Abstract
Oscillating clock gene expression gives rise to a molecular clock that is present not only in the body’s master circadian pacemaker, the hypothalamic suprachiasmatic nucleus (SCN), but also in extra-SCN brain regions. These extra-SCN molecular clocks depend on the SCN for entrainment to a light:dark cycle. The SCN has limited neural efferents, so it may entrain extra-SCN molecular clocks through its well-established circadian control of glucocorticoid hormone secretion. Glucocorticoids can regulate the normal rhythmic expression of clock genes in some extra-SCN tissues. Untimely stress-induced glucocorticoid secretion may compromise extra-SCN molecular clock function. We examined whether acute restraint stress during the rat’s inactive phase can rapidly (within 30 min) alter clock gene (Per1, Per2, Bmal1) and cFos mRNA (in situ hybridization) in the SCN, hypothalamic paraventricular nucleus (PVN), and prefrontal cortex (PFC) of male and female rats (6 rats per treatment group). Restraint stress increased Per1 and cFos mRNA in the PVN and PFC of both sexes. Stress also increased cFos mRNA in the SCN of male rats, but not when subsequently tested during their active phase. We also examined in male rats whether endogenous glucocorticoids are necessary for stress-induced Per1 mRNA (6–7 rats per treatment group). Adrenalectomy attenuated stress-induced Per1 mRNA in the PVN and ventral orbital cortex, but not in the medial PFC. These data indicate that increased Per1 mRNA may be a means by which extra-SCN molecular clocks adapt to environmental stimuli (e.g. stress), and in the PFC this effect is largely independent of glucocorticoids.
Introduction
Properly entrained circadian rhythms are essential for healthy behavior and physiology (Reppert & Weaver, Citation2002). Molecular clocks arising from oscillatory expression of core clock genes underlie these circadian rhythms. The positive clock gene components (Bmal1, Clock/Npas2) encode protein transcription factors that increase the expression of the negative clock gene components (Period1,2,3 and Cryptochrome1,2). PER and CRY repress the actions of the positive component. This self-regulating transcription–translation cycle approximates 24 h in duration (Reppert & Weaver, Citation2002). An oscillating molecular clock is well established in the body’s master clock, the hypothalamic suprachiasmatic nucleus (SCN) (Oishi, Sakamoto, Okada, Nagase, & Ishida, Citation1998; Welsh, Takahashi, & Kay, Citation2010). Molecular clocks also exist in extra-SCN tissues (Chun, Woodruff, Morton, Hinds, & Spencer, Citation2015; Harbour, Weigl, Robinson, Amir, & Bartell, Citation2014; Rath, Rohde, Fahrenkrug, & Møller, Citation2013; Yamamoto et al., Citation2004), however, their normal operation requires daily entrainment signals originating from the SCN.
The importance of molecular clocks in normal health and behavior is demonstrated by rodent and human studies. Global knockdown of Bmal1 or Per1/Per2 expression leads to behavioral arrhythmicity (Bae et al., Citation2001; Bunger et al., Citation2000). Selective disruption of clock gene expression in extra-SCN brain regions is sufficient to produce manic-like (Mukherjee et al., Citation2010) or anxiety-like behavior in rats (Spencer et al., Citation2013). In humans, clock gene polymorphisms or rare variants are associated with mood disorders (Bunney et al., Citation2015; Li et al., Citation2013; McCarthy & Welsh, Citation2012).
The SCN has relatively limited neural projections (Watts, Swanson, & Sanchez-Watts, Citation1987), and thus may communicate with extra-SCN clocks through the release of glucocorticoid hormones (corticosterone in the rat). Corticosterone is released upon activation of the hypothalamic-pituitary-adrenal (HPA) axis. Neural input to the hypothalamic paraventricular nucleus (PVN) initiates a neuroendocrine cascade that ultimately drives the release of corticosterone. The HPA axis receives both stress-related (Ulrich-Lai & Herman, Citation2009) and circadian-related neural input (Dickmeis, Citation2009). Corticosterone has an SCN-dependent diurnal rhythm (Kalsbeek, Liu, et al., Citation2012) that is due to SCN neural input to both the PVN and adrenal elements of the HPA axis. The SCN projects both directly and indirectly (via the subparaventricular zone of the PVN and via the dorsomedial hypothalamic nucleus) to corticotropin-releasing hormone neurons in the PVN (Buijs, Markman, Nunes-Cardoso, Hou, & Shinn, Citation1993; Kalsbeek, van der Spek, et al., Citation2012; Watts et al., Citation1987). The SCN also regulates the circadian release of corticosterone by modulating adrenal sensitivity to ACTH via the splanchnic nerve (Buijs et al., Citation2003; Ulrich-Lai, Arnhold, & Engeland, Citation2006).
