Abstract
Adverse childhood experiences (ACEs) affect the development of social cognition (and identify a risk factor for several physical and mental disorders). Theory of Mind (ToM) is a key predictor of social functioning, mental health, and quality of life. No previous study explored the effect of mild ACEs on the neural correlates of ToM in healthy humans. In 23 healthy participants, we used brain blood oxygen level-dependent fMRI to study the effect of ACEs on the neural responses to tasks targeting affective and cognitive ToM. Results pointed out an association between ACEs and a lower neural response in the vermis of the cerebellum (r = −.85), precentral gyrus, and inferior frontal operculum (putative Mirror Neural System, r = −.78) during affective ToM. A lower recruitment of these brain regions, paralleled by the same performance, could express an increased neural efficacy in inferring affective mental states driven by previous experience, in this case, ACEs.
Introduction
In Western countries about 30–40% of the adult population experienced at least some form of maltreatment or adversities during childhood, including abuse, neglect, and family dysfunction. Adverse childhood experiences (ACEs) increase the lifetime risk for physical and psychiatric disorders including cancer, heart disease, depression, anxiety, and substance abuse (Teicher, Samson, Anderson, & Ohashi, Citation2016). In addition, ACEs negatively influence the development of social skills, response to stress, and the ability to control and regulate emotions, all of which are important when interacting with others (Repetti et al., Citation2002; Taylor, Lerner, Sage, Lehman, & Seeman, Citation2004). Understanding how ACEs affect the ability to relate to others and increase the risk of physical and mental illness is of paramount importance to prevent, pre-empt, and treat the consequences of maltreatment and neglect.
Human life critically depends on understanding the internal causes of behavior: Theory of Mind (ToM) is defined as the ability to make inferences upon one's own and other people’s mental states, it is crucial in making people’s behavior meaningful and predictable. This ability is involved in adaptively responding to particular interpersonal transactions, in affect regulation, impulse control, and self-monitoring, identifying a key predictor of social functioning outcomes, mental health, and quality of life. ToM gradually develops during childhood and adolescence: environmental experiences and biological maturation of the involved brain regions co-occur and interact in their development. ToM can be divided into cognitive (CToM) and affective subcomponents (AToM) (Shamay-Tsoory, Citation2011): the former primarily concerns the representations of others’ knowledge, beliefs, intentions, and other neutral states, while the latter is involved in others’ affective states (i.e. emotions, feelings, and desires). Several brain areas have been implicated in ToM processes: superior temporal sulcus (STS), temporoparietal junction (TPJ), posterior cingulate cortex, and medial prefrontal cortex (MPFC) (Van Overwalle & Baetens, Citation2009). Furthermore, AToM has been associated with a deeper recruitment of ventral prefrontal cortex (Shamay-Tsoory & Aharon-Peretz, Citation2007).
A previous study in 5000 adults confirmed that ACEs influence ToM performances in adulthood (Germine, Dunn, McLaughlin, Smoller, & Allen, 2015) paralleled by effects on brain structure and function in regions putatively recruited during ToM tasks. A wide literature confirmed the detrimental effects of ACEs on brain development, detectable also in maltreated adults with structural and functional effects predominantly in cortico-limbic networks, including dorsolateral prefrontal cortex, MPFC, orbitofrontal cortex, anterior cingulate cortex, hippocampus, striatal, cerebellar, and parieto-temporal areas (Teicher et al., Citation2016). The synaptic reorganization that is evident during developmental stages, paralleled by myelination and pruning of synapses, makes these brain regions especially sensitive to environmental experiences (Teicher & Samson, Citation2016).
Most studies explored the effect of severe trauma (i.e. sexual and physical abuse), but also a dysfunctional family environment such as neglectful or harsh parenting and chaotic home life can markedly impact adult well-being (Repetti, Robles, & Reynolds, Citation2011). A cumulative and constant stressful environment for the children can induce long-lasting changes in the brain, which have been interpreted as stress-induced damage, but, in the case of mild stress, could also represent a potential adaptive mechanism to the stressful environment (Teicher et al., Citation2003). Previous findings showed that moderate adversities were associated with a heightened ability in emotional regulation paralleled by neural efficacy in prefrontal and temporal cortexes: mild ACEs are a more likely context for the enhancement of social cognition capacity, relative to severe early life stress (Schweizer et al., Citation2016). These brain effects were never explored in the context of ACE and ToM. In this study, we explored if ACEs and household dysfunction are associated with detrimental effects or adaptive responses on functional neural correlates of cognitive and affective ToM in participants not affected by psychopathology and exposed to a mild load of ACEs.
