indexed by HCC. Salivary cortisol AUCg was correlated with children’s HCC (r(53) = 0.32, p = 0.021; see ), such that children with higher total salivary cortisol output during the laboratory visit had higher levels of cumulative cortisol exposure. Salivary cortisol AUCi was also correlated with children’s HCC (r(53) = 0.29, p = 0.037, see ) indicating that children who had a stronger biological reaction to the stressor also had higher cumulative cortisol exposure. Additionally, we found that parent and child HCC were positively correlated (r(51) = 0.32, p = 0.022).
Figure 3. Scatterplot showing (a) the relationship between children’s HCC and salivary cortisol total output (AUCg) and (b) the relationship between children’s HCC and salivary cortisol reactivity (AUCi).
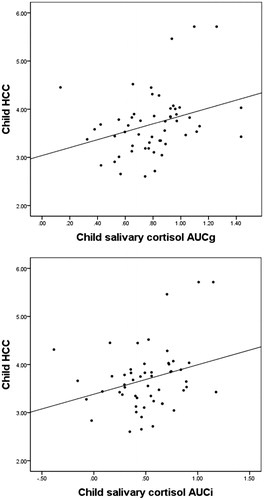
Table 3. Correlations amongst Measures.
Salivary cortisol, HCC, and behavioral problems
Salivary cortisol AUCi was positively correlated with externalizing problems, r(58) = 0.27, p = 0.045) such that children with greater reactivity to the stressor had more parent-reported externalizing behaviors. Salivary cortisol AUCi was not related to internalizing problems. Salivary cortisol AUCg and HCC were not associated with internalizing or externalizing problems.
Discussion
We examined preschool children’s salivary cortisol reactivity in relation to HCC, a marker of cumulative cortisol exposure, and the association of these HPA markers with behavioral problems. Children who had high cortisol reactivity to challenge had higher levels of cumulative cortisol exposure. Salivary cortisol reactivity was also related to behavioral problems, such that children with a greater salivary cortisol response to challenge were reported to have more externalizing problems. Findings have implications for children who have high stress reactivity, hence physiological dysregulation in early childhood can be a marker both of risk for chronically elevated cortisol and for externalizing problems. Results support the value in measuring both acute and long term levels of cortisol to better understand the interplay of how cortisol reacts to a specific stressor and long-term cumulative cortisol exposure could contribute to an understanding of the development of the HPA axis with implications for children's later outcomes.
Other studies have found a relationship between salivary cortisol reactivity and HCC in a longitudinal sample of girls (Ouellette et al., Citation2015) and between salivary AUCg and HCC in a sample of obese girls (Papafotiou et al., Citation2017). In our sample, children with higher salivary cortisol reactivity had higher HCC. Specifically, children with greater salivary cortisol reactivity when faced psychosocial stressors in a lab setting (e.g. listening to adults quarrel, performing under difficult circumstances) also had higher levels of cumulative cortisol exposure across several months. Not only can individual differences in responsivity lead to more frequent activation and higher cumulative exposure, but also different environmental exposures can contribute to higher cumulative cortisol exposure. Children face numerous challenges on a daily basis in the classroom and in their home environments. How children respond to those challenges, including their physiological threshold for a stress response and the magnitude, and duration of their response to the stressor, may have a cumulative impact on their overall physiological stress toll (McEwen, Citation2006). This has important implications for children growing up in poverty who may face frequent stressors and subsequently endure repeated cortisol elevation, contributing to chronic HPA dysregulation.
Preschoolers who had higher cumulative cortisol exposure also had higher salivary cortisol AUCi, which indexes specifically the change in cortisol over time in response to challenge after controlling for baseline levels. Thus, it is not just that reactive children generally have higher cortisol all the time. One might imagine a compensatory mechanism where children who are more reactive have lower baseline levels and more context-specific cortisol levels with no difference in overall HCC exposure. However, our data show that was not the case. The specific association of HCC with salivary cortisol reactivity controlling for baseline salivary levels suggests that a lower threshold for cortisol responses or a larger, or more prolonged response may add up to higher cortisol exposure across time. This has implications not only for children exposed to frequent or intense stressors but also for children who are more reactive to mild stressors. Thus, there could be multiple risk factors for high cumulative cortisol exposure. Our results are relevant for children experiencing adverse environments as well as highly stress reactive children growing up in supportive environments who could still be prone to high cumulative cortisol exposure due to their reactivity to mild stressors.