Corticosterone is a systemic hormone that crosses the blood–brain barrier, and its glucocorticoid receptors (GRs) are ubiquitously expressed throughout the body, with the one notable exception being the SCN (Balsalobre et al., Citation2000). The Per1 gene has a well-characterized functional glucocorticoid response element (GRE) (So, Bernal, Pillsbury, Yamamoto, & Feldman, Citation2009; Yamamoto et al., Citation2005). Acute glucocorticoid treatment has been shown to rapidly increase Per1 expression in various cell lines, peripheral tissues, and hippocampus (Balsalobre et al., Citation2000; Burioka et al., Citation2005; Conway-Campbell et al., Citation2010; Yamamoto et al., Citation2005). The Per2 and Bmal1 genes are also associated with putative GRE sequences (Cheon, Park, Cho, & Kim, Citation2013; Reddy et al., Citation2007; So et al., Citation2009), but they have not been found to be rapidly increased in vivo by glucocorticoids (Yamamoto et al., Citation2005). Interestingly, an appropriately timed diurnal peak in corticosterone is necessary for normal rhythmic clock gene expression in the PFC of male rats (Woodruff, Chun, Hinds, & Spencer, Citation2016). The rapid regulation of Per1 expression by corticosterone may be the primary mechanism by which the molecular clock in extra-SCN tissue is modulated by this SCN-controlled corticosterone signal. If a circadian peak in corticosterone modulates entrainment of extra-SCN molecular clocks, then untimely stress-induced surges in corticosterone may compromise extra-SCN molecular clock function.
Chronic or repeated stress alters the phase or amplitude of Per1 and Per2 mRNA in peripheral and extra-SCN brain tissue (Logan et al., Citation2015; Tahara et al., Citation2015). These changes to Per1 and Per2 rhythmic expression may be due to the repeated or cumulative effects of each single stressor on clock gene expression. Few studies have examined whether acute stress is sufficient to affect clock gene expression. Acute stress (45–60 min after stressor onset) increased Per1, but not Per2 mRNA, in the PVN of mice (Takahashi et al., Citation2001), increased Per1 mRNA in the hippocampus of male and female mice (Bohacek, Manuella, Roszkowski, & Mansuy, Citation2015) and increased PER1 protein in the PVN, dorsomedial hypothalamus, and piriform cortex in rats (Al-Safadi et al., Citation2014). Stress-altered clock gene expression has yet to be examined in the PFC, a brain region important in emotional control and stress modulation (Arnsten & Rubia, Citation2012). PFC dysfunction is linked to disorders associated with both stress and disruptions in circadian rhythms (Mayberg et al., Citation1999), and stress-induced alteration of PFC clock gene expression may be a contributing factor to these disorders.
Mood disorders (e.g. depression, post-traumatic stress disorder) associated with stress and disruptions in circadian rhythms have a greater prevalence in women (Kessler, Citation2003). Stress may exacerbate mood disorders by disrupting extra-SCN molecular clocks via untimely glucocorticoid surges. Female rats also have greater stress-induced plasma corticosterone compared to males (Babb, Masini, Day, & Campeau, Citation2014; Viau & Meaney, Citation1991), which may contribute to the greater prevalence of stress-related mood disorders in females, possibly through disruptions in the molecular clock. Most of the studies examining the effects of stress on clock gene expression have only examined male rodents.
This study examined whether acute restraint stress can alter clock gene expression (Per1, Per2, Bmal1 mRNA) in the SCN, PFC, and PVN of male and female rats. These brain regions have rhythmic clock gene expression in male and female rats (Chun et al., Citation2015). The SCN was examined as the body’s master clock. Although clock gene expression in the SCN is finely tuned to changes in light:dark cycles, previous studies found that its expression is largely resistant to other environmental alterations such as stress (Al-Safadi et al., Citation2014; Takahashi et al., Citation2001). Furthermore, if acute stress alters clock gene expression via increased corticosterone, then the SCN should not exhibit stress-induced changes in clock gene expression because of its lack of GRs (Balsalobre et al., Citation2000). Previous studies have shown that acute stress increased Per1 expression in the PVN, the head of the HPA axis, in male rodents (Al-Safadi et al., Citation2014; Takahashi et al., Citation2001). Stress-induced clock gene expression has yet to be examined in the PFC, a brain region critical in stress reactivity, emotional control, and normal mood behaviors (Arnsten & Rubia, Citation2012; Mayberg et al., Citation1999). cFos, an immediate early gene (IEG) rapidly induced by restraint stress, was also examined in each brain region for comparison purposes.
We hypothesized that given the well-characterized functional GRE associated with the Per1 gene that acute restraint stress would directly and rapidly (within 30 min) increase Per1 expression in the PFC and PVN of male and female rats. Female rats would have greater stress-induced Per1 expression compared to male rats because female rats have greater stress-induced plasma corticosterone. In contrast, given our assumption that the Per2 or Bmal1 genes are not directly regulated by corticosterone, we predicted that their mRNAs would not be rapidly altered by restraint. To examine if untimely stress-induced corticosterone is necessary for stress-induced changes in clock gene expression, male rats were adrenalectomized to see if that abolishes stress-induced clock gene expression. Because normal rhythmic clock gene expression in male rat PFC is dependent on the presence of a circadian pattern of circulating corticosterone (Woodruff et al., Citation2016), we hypothesized that stress-induced alterations of clock gene expression would be absent in adrenalectomized rats.
Methods
Animals
Sprague-Dawley rats (Harlan, Indianapolis, IN), aged ∼3 months at the time of euthanasia, were used for all experiments. Female rats weighed 180–205 g and male rats weighed 260–325 g at the time of euthanasia. Upon arrival at the facility, rats were pair-housed with same sex cage mates and evenly distributed between two adjacent individual rooms that were humidity and temperature controlled. Rooms were kept at 23 °C and 50% humidity. Rats were kept on a standard 12:12 h light:dark cycle. For logistic purposes, zeitgeber time (ZT) 0, time of lights on, was 10:00 h for Experiment 1 and 06:00 h or 08:00 h for Experiment 2 in order to allow the time of euthanasia to center around ZT4, whereas it was 23:00 h for Experiment 3 in order to allow the time of euthanasia to be centered around ZT16. Rats were given 2 weeks to acclimate to the animal facility prior to experimental manipulations. Food and water were available ad libitum. All procedures were approved by the University of Colorado Boulder’s Institutional Animal Care and Use Committee, and were in accordance with the guidelines found within the Guide for the Care and Use of Laboratory Animals (DHHS Publication No. [NIH] 80-23, revised 2010 eighth edition).