This perspective could help in better understanding the mechanisms by which ACE affect brain developing and ToM, providing new evidence for identifying vulnerable populations, and new targets for preventive treatments.
Method
Participants
Twenty-three healthy participants (14 females) were recruited from the general population (). Inclusion criteria were absence of mental retardation, pregnancy, major medical/neurological, or psychiatric disorders. After a complete description of the study, approved by the local ethical committee, a written informed consent was obtained. Our sample showed lower adverse childhood adversities (t = 2.02; p < .044) compared with an independent sample of 140 healthy subjects recruited from general population (N = 140; RFQ mean = 27.76, SD = 10.55) (Janicki-Deverts, Cohen, Doyle, Marsland, & Bosch, Citation2014).
Table 1. Data are expressed in mean ± standard deviation.
Adverse childhood experiences
ACEs were rated on the Risky Families Questionnaire (RFQ) (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, Citation2006) after fMRI scanning. The 13 items RFQ was used to assess the extent of family dysfunction during childhood and adolescence. Using a 5-point frequency scale respondents rank the extent to which their family was lacking in nurturance and affection (i.e. how often did a parent or other adult make you feel loved, supported, and cared for?), characterized by overt conflict and aggression (i.e. how often was there quarreling, arguing, or shouting between your parents?), chaotic (i.e. was the household chaotic and disorganized?), and/or abusive (i.e. how often did a parent or other adult push, grab, shove, or slap you?). For all items, participants were instructed to respond with reference to ages 5–15.
Image acquisition and activation paradigms
Gradient echo and echo-planar images (EPI) were acquired on a 3.0 T scanner (Gyroscan Intera; Philips, Amsterdam, The Netherlands) using a six-channel sensitivity encoding head coil. fMRI task comprised a visual activation paradigm composed of a series of comic strips, each depicting a short story. The fMRI task was derived from Vollm et al. (Citation2006). Stimuli were presented in blocks. There were four categories of stories: CToM, with one character whose intentions had to be inferred by the subject; AToM, with interactions between two story characters which required the participant to understand the emotional state of the protagonist; and two control conditions based on the comprehension of physical causality, with one or two characters. Each condition was presented twice, in a random order, so that the task consisted of eight blocks. In each block, five different comic strips (three pictures each) depicting a short story were presented. Each picture was shown for 3 s, and then a picture showing two possible outcomes of the scenario was shown for further 8 s. Participants were required to make a choice between the two stories endings using a response box. Only one of the outcomes represented a plausible story ending. At the beginning of each block, a short question, designed to engage the corresponding mental construct, was shown. The total duration of the task was 13 min. Each block lasted about 1 min and 37 s. For functional run, 256 T2*-weighted volumes were acquired using an EPI pulse sequence [repetition time = 3000 ms, echo time = 35 ms, flip angle = 90°, field of view = 230 mm, number of axial slices = 40, slice thickness = 5 mm, matrix size = 80 × 80 reconstructed up to 128 × 128 pixels]. Two dummy scans before fMRI acquisition allowed us to obtain longitudinal magnetization equilibrium. Participants’ task performances were recorded.
Data analysis
All images were analyzed using Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuroscience, Institute of Neurology and the National Hospital for Neurology and Neurosurgery; London, England). fMRI scans were corrected for slice timing and realigned for head movements. Images were then normalized to a standard EPI template volume based on the Montreal Neurological Institute reference brain and smoothed using an 8-mm full-width at half-maximum isotropic Gaussian kernel. The evoked hemodynamic responses were convolved with a hemodynamic response and its temporal and spatial derivatives within the context of the general linear model (GLM). A high pass filtering was applied on time courses (128 Hz) in each voxel, serial correlations due to aliased biorhythms and unmodeled neural activity was accounted by using an autoregressive process (AR 1).