This is the first study to report parent-child concordance in HCC within a preschool population. Our finding is consistent with patterns of mother-child HCC association reported in 12-month-old infants (Flom et al., Citation2017) and in older children (Ouellette et al., Citation2015), but divergent from a study that found no relation of mother-infant HCC at 9 or 12 months of age (Liu, Snidman, Leonard, Meyer, & Tronick, Citation2016). There are several possible explanations for HCC synchrony between preschool children and their parents. First, young children rely on their caregivers for help in regulating emotions and managing stress (Loman & Gunnar, Citation2010). Parents who themselves have difficulty regulating their own stress may in turn be less able to help their child regulate their stress (Gunnar & Talge, Citation2008). This explanation might lead one to anticipate an association between parent HCC and children’s salivary cortisol AUCi, which we did not find in the current study. However, parents coping skills to long term stressors may be different from their coping skills during acute stressors. Therefore, to examine the possible implications of parents daily biological stress regulation for their children’s capacity to regulate biological stress, future studies should incorporate a stress reactivity measure for parents in addition to measuring their long term cortisol exposure. This would allow examination of the interplay of parent HCC and salivary cortisol reactivity to a stressor with children’s HCC and salivary cortisol reactivity to a stressor. A second explanation of HCC parent-child synchrony is that, parents and young children may be exposed to similar environmental stressors, resulting in more similar cumulative cortisol exposure (Stenius et al., Citation2008). Finally, it is possible that parents and children have related HCC because HCC is genetically influenced. Twin studies have demonstrated the heritability of basal salivary cortisol (Bartels, de Geus, Kirschbaum, Sluyter, & Boomsma, Citation2003; Ouellet-Morin et al., Citation2008, Citation2009) and animal studies have indicated the heritability of HCC in monkeys (Fairbanks et al., Citation2011). Recently, the first genetic study examining the role of genetic variation of long-term cortisol exposure revealed that genetic factors accounted for about half the variation in HCC (Tucker-Drob et al., Citation2017). Thus, the relation of parent and child HCC may be due, in part, to shared genetic alleles. While the mechanisms underlying the parent-child HCC association have not been determined, our results indicate that young children whose parents have high biological stress are themselves at risk for higher cumulative cortisol exposure.
We found that children with higher levels of salivary AUCi had more parent-reported externalizing symptoms. This result is consistent with a past study showing that higher cortisol reactivity was related to mother’s report of behavioral problems in a 4.5 year old (Spinrad et al., Citation2009). One possibility for this relation is that children who are emotionally dysregulated tend to have both a dysregulated behavioral reaction and high cortisol response to challenges. However, Spinrad et al. (Citation2009) did not find a relation between cortisol reactivity and children’s behavioral maladjustment, measured by a laboratory temperament battery. The literature on cortisol reactivity and externalizing behaviors is quite complex. Some researchers report an inverse relationship between cortisol reactivity and externalizing behaviors (Lahey, McBurnnett, Loeber, & Hart, Citation1995; Van Goozen, Fairchild, Snoek, & Harold, Citation2007). In a meta-analysis, Alink et al. (Citation2008) offer many factors that moderate the relationship between cortisol reactivity and externalizing behaviors. For example, differences in methodologies, such as observational measures or questionnaires, used to measure externalizing behaviors may contribute to different outcomes. Specifically, different methods may capture different aspects of externalizing behavior (Karp, Serbin, Stack, & Schwartzman, Citation2004; McEvoy, Estrem, Rodriguez, & Olson, Citation2003) and may lead to differences in the relation between cortisol and externalizing behavior (Alink et al., Citation2008). We used parent-report to measure externalizing behaviors and may have found a different relationship if we had a laboratory-based measure of externalizing.
In our sample, children’s HCC was unrelated to parent-report of children’s externalizing and internalizing symptoms. This is consistent with adult research, which typically does not find relations between HCC and questionnaire measures assessing mental health such as depression and anxiety (see for review Staufenbiel et al., Citation2013). It may be that long term cortisol exposure does not capture the aspect of HPA function most relevant to behavior problems. Other indices of HPA function, such as cortisol reactivity in the current study, and also diurnal cortisol regulation across the day, may be more associated with behavioral dysregulation. AUCi specifically reflects the child’s response to the stressor and similarly, externalizing problems often refer to children “acting out” or reacting to an external stimuli in the environment. This parallel may help explain why we observed a distinct relationship of externalizing problems with AUCi but not with HCC. The absence of an association between HCC and behavioral problems further emphasizes the need to include multiple measures of HPA function in future studies, as they may have distinct implications for children’s behavioral development.