Terminal procedures
For each experiment, rats were stressed for 30 min in Plexiglas cylinder restraint tubes (6.3 cm diameter × 16 cm length for males; 5 cm diameter × 15.5 cm length for females; all had four ventilation holes at the front end of the restraint tube) and then immediately killed by rapid guillotine decapitation (within 30 s of removal from the restraint tube or home cage). Rapid decapitation of unanesthetized rats does not induce a confounding HPA axis or IEG activation at the time of euthanasia, unlike anesthesia (Spencer & Deak, Citation2017). Non-stressed control rats remained in their home cage until time of day-matched decapitation. Restraint stress was administered in a separate room than the rats’ home room. Trunk blood was collected in EDTA-coated tubes and immediately put on wet ice before being centrifuged at 3095 g at 4 °C for 10 min. Plasma was then aliquoted, snap frozen on dry ice, and stored at −70 °C until further use. Brains were extracted and then immediately flash frozen in isopentane that was cooled to −20 °C to −30 °C with dry ice. Brains were then stored at −70 °C until further use.
Brain processing
Brains were sliced using a cryostat (Leica CM1850); 12 μm coronal sections were taken at the level of the PFC (2.2–3.2 mm anterior to bregma), the SCN (1.3–1.4 mm posterior to bregma), and the PVN of the hypothalamus (1.80–1.88 mm posterior to bregma) (Paxinos & Watson, Citation1998). Brain slices were thaw-mounted onto Colorfrost Plus microscope slides, then stored at −70 °C until use for in situ hybridization.
Experiment 1: Does acute stress rapidly alter clock gene expression in male and female rats at ZT4?
Twelve male and 12 female Sprague-Dawley rats were used. Males and females were housed in separate rooms. Female rats’ estrous cycles were not tracked. Thus, subsequent data were pooled regardless of estrous cycle stage. After 2 weeks of acclimation, rats received 30 min of restraint stress, or remained in their home cage until time of euthanasia, which centered around ZT4 (ZT = hours after lights on), during the rats’ inactive phase (2 × 2 factorial design, 6 rats per treatment group).
Experiment 2: Does stress-altered clock gene expression depend on the presence of endogenous corticosterone in male rats?
Twenty-five male Sprague-Dawley rats (Harlan, Indianapolis, IN) were used; 13 of the rats received bilateral adrenalectomy surgery. Rats were anesthetized with halothane inhalation before bilateral 1 cm incisions were made through the skin and the underlying peritoneal wall positioned 1 cm ventral from the spine and immediately posterior to the ribcage. The adrenal glands were carefully isolated with intestinal tissue forceps and removed from the surrounding adipose tissue. The remaining 12 rats received sham surgery, which followed the same procedure for adrenalectomy, but the adrenals were left undisturbed. The peritoneal wall was closed with 2–3 sutures. The skin was closed with surgical staples. Immediately after surgery and once a day for the following 3 days rats were given buprenorphine (0.01 mg/kg, s.c.) as a prophylactic analgesic treatment. Surgery was performed 2 weeks after arrival at the facility and rats were given 1 week to recover before the test day. After surgery, adrenalectomized rats were maintained on 0.9% saline instead of water to drink. On the test day, rats were acutely exposed to 30 min of restraint stress, or remained in their home cage until they were euthanized by rapid decapitation without anesthetic (ZT4; 2 × 2 factorial design, 6–7 rats per treatment group).
Experiment 3: Test of the effect of acute stress on Per1 and cFos mRNA in the SCN and PVN of male and female rats at ZT16
It has been previously reported that non-photic stimulation of the SCN occurs during the rats’ inactive, or light phase, but not during their active phase (Edelstein & Amir, Citation1995; Dibner, Schibler, & Albrecht, Citation2010). In order to examine if acute stress could alter cFos or clock gene expression in the SCN and PVN of male and female rats at ZT16, during the rats’ active phase, 12 male and 12 female Sprague-Dawley rats (Harlan, Indianapolis, IN) were used for this experiment. Males and females were housed in separate rooms. Female rats’ estrous cycles were not tracked and subsequent data pooled regardless of estrous cycle stage. Rats were taken from their home cage, or were exposed to 30 min of restraint stress under complete darkness and euthanasia was centered around ZT16 (2 × 2 factorial design, 6 rats per treatment group).
In situ hybridization
In situ hybridization for Per1, Per2, Bmal1, and cFos mRNA followed procedures previously reported (Chun et al., Citation2015). Briefly, sections on slides were fixed with 4% paraformaldehyde, went through a series of standard saline citrate (SSC) solution washes, then bathed in triethanolamine and acetic anhydride solution. Sections were dehydrated in increasing concentrations of ethanol baths before being air dried. Hybridization buffer containing the 35S-labelled riboprobe for each gene of interest was applied to each slide. Hybridization occurred in humidified chambers with 50% formamide and 50% water for 16–20 h. Coverslips were gently removed in SSC baths. Tissue was then exposed to 0.02 g/L RNase at 37 °C, washed in decreasing concentrations of SSC, and incubated in SSC at 65 °C for 1 h. Sections were dehydrated in increasing concentrations of ethanol, then air dried. Slides were set on Kodak BioFilm Maximum Resolution Autoradiography Film (Carestream Health, Windsor, CO) for 2–4 weeks, then developed in a medical film processor SRX-101A (Konica Minolta, Tokyo, Japan). Each in situ hybridization assay was separated by experiment and by region of interest (ROI; SCN and PVN were run together).