At the individual level, we compared the CToM and the AToM condition with the corresponding control condition (one or two characters), thereby producing two contrasted images for each subject (one for CToM and one for AToM).
Images contrasted at first level were entered as the dependent variable, RFQ scores as continuous regressor, age, and sex as nuisance covariates (no missing data were detected among the sample). Age was entered by considering that ACEs could have different effects depending on life-cycle stages and its effect could be affected or reversed over time. These analyses were performed in the whole brain and separately for ATom and CToM. Whole Brain results were false discovery rate (FDR) corrected for multiple comparisons at cluster level p < .05 (voxelwise thresholded at p < .001, uncorrected). Further analyses were performed where RFQ showed significant effects, by masking one samples t-tests for the results of regression analyses. Two different one samples t-tests were performed by adding or not RFQ scores as a nuisance covariate. This procedure allowed us to observe if the region is significantly activated by the task, controlling for the effect of RFQ in terms of predicted values. This was done by centering RFQ scores at the value of 13, which is the score of a subject who never experienced ACEs as listed in RFQ. Automated Anatomical Labeling toolbox (AAL, http://www.gin.cnrs.fr/AAL) was used to list the regions included into significant clusters.
Results
ACE significantly influenced the neural responses to the ToM task. Higher RFQ scores were significantly associated with lower neural response in the thalamus, cerebellum, cingulate cortex, and Precentral gyrus during AToM (pFDR < .001; and ). Moreover, task-induced activations of the frontal cluster during AToM were revealed when RFQ scores were entered as nuisance covariate (MNI coordinates 38 10 36, t = 4.76, Z = 3.81, p = .04) but disappeared when the effect of RFQ was not considered. RFQ scores did not significantly affect the BOLD signal during cToM.
Figure 1. Association between ACEs on neural response during affective Theory of Mind. Threshold at cluster level pFDR < .05.
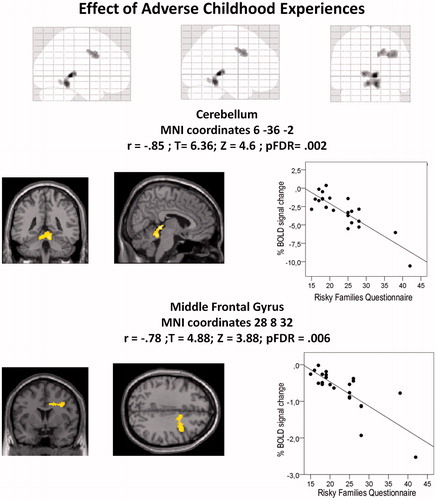
Table 2. fMRI results are reported at cluster level: pFDR, k, cluster size in voxels; peak level: pFDR, MNI coordinates (x, y, z) of voxels with higher T and Z values (signal peaks) and regions included in the activated cluster.
Discussion
We observed an inverse relationship between ACEs and BOLD signal in the vermis of the cerebellum, in the precentral gyrus, and in inferior frontal operculum during AToM.
These regions are all considered being part of the putative mirror neural system (MNS) in humans (Caspers, Zilles, Laird, & Eickhoff, Citation2010), which is proposed as a shared basis for social cognition domains that can be involved in a direct simulation of social events. Mirror processes have been implicated in imitation, action observation, intention understanding, and in identifying action goals, for instance when participants have to process action trajectory or think about how the actions were being performed. MNS may provide a neural underpinning for “mentalizing” with others’, rapidly identifying the actions and their kinematic.
Cerebellum, especially the vermis, is deeply involved in emotional processing and ToM (Van Overwalle, Baetens, Marien, & Vandekerckhove, Citation2014). Cerebellar damage is associated with emotional deficits, impaired working memory, reasoning, and planning, ToM, flattened affect and inappropriate behavior (Manto & Marien, Citation2015). The cerebellum is also recruited in constructing internal models of planned action and event sequences. This effect could also be observed in social cognition: social event implications generate internal models that are “stored” into cerebellum, allowing humans to anticipate action sequences during social interaction in an automatic and intuitive way, continuously checking whether an anticipated event matches with the observed event (Van Overwalle, D'Aes, & Mariën, Citation2015). This process might be relatively automated during typical events (revealing minimal neural activity), but an increased effort is required in reconstructing novel past, future or more complex events (suggesting more activity) (Van Overwalle et al., Citation2015).