The current study was limited to the use of parent-report measures on children’s externalizing and internalizing symptoms. Future studies should incorporate observational measures to fully capture children’s behavioral regulation. While previous studies have shown that variables such as income, ethnicity, and parent education are related to HCC in children (Rippe et al., Citation2016; Vaghri et al., Citation2013), these variables were not related in our sample. Our particular population was relatively privileged and socioeconomically homogenous. Future studies should investigate current findings in a more socio-economically diverse sample to explore whether findings would generalize across different socioeconomic backgrounds. Future work would also benefit using a longitudinal design to help better understand the development trajectory of early cumulative cortisol exposure and physiological reactivity with later mental and physical health problems. Longitudinal designs could also further explore how the HPA axis changes with age and how this may influence relationships with physical and mental health. In addition, studies specifically examining HPA functioning with a high risk sample may help to clarify how these findings apply to young children who face daily psychosocial stressors and what that means for their physiological stress system. Although our sample was predominantly low risk and well adjusted, we still found a link between salivary cortisol reactivity and HCC. It is striking that even in a low risk sample, higher cortisol reactivity contributed to chronic cortisol exposure. Among children exposed to more frequent and intense stressors, those who are stress reactive may be particularly prone to elevated HCC with potential long term health implications.
Acknowledgements
The authors thank Srishti Nayak, Fang Hong, Sonja Jasinski, Maitreyi Choski, and Megan Flom for assisting with data collection and Kendra Rosenberg for assaying the hair samples. We are grateful to the families who participated.
Disclosure statement
All authors report no conflict of interest.
References
- Achenbach, T.M., & Rescorla, L.A. (2000). ASEBA preschool forms & profiles. Burlington (VT): University of Vermont, Research Center for Children, Youth and Families.
- Alink, L.R., van IJzendoorn, M.H., Bakermans‐Kranenburg, M.J., Mesman, J., Juffer, F., & Koot, H.M. (2008). Cortisol and externalizing behavior in children and adolescents: mixed meta‐analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology, 50, 427–450. doi: 10.1002/dev.20300
- Allwood, M.A., Handwerger, K., Kivlighan, K.T., Granger, D.A., & Stroud, L.R. (2011). Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological Psychology, 88, 57–64. doi: 10.1016/j.biopsycho.2011.06.008
- Bartels, M., de Geus, E.J., Kirschbaum, C., Sluyter, F., & Boomsma, D.I. (2003). Heritability of daytime cortisol levels in children. Behavior Genetics, 33, 421–433. doi: 10.1023/A:1025321609994
- Blair, C., Granger, D.A., Kivlighan, K.T., Mills-Koonce, R., Willoughby, M., Greenberg, M.T., … Fortunato, C.K. (2008). Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology, 44, 1095–1107. doi: 10.1037/0012-1649.44.4.1095
- Blair, C., Granger, D., Willoughby, M., & Kivlighan, K. (2006). Maternal sensitivity is related to hypothalamic-pituitary-adrenal axis stress reactivity and regulation in response to emotion challenge in 6-month-old infants. Annals of the New York Academy of Sciences, 1094, 263–267.
- Brummelte, S., Chau, C.M., Cepeda, I.L., Degenhardt, A., Weinberg, J., Synnes, A.R., & Grunau, R.E. (2015). Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology, 51, 151–163. doi: 10.1016/j.psyneuen.2014.09.018
- Brummelte, S., Grunau, R.E., Zaidman‐Zait, A., Weinberg, J., Nordstokke, D., & Cepeda, I.L. (2011). Cortisol levels in relation to maternal interaction and child internalizing behavior in preterm and full‐term children at 18 months corrected age. Developmental Psychobiology, 53, 184–195. doi: 10.1002/dev.20511
- Daughters, S.B., Gorka, S.M., Matusiewicz, A., & Anderson, K. (2013). Gender specific effect of psychological stress and cortisol reactivity on adolescent risk taking. Journal of Abnormal Child Psychology, 41, 749–758. doi: 10.1007/s10802-013-9713-4
- Daughters, S.B., Reynolds, E.K., MacPherson, L., Kahler, C.W., Danielson, C.K., Zvolensky, M., & Lejuez, C.W. (2009). Distress tolerance and early adolescent externalizing and internalizing symptoms: The moderating role of gender and ethnicity. Behaviour Research and Therapy, 47, 198–205. doi: 10.1016/j.brat.2008.12.001
- Davenport, M.D., Tiefenbacher, S., Lutz, C.K., Novak, M.A., & Meyer, J.S. (2006). Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology, 147, 255–261. doi: 10.1016/j.ygcen.2006.01.005