Optical density
Brain images from X-ray film were captured using a lightbox Northern Light model B95 (Imaging Res Inc., St. Catharines, Ontario, Canada) and a Sony CCD video camera model XC-ST70 fitted with a Navitar 7000 zoom lens (Rochester, NY) and connected to LG3-01 frame grabber (Scion Corp., Frederick, MD) inside a Dell Dimension 500, and captured with Scion Image beta rel. 4.0.2. Once digitized, images were analyzed using ImageJ Software (NIH). Mean gray values were converted to uncalibrated optical density, and densitometry was performed on images whose gray levels fell within a linear range of the gray level to optical density ratio.
An experimenter blind to the treatment group of the rats took each measurement. ROIs were selected by using anatomical landmarks from Paxinos and Watson (Citation1998, 4th edition). ROIs at the level of the PFC include: anterior cingulate cortex (AC), prelimbic medial prefrontal cortex (PL), infralimbic medial prefrontal cortex (IL), ventral orbital cortex (VO), and insula. A virtual tool was used to draw a circle centered within each ROI to measure representative portions of each ROI. Six slices/brain were used for analysis of the PFC and insula subregions. In more caudal sections, SCN and PVN were measured by the experimenter using a free-hand outline of the visually apparent ROI; four slices/brain were used for analysis of the SCN and PVN. Measurements were taken from both hemispheres for all brain slices analyzed. The average optical density for each ROI for each brain was used for treatment group averages and statistical analysis.
Corticosterone assay
An enzyme-linked immunosorbent assay kit (Cat. No. K014-H1, Arbor Assays, Ann Arbor, MI) was used according to the manufacturer’s instructions. Plasma samples were run in duplicate and diluted 1:50 in assay buffer. Samples were then heated for 1 h at 65 °C to inactivate corticosteroid-binding globulin. Intra- and inter-assay coefficients of variation for 5 µg/100 mL (low corticosterone) and 20 µg/100 mL (high corticosterone) were ≤10%. Assay sensitivity was 35 pg/mL.
Statistical analysis
Statistical Package for Social Sciences (SPSS; IBM Mac version 22, 2012) was used for statistical analysis. Multifactorial between-group analysis of variance (ANOVA) was used to determine whether there were significant main effects and interactions for the various treatment factors of each experiment. Fischer’s least significant difference (FLSD) post-hoc analysis was used for pair-wise group comparisons of interest. Significance level was a priori set at p < .05. Outliers were determined using the Grubbs’ Test criteria with GraphPad Software QuickCalcs (α = 0.05).
Results
Experiment 1: Does acute stress rapidly alter clock gene expression in male and female rats at ZT4?
Corticosterone
There were significant main effects for stress and sex (, bottom row). As expected, acute restraint stress significantly increased plasma corticosterone concentrations for both sexes (FLSD, p < .001) (). In line with what is well established (Babb et al., Citation2014; Viau & Meaney, Citation1991), females had significantly higher basal and stress-induced plasma corticosterone concentrations than males (FLSD, p < .001) (). Females had much greater variability in basal plasma corticosterone concentrations than males, consistent with previous findings that female but not male Sprague-Dawley rats have pronounced ultradian variations in basal plasma corticosterone concentrations in the morning (Spiga, Walker, Terry, & Lightman, Citation2014).
Figure 1. Experiment 1: Effect of stress and sex on plasma corticosterone concentrations. Plasma corticosterone concentration was increased by 30 min of acute restraint stress. Females had greater plasma corticosterone concentrations compared to males during both basal and stressed conditions. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). See for statistical details.
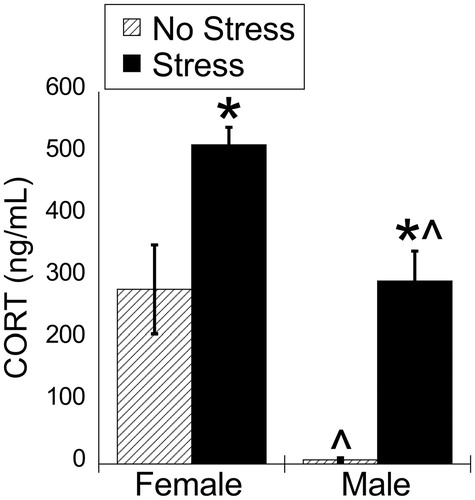
Table 1. Two-way ANOVA results for Experiment 1.