It can be hypothesized that a lower recruitment of these brain regions, with the same performance, could express an increased neural efficacy in inferring affective mental states driven by previous experience, in this case, ACEs. Conflictual and neglecting parenting could also provide more opportunities to heighten and refine social cognition skills and acquire internal social models, by stimulating the attribution of affective mental states in order to successfully cope with situations. These data support the hypothesis that forms of mild adversity may provide more opportunities to learn and practice social cognition skill and, when overcome, produce competence in the management, and enhanced resistance to, subsequent stressors is sustained by previous evidence (Seery, Leo, Lupien, Kondrak, & Almonte, Citation2013). Our findings extended this research perspective by suggesting that ACEs could induce neural efficacy in MNS and in the vermis of cerebellum during AToM in humans not affected by psychopathology.
No significant effects for CToM were found. It is not surprising that mild harsh, conflictual parenting and neglect mainly affect, in terms of local neural efficacy, the ability to infer affective mental states rather than cognitive ones. This is supported by considering that AToM mainly develops from late childhood (7 years old – affective second order beliefs) to adulthood with a longer developmental trajectory compared with CToM (Sebastian et al., Citation2012), which mainly end in early adolescence. Moreover, CToM is a prerequisite for AToM (Shamay-Tsoory, Tibi-Elhanany, & Aharon-Peretz, Citation2006), which relies on emotional regulation abilities and a further cognitive control, making AToM a more complex social cognition ability. Future research could explore possible effects of neural efficacy on structural changes in the brain. ACEs were associated with the decreased volume, thickness, or blood flow in corticolimbic circuitry and cerebellum (Teicher et al., Citation2016).
The effect of ACEs on brain development was initially interpreted as stress-induced damage of the brain. However, Teicher et al. (Citation2003) proposed a different perspective: brain alterations could represent a potential adaptive mechanism to the stressful environment, leading to the emergence of alternative developmental pathways in order to survive or adapt in what, based on experience, appears to be a malevolent stress-filled world (Teicher et al., Citation2016). As pointed out by Teicher et al. (Citation2016), a critical issue is to determine which factors could increase vulnerability against resilience (defined as presence or absence of overt psychopathology in maltreated individuals): for example, the detrimental effects related to ACEs could be masked through alterations in molecular expression and by compensatory changes in other regions (Teicher et al., Citation2016) as our data suggest.
Limitations of the present study, which is retrospective, uncontrolled, and correlational in nature, include issues of generalizability and population stratification. ACEs were retrospectively measured with a self-report questionnaire: this could be associated to false or “recovered” memories and recall bias. Previous research, however, indicates that risky family assessments are stable across time (Taylor et al., Citation2004). Furthermore, the cross-sectional nature of this study constitutes a limiting factor regarding the causal interpretation of reported results: altered neural response and brain connectivity could also be a pre-existing factor, which could contribute to vulnerability to ACEs. Finally, here we observed lower ACEs compared with previous studies. Future studies in larger samples are needed to replicate this finding.
Conclusion
In healthy subjects, ACEs do not seem to be associated with detrimental effects, but they mainly induce local neural efficacy, probably exerting a facilitating effect on social cognition performances. Notably, a history of mild ACEs did not allow us to make inference in terms of resilience; future studies should be performed in order to explore possible neurobiological underpinnings of this protective mechanism.