- Dressendörfer, R.A., Kirschbaum, C., Rohde, W., Stahl, F., & Strasburger, C.J. (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology, 43, 683–692. doi: 10.1016/0960-0760(92)90294-S
- El-Sheikh, M. (1994). Children's emotional and physiological responses to interadult angry behavior: the role of history of interparental hostility. Journal of Abnormal Child Psychology, 22, 661–678. doi: 10.1007/BF02171994
- El-Sheikh, M., Cummings, E.M., & Reiter, S. (1996). Preschoolers' responses to ongoing interadult conflict: the role of prior exposure to resolved versus unresolved arguments. Journal of Abnormal Child Psychology, 24, 665–679. doi: 10.1007/BF01670106
- Epel, E.S., McEwen, B., Seeman, T., Matthews, K., Castellazzo, G., Brownell, K.D., … Ickovics, J.R. (2000). Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine, 62, 623–632. doi: 10.1097/00006842-200009000-00005
- Fairbanks, L.A., Jorgensen, M.J., Bailey, J.N., Breidenthal, S.E., Grzywa, R., & Laudenslager, M.L. (2011). Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology, 36, 1201–1208. doi: 10.106/j.psyneuen.2011.02.013
- Fekedulegn, D.B., Andrew, M.E., Burchfiel, C.M., Violanti, J.M., Hartley, T.A., Charles, L.E., & Miller, D.B. (2007). Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine, 69, 651–659. doi: 10.1097/PSY.0b013e31814c405c
- Flom, M., St John, A.M., Meyer, J.S., & Tarullo, A.R. (2017). Infant hair cortisol: associations with salivary cortisol and environmental context. Developmental Psychobiology, 59, 26–38. doi: 10.1002/dev.21449
- Glenn, A.L., Remmel, R.J., Raine, A., Schug, R.A., Gao, Y., & Granger, D.A. (2015). Alpha-amylase reactivity in relation to psychopathic traits in adults. Psychoneuroendocrinology, 54, 14–23. doi: 10.1016/j.psyneuen.2015.01.012
- Granger, D.A., Weisz, J.R., McCracken, J.T., Ikeda, S.C., & Douglas, P. (1996). Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic‐referred children. Child Development, 67, 3250–3262. doi: 10.1111/j.1467-8624.1996.tb01912.x
- Gunnar, M., & Quevedo, K. (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. doi: 10.1146/annurev.psych.58.110405.85605
- Gunnar, M., & Talge, N.M. (2008). Neuroendocrine measures in developmental research. In L. A. Schmidt, & S. J. Segalowitz (Eds.). Developmental psychophysiology: Theory, systems, and methods ( pp. 343–364). New York (NY): Cambridge University Press.
- Hoffman, M.C., Karban, L.V., Benitez, P., Goodteacher, A., & Laudenslager, M.L. (2014). Chemical processing and shampooing impact cortisol measured in human hair. Clinical & Investigative Medicine, 37, E252–E257. doi: 10.25011/cim.v37i4.21731
- Karp, J., Serbin, L.A., Stack, D.M., & Schwartzman, A.E. (2004). An observational measure of children's behavioural style: evidence supporting a multi‐method approach to studying temperament. Infant and Child Development, 13, 135–158. doi: 10.1002/icd.346
- Kirschbaum, C., & Hellhammer, D.H. (1994). Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinoligy, 19, 313–333. doi: 10.1016/0306-4530(94)90013-2
- Lahey, B.B., McBurnnett, K., Loeber, R., & Hart, E.L. (1995). Psychobiology. In G.P. Sholever (Ed.), Conduct disorders in children and adolescents(pp. 27–44). Washington (DC): American Psychiatric Press.
- LeBeau, M.A., Montgomery, M.A., & Brewer, J.D. (2011). The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Science International, 210, 110–116. doi: 10.1016/j.forsciint.2011.02.015
- Lejuez, C.W., Daughters, S.B., Danielson, C.W., & Ruggiero, K. (2006). The behavioral indicator of resiliency to distress (BIRD). Unpublished manual.
- Liu, C.H., Snidman, N., Leonard, A., Meyer, J., & Tronick, E. (2016). Intra‐individual stability and developmental change in hair cortisol among postpartum mothers and infants: implications for understanding chronic stress. Developmental Psychobiology, 58, 509–518. doi: 10.1002/dev
- Loman, M.M., & Gunnar, M.R. (2010). Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews, 34, 867–876. doi: 10.1016/j.neubiorev.2009.05.007
- Lupien, S.J., King, S., Meaney, M.J., & McEwen, B.S. (2000). Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry, 48, 976–980. doi:10.1016/S0006-3223(00)00965-3
- McEvoy, M.A., Estrem, T.L., Rodriguez, M.C., & Olson, M.L. (2003). Assessing relational and physical aggression among preschool children: Inter method agreement. Topics in Early Child Special Education, 23, 51–61. doi: 10.1177/02711214030230020101
- McEwen, B.S. (1998). Protective and damaging effects of stress mediators. The New England Journal of Medicine, 338, 171–179. doi: 10.1056/NEJM199801153380307
- McEwen, B.S. (2006). Protective and damaging effects of stress mediators: central role of the brain. Dialogues in Clinical Neuroscience, 8, 367–377.