Suprachiasmatic nucleus
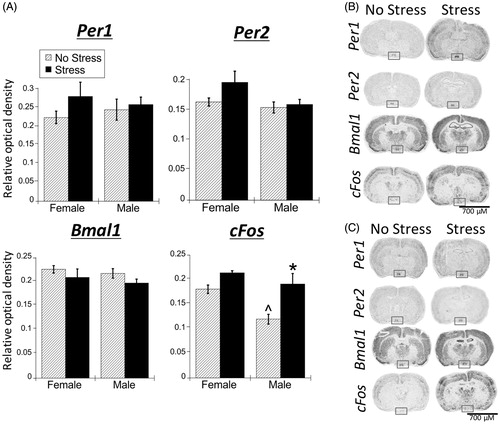
Acute stress had no significant main effects for Per1, Per2, and Bmal1 mRNA expression within the SCN of both male and female rats. However, there were main effects for both stress and sex for cFos mRNA in the SCN (; ). Pair-wise comparisons showed that under no stress conditions, female rats had greater cFos mRNA expression compared to males (FLSD, p < .01). Under stress conditions, cFos mRNA was significantly increased in male rats (FLSD, p < .001).
Figure 2. Experiment 1: Effect of stress and sex on gene expression in the SCN. (A) There were no significant main effects of stress or sex for Per1, Per2, and Bmal1 mRNA in the suprachiasmatic nucleus (SCN) of male and female rats. cFos mRNA was increased by stress in only male SCN. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). (B and C) Representative autoradiographs of gene expression under no stress and stress conditions of female (B) and male (C) rats. The SCN is located within the box. See for statistical details.
Paraventricular nucleus of the hypothalamus
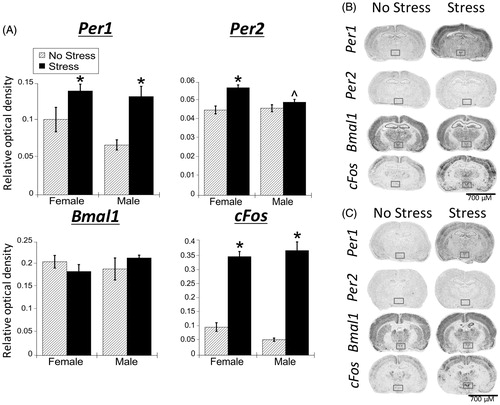
Stress significantly increased Per1 and cFos mRNA in the PVN of both male and female rats (, ). Per2 mRNA also showed a significant main effect of stress, and a significant stress × sex interaction. Post-hoc analysis showed that stress increased Per2 mRNA in the PVN of female rats only (FLSD, p < .001). There were no sex differences in stress-induced Per1 or cFos mRNA despite the significantly greater stress-induced corticosterone concentrations ().
Figure 3. Experiment 1: Effect of stress and sex on gene expression in the PVN. (A) 30 min of acute restraint stress increased Per1 and cFos mRNA in the paraventricular nucleus of the hypothalamus (PVN) of male and female rats. There were no sex differences in stress-induced Per1 and cFos mRNA. Stress also increased Per2 mRNA only in female rat PVN. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). (B and C) Representative autoradiographs of gene expression under no stress and stress conditions of female (B) and male (C) rats. The PVN is located within the box. See for statistical details.
Prefrontal cortex
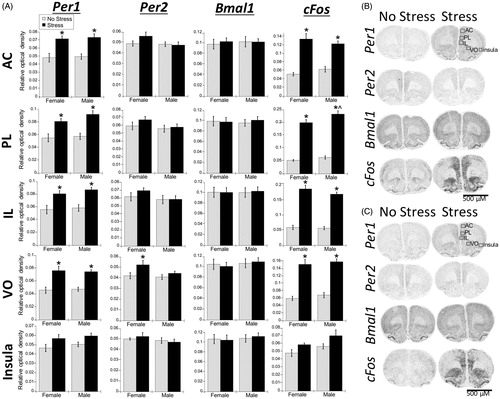
Stress significantly increased Per1 and cFos mRNA in all subregions of the PFC (AC, PL, IL, and VO) (; ). This stress effect was significant for both males and females (FLSD, p < .05). In contrast, there was no significant effect of stress on Bmal1 mRNA, and a restricted effect on Per2 mRNA (only in the VO subregion); post-hoc analysis revealed that, similar to the PVN (), stress increased Per2 mRNA in the VO only in females (FLSD, p < .05). There was also a significant main effect of sex for cFos mRNA in the PL subregion (; ), where, similar to the SCN (), males had greater cFos mRNA expression than females under stress conditions (FLSD, p < .05).
Figure 4. Experiment 1: Effect of stress and sex on gene expression in the PFC and rostral agranular insula (RAI). (A) Acute stress increased Per1 and cFos mRNA throughout the prefrontal cortex (PFC) subregions (anterior cingulate, AC; prelimbic cortex, PL; infralimbic cortex, IL; ventral orbital cortex, VO), and to a lesser extent in the RAI, of male and female rats. Per2 mRNA was also increased by stress, but only in the VO subregion. There was no effect of stress on Bmal1 mRNA. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). (B and C) Representative autoradiographs of gene expression under no stress and stress conditions of female (B) and male (C) rats. See for statistical; details.
Rostral agranular insula
There was a small but significant main effect of stress for Per1 and cFos mRNA in the rostral agranular insula (RAI) (; , Insula). There was also a significant main effect of sex on cFos mRNA, with overall greater cFos mRNA in males compared to females.
Experiment 2: Does stress-altered clock gene expression depend on the presence of endogenous corticosterone in male rats?
In the first experiment, we found that acute restraint stress produced a rapid increase in Per1 mRNA in the PVN and each subdivision of the PFC. This experiment tested whether that increase required the presence of endogenous corticosterone by including an adrenalectomy comparison group. Because we did not observe a sex difference in stress-induced Per1 mRNA in the first experiment, this experiment examined only male rats.