Lay summary
In healthy adults, mild family dysfunction experienced during the childhood does not seem to be associated with detrimental effects, but they induce local neural efficacy (a lower recruitment of brain regions, paralleled by the same performance) in the circuitry involved in inferring other people’s affective mental states, such as emotions, feelings, and desires, probably exerting a facilitating effect on social cognition performances.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Caspers, S., Zilles, K., Laird, A.R., & Eickhoff, S.B. (2010). ALE meta-analysis of action observation and imitation in the human brain. NeuroImage, 50, 1148–1167. doi:10.1016/j.neuroimage.2009.12.112
- Germine, L., Dunn, E.C., McLaughlin, K.A., Smoller, J.W., & Allen, P. (2015). Childhood adversity is associated with adult theory of mind and social affiliation, but not face processing. PLoS One, 10, e0129612. doi:10.1371/journal.pone.0129612
- Janicki-Deverts, D., Cohen, S., Doyle, W.J., Marsland, A.L., & Bosch, J. (2014). Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain, Behavior, and Immunity, 40, 174–181. doi:10.1016/j.bbi.2014.03.010
- Manto, M., & Marien, P. (2015). Schmahmann's syndrome – identification of the third cornerstone of clinical ataxiology. Cerebellum & Ataxias, 2, 2. doi:10.1186/s40673-015-0023-1
- Repetti, R.L., Robles, T.F., & Reynolds, B. (2011). Allostatic processes in the family. Development and Psychopathology, 23, 921–938. doi:10.1017/S095457941100040X
- Repetti, R.L., Taylor, S.E., Seeman, T.E. (2002). Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull, 128, 330–366. doi:10.1037/0033-2909.128.2.330
- Schweizer, S., Walsh, N.D., Stretton, J., Dunn, V.J., Goodyer, I.M., & Dalgleish, T. (2016). Enhanced emotion regulation capacity and its neural substrates in those exposed to moderate childhood adversity. Social Cognitive and Affective Neuroscience, 11, 272–281. doi:10.1093/scan/nsv109
- Sebastian, C.L., Fontaine, N.M., Bird, G., Blakemore, S.J., Brito, S.A., McCrory, E.J., & Viding, E. (2012). Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Social Cognitive and Affective Neuroscience, 7, 53–63. doi:10.1093/scan/nsr023
- Seery, M.D., Leo, R.J., Lupien, S.P., Kondrak, C.L., & Almonte, J.L. (2013). An upside to adversity?: Moderate cumulative lifetime adversity is associated with resilient responses in the face of controlled stressors. Psychological Science, 24, 1181–1189. doi:10.1177/0956797612469210
- Shamay-Tsoory, S.G. (2011). The neural bases for empathy. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 17, 18–24. doi:10.1177/1073858410379268
- Shamay-Tsoory, S.G., & Aharon-Peretz, J. (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia, 45, 3054–3067. doi:10.1016/j.neuropsychologia.2007.05.021
- Shamay-Tsoory, S.G., Tibi-Elhanany, Y., & Aharon-Peretz, J. (2006). The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Soc Neurosci, 1, 149–166. doi:10.1080/17470910600985589
- Taylor, S., Eisenberger, N., Saxbe, D., Lehman, B., & Lieberman, M. (2006). Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry, 60, 296–301. doi:10.1016/j.biopsych.2005.09.027
- Taylor, S.E., Lerner, J.S., Sage, R.M., Lehman, B.J., & Seeman, T.E. (2004). Early environment, emotions, responses to stress, and health. Journal of Personality, 72, 1365–1393. doi:10.1111/j.1467-6494.2004.00300.x
- Teicher, M.H., Andersen, S.L., Polcari, A., Anderson, C.M., Navalta, C.P., & Kim, D.M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews, 27, 33–44. doi:10.1016/S0149-7634(03)00007-1
- Teicher, M.H., & Samson, J.A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, 57, 241–266. doi:10.1111/jcpp.12507
- Teicher, M.H., Samson, J.A., Anderson, C.M., & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17, 652–666. doi:10.1038/nrn.2016.111
- Van Overwalle, F., & Baetens, K. (2009). Understanding others' actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage, 48, 564–584. doi:10.1016/j.neuroimage.2009.06.009
- Van Overwalle, F., Baetens, K., Marien, P., & Vandekerckhove, M. (2014). Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. NeuroImage, 86, 554–572. doi:10.1016/j.neuroimage.2013.09.033
- Van Overwalle, F., D'aes, T., & Mariën, P. (2015). Social cognition and the cerebellum: A meta-analytic connectivity analysis. Human Brain Mapping, 36, 5137–5154. doi:10.1002/hbm.23002
- Vollm, B.A., Taylor, A.N., Richardson, P., Corcoran, R., Stirling, J., McKie, S., … Elliott, R. (2006). Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage, 29, 90–98. doi:10.1016/j.neuroimage.2005.07.022