- Meyer, J., Novak, M., Hamel, A., & Rosenberg, K. (2014). Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments, 83, e50882. doi: 10.3791/50882
- Meyer, J.S., & Novak, M.A. (2012). Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology, 153, 4120–4127. doi: 10.1210/en.2012-1226
- Ouellet-Morin, I., Boivin, M., Dionne, G., Lupien, S.J., Arseneault, L., Barr, R.G., … Tremblay, R.E. (2008). Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19- month-old twins. Archives of General Psychiatry, 65, 211–218. doi: 10.1001/archgenpsychiatry.2007.27
- Ouellet-Morin, I., Dionne, G., Pérusse, D., Lupien, S.J., Arseneault, L., Barr, R.G., … Boivin, M. (2009). Daytime cortisol secretion in 6-month-old twins: genetic and environmental contributions as a function of early familial adversity. Biological Psychiatry, 65, 409–416. doi: 10.1016/j.biopsych.2008.10.003
- Ouellette, S.J., Russell, E., Kryski, K.R., Sheikh, H.I., Singh, S.M., Koren, G., & Hayden, E.P. (2015). Hair cortisol concentrations in higher‐and lower‐stress mother–daughter dyads: A pilot study of associations and moderators. Developmental Psychobiology, 57, 519–534. doi: 10.1002/dev.21302
- Papafotiou, C., Christaki, E., van den Akker, E.L., Wester, V.L., Apostolakou, F., Papassotiriou, I., … Pervanidou, P. (2017). Hair cortisol concentrations exhibit a positive association with salivary cortisol profiles and are increased in obese prepubertal girls. Stress, 20, 217–222. doi: 10.1002/dev.21302
- Pruessner, J.C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D.H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. doi: 10.1016/S0306-4530(02)00108-7
- Quirin, M., Pruessner, J.C., & Kuhl, J. (2008). HPA system regulation and adult attachment anxiety: individual differences in reactive and awakening cortisol. Psychoneuroendocrinology, 33, 581–590. doi: 10.1016/j.psyneuen.2008.01.013
- Rippe, R.C., Noppe, G., Windhorst, D.A., Tiemeier, H., van Rossum, E.F., Jaddoe, V.W., … van den Akker, E.L. (2016). Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology, 66, 56–64. doi: 10.1016/j.psyneuen.2015.12.016
- Spinrad, T.L., Eisenberg, N., Granger, D.A., Eggum, N.D., Sallquist, J., Haugen, R.G., … Hofer, C. (2009). Individual differences in preschoolers salivary cortisol and alpha-amylase reactivity: relations to temperament and maladjustment. Hormones and Behavior, 56, 133–139. doi: 10.106/j.yhbeh.2009.03.020
- Staufenbiel, S.M., Penninx, B.W., Spijker, A.T., Elzinga, B.M., & van Rossum, E.F. (2013). Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology, 38, 1220–1235. doi: 10.106/j.psyneuen.2012.11.015
- Stenius, F., Theorell, T., Lilja, G., Scheynius, A., Alm, J., & Lindblad, F. (2008). Comparisons between salivary cortisol levels in six-months-olds and their parents. Psychoneuroendocrinology, 33, 352–359. doi: 10.1016/j.psyneuen.2007.12.001
- Tucker-Drob, E.M., Grotzinger, A.D., Briley, D.A., Engelhardt, L.E., Mann, F.D., Patterson, M., … Harden, K.P. (2017). Genetic influences on hormonal markers of chronic hypothalamic–pituitary–adrenal function in human hair. Psychological Medicine, 47, 1389–1401. doi: 10.1017/S0033291716003068
- Vaghri, Z., Guhn, M., Weinberg, J., Grunau, R.E., Yu, W., & Hertzman, C. (2013). Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology, 38, 331–340. doi: 10.1016/j.psyneuen.2012.06.009
- Van Goozen, S.H.M., Fairchild, G., Snoek, H., & Harold, G.T. (2007). The evidence for a neurobiological model of childhood antisocial behavior. Psychical Bulletin, 133, 149–182.