Corticosterone
Plasma corticosterone concentrations for adrenalectomized rats were below detection levels, thus only plasma corticosterone concentrations for SHAM rats were analyzed. Stress increased plasma corticosterone concentrations similarly to those seen in male rats in Experiment 1 (). Mean (±SEM) plasma corticosterone concentrations were as follows: no stress = 38.97 (±11.87) ng/mL and stress = 359.5 (±76.55) ng/mL.
Table 2. Two-way ANOVA results for Experiment 2.
Suprachiasmatic nucleus
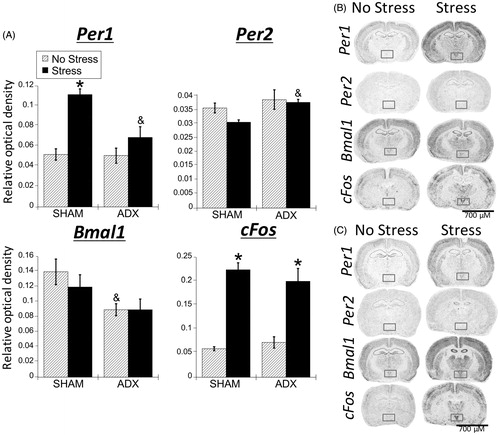
There were no significant main effects of stress or adrenal status for Per1, Per2, or Bmal1 mRNA in the SCN (; ). Similar to Experiment 1, there was a significant main effect of stress on cFos mRNA in the SCN (), where stress increased cFos mRNA expression. This effect was only significant in the SHAM rats (FLSD, p < .05) ().
Figure 5. Experiment 2: Effect of stress and adrenal status on gene expression in the SCN. (A) Acute restraint stress had no effect on Per1, Per2, and Bmal1 mRNA expression. Stress increased cFos mRNA in the suprachiasmatic nucleus (SCN) of SHAM rats only. Data are presented as mean ± SEM (*stress effect within same adrenal status conditions [sham or adrenalectomized]; p < .05, FLSD, n = 4–7 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of SHAM (B) and adrenalectomized (ADX) (C) rats; the SCN is located within the box. See for statistical details.
![Figure 5. Experiment 2: Effect of stress and adrenal status on gene expression in the SCN. (A) Acute restraint stress had no effect on Per1, Per2, and Bmal1 mRNA expression. Stress increased cFos mRNA in the suprachiasmatic nucleus (SCN) of SHAM rats only. Data are presented as mean ± SEM (*stress effect within same adrenal status conditions [sham or adrenalectomized]; p < .05, FLSD, n = 4–7 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of SHAM (B) and adrenalectomized (ADX) (C) rats; the SCN is located within the box. See Table 2 for statistical details.](/cms/asset/bd0999d9-01dc-481a-a3d2-a492edb7f979/ists_a_1404571_f0005_b.jpg)
Paraventricular nucleus of the hypothalamus
Consistent with Experiment 1, there was a significant main effect of stress for Per1 and cFos mRNA expression in the PVN (; ), where both were increased by stress. There was also a significant main effect of adrenal status and a significant interaction, where stress increased Per1 mRNA only in SHAM rats, suggesting that stress-induced Per1 mRNA expression is dependent on the presence of endogenous corticosterone. This corticosterone-dependency did not extend to cFos mRNA, as stress increased cFos mRNA expression for both SHAM and adrenalectomized rats (; FLSD, p < .05). There was a significant main effect of adrenal status for Per2 mRNA. Post-hoc tests indicate that adrenalectomized rats had greater Per2 mRNA expression compared to SHAM rats under conditions of stress (FLSD, p < .05). There was also a significant effect of adrenal status for Bmal1 mRNA. Post-hoc tests indicated that no stress SHAM rats had greater Bmal1 mRNA expression compared to no stress adrenalectomized rats (FLSD, p < .05).
Figure 6. Experiment 2: Effect of stress and adrenal status on gene expression in the PVN. (A) Acute restraint stress increased Per1 and cFos, but not Per2 or Bmal1 mRNA in the paraventricular nucleus of the hypothalamus (PVN), replicating the male data from Experiment 1. Stress increased cFos mRNA occurred regardless of adrenal status (sham or adrenalectomized). However, stress-induced Per1 mRNA was attenuated by adrenalectomy (ADX). Data are presented as mean ± SEM (*stress effect within same adrenal status condition; &adrenal status effect within same stress conditions; p < .05, FLSD, n = 5–6 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of sham (B) and ADX (C) rats; the PVN is located within the box. See for statistical details.
Prefrontal cortex
Similar to the PVN, in all subregions examined (AC, PL, IL, and VO), stress increased Per1 and cFos mRNA (; ). For cFos mRNA, this stress induction was evident in both SHAM and adrenalectomized rats, however on average the induction was somewhat less in adrenalectomized rats. In the PL and IL subregions, there was a significant main effect of adrenal status and a significant stress × adrenal status interaction (), with SHAM rats exhibiting greater stress-induced cFos mRNA compared to adrenalectomized rats (FLSD, p < .05). Stress increased Per1 mRNA in the AC and PL for both SHAM and adrenalectomized rats, but only for SHAM rats in the VO (FLSD, p < .05), indicating that stress-induced Per1 mRNA throughout the medial subregions of the PFC (AC, PL, and IL), but not the more lateral VO, is largely independent from the presence of endogenous corticosterone. There was also a main effect of adrenal status for Bmal1 mRNA in the AC, PL, and VO subregions, where SHAM rats had greater Bmal1 mRNA than adrenalectomized rats under no stress conditions (FLSD, p < .05). This may be due to possible phase shifts or blunting of diurnal amplitude of Bmal1 mRNA in the PFC of adrenalectomized rats, as has been previously shown (Woodruff et al., Citation2016). The IL and VO both displayed a significant stress effect for Bmal1, where stress decreased Bmal1 mRNA, but in SHAM rats only (FLSD, p < .05). However, there was no significant main effect of adrenal status, indicating that stress can rapidly decrease Bmal1 mRNA expression. This decrease may be largely independent of corticosterone.
Figure 7. Experiment 2: Effect of stress and adrenal status on gene expression in the PFC and rostral agranular insula (RAI). (A) Acute stress increased Per1 and cFos mRNA throughout the prefrontal cortex (PFC) subregions (anterior cingulate, AC; prelimbic cortex, PL; infralimbic cortex, IL; ventral orbital cortex, VO), but not in the RAI. In the AC and PL subregions, stress increased Per1 mRNA in both SHAM and adrenalectomized (ADX) rats, suggesting corticosterone-independent effects. The VO subregion had stress-induced Per1 mRNA only in SHAM rats. cFos mRNA was induced by stress regardless of adrenal status, but the increase was attenuated by adrenalectomy in the PL and IL. There was also a main effect for stress to decrease Bmal1 mRNA levels in the IL, VO, and RAI. Data are presented as mean ± SEM (*stress effect within same adrenal status conditions; &adrenal status effect within same stress conditions; p < .05, FLSD, n = 5–6 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of sham (B) and ADX (C) rats. See for statistical details.
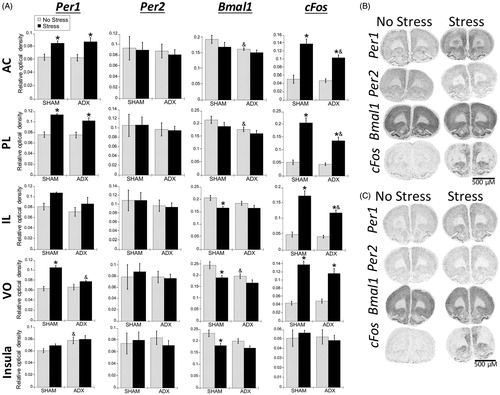
Rostral agranular insula
There was no effect of stress on Per1 or cFos mRNA. There was a significant main effect of adrenal status for Per1 mRNA in the RAI (; , insula). Post-hoc analysis showed that under no stress conditions, Per1 mRNA expression was greater in adrenalectomized rats compared to SHAM rats (FLSD, p < .05). Bmal1 mRNA had a significant main effect of stress, where similar to the IL and VO subregions of the PFC, stress decreased Bmal1 mRNA in SHAM rats.
Experiment 3: Test of acute stress on Per1 and cFos mRNA in the SCN and PVN of male and female rats at ZT16
We found in the first two experiments that stress increased cFos mRNA in the SCN of male rats. It has been suggested that non-photic stimuli (e.g. acute stress) can influence the SCN only during the animals’ inactive phase (Dibner et al., Citation2010). A previous study observed an increase in Fos protein immunoreactivity after acute stress in the SCN that was more pronounced when administered during the rat’s inactive phase (Edelstein & Amir, Citation1995). This experiment sought to determine if stress could induce cFos mRNA in the SCN of male and female rats during the rats’ active period (ZT16).
Corticosterone
There were significant main effects of stress and sex, and a significant stress × sex interaction on plasma corticosterone concentrations (; ). Stress significantly increased plasma corticosterone concentrations in both males and females, and females had greater plasma corticosterone concentrations than males under no stress conditions (FLSD, p < .05).
Figure 8. Experiment 3: Effect of stress at zeitgeber time (ZT)16 on plasma corticosterone concentrations in male and female rats. 30 min of acute restraint stress increased plasma corticosterone concentrations in both male and female rats. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress conditions; p < .05, FLSD, n = 6 rats per treatment group). See for statistical details.
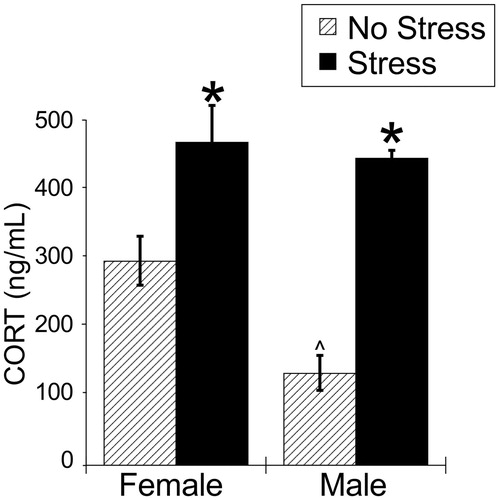
Table 3. Two-way ANOVA results for Experiment 3.
Suprachiasmatic nucleus
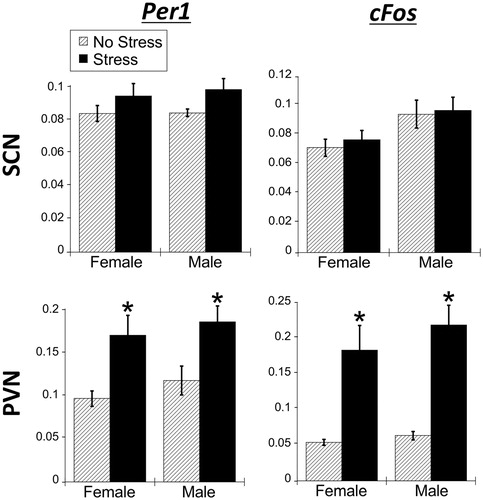
There was no overall main effect of stress on Per1 or cFos mRNA in the SCN of both male and female rats (; ). These results differ from the stress-induced cFos mRNA in the SCN seen in Experiments 1 and 2. Also similar to the first two experiments, stress had no effect on Per1 mRNA in male and female rat SCN. There was a significant main effect of sex on cFos mRNA, where males had greater cFos mRNA than females (). Interestingly, males had less cFos mRNA than females in Experiment 1, indicating that this sex difference may reflect an interaction with time of day.
Figure 9. Experiment 3: Effect of stress at ZT16 on SCN and PVN Per1 and cFos mRNA in male and female rats. When rats were exposed to 30 min of acute restraint stress at ZT16, there was no increase of Per1 and cFos mRNA in the suprachiasmatic nucleus (SCN) of both male and female rats. However, in the paraventricular nucleus of the hypothalamus (PVN), both males and females showed a significant increase in both Per1 and cFos mRNA. Data are presented as mean ± SEM (*stress effect within same sex conditions, p < .05, FLSD, n = 6 rats per treatment group). See for statistical details.
Paraventricular nucleus of the hypothalamus
As expected, there was a significant main effect of stress, where restraint stress significantly increased Per1 and cFos mRNA expression in the PVN for both males and females (; ; FLSD, p < .05), which is consistent with the results seen in Experiment 1 and 2. There was no main effect of sex.
Discussion
This study demonstrates that 30 min of acute restraint stress rapidly increases Per1 mRNA expression in male and female rat PFC and PVN, and the extent to which this effect is adrenal-dependent varies with brain region. These data extend the range of extra-SCN brain tissues (hippocampus, dorsal medial hypothalamus, and piriform cortex) (Al-Safadi et al., Citation2014; Bohacek et al., Citation2015; Mifsud & Reul, Citation2016), and also peripheral tissues (liver, heart, lung, and stomach) (Yamamoto et al., Citation2005) that exhibit stress-induced Per1 expression. As expected, the SCN lacked stress-induced Per1 mRNA, which corroborates evidence that SCN molecular clock function is largely resistant to non-photic cues (Al-Safadi et al., Citation2014; Takahashi et al., Citation2001). While there was no effect of stress on clock gene function in the SCN, stress did increase cFos mRNA, but only at ZT4. Stress also rapidly increased Per2 mRNA in female rat VO and PVN (Experiment 1), and decreased Bmal1 mRNA in the IL, VO, and RAI (Experiment 2). Stress-induced Per1 mRNA in the medial subregions of the PFC largely did not require the presence of endogenous corticosterone, indicating that this effect is mediated independently of corticosterone.
Rapid alteration of clock gene expression by acute stress
This study, and two other studies using male mice, found that acute stress reliably increases Per1 mRNA, but that acute stress has a more limited effect on other clock gene expression (Takahashi et al., Citation2001; Yamamoto et al., Citation2005). The direct modulation of Per1 expression may be a main mechanism by which stress can alter the molecular clock in extra-SCN tissue. This action may be related to the mechanism by which light entrains the phase of the molecular clock in the SCN. A light pulse during the dark phase rapidly induces SCN Per1 mRNA, resulting in a subsequent phase shift in SCN clock gene expression and the animal’s circadian rhythm (Oster et al., Citation2003; Shigeyoshi et al., Citation1997). Light also increases Per2 expression in the SCN, but the increase is delayed (∼180 min) compared to Per1 (Shearman, Zylka, Weaver, Kolakowski, & Reppert, Citation1997). This delay in light-induced Per2 expression in the SCN may extend to Per2 induction in extra-SCN tissue. Glucocorticoid treatment of cell lines produces a delayed increase in Per2 mRNA relative to Per1 mRNA (Cheon et al., Citation2013; So et al., Citation2009). Thus, our examination of gene expression 30 min after stressor onset may have been too short a period of time to see robust induction of clock genes other than Per1 that may require more than 30 min to reflect a significant increase in mRNA levels. Interestingly, the current experiments demonstrated that acute stress produced modest increases in Per2 mRNA in female VO and PVN, and decreases in Bmal1 mRNA in select PFC subregions and RAI. This rapid stress-induced decrease in Bmal1 mRNA in Experiment 2 may be due to direct actions on Bmal1 transcription, or actions via stress-induced alterations in Rev-erbα or Rorα expression, components of an accessory loop that regulates Bmal1 transcription (Hastings, O’Neill, & Maywood, Citation2007). The stress-induced increase in Per2 mRNA in the VO and PVN of female rats may be a reflection of the greater stress-induced corticosterone secretion in females. It is possible, though unexplored, that rapid Per2 gene induction may require a threshold level of corticosterone to be present.
There were small variations in stress-induced clock gene expression throughout the different PFC subregions. While Per1 mRNA was uniformly increased in each subregion by restraint stress for both males and females, Per2 mRNA was only increased in the VO of females and Bmal1 mRNA was decreased by stress only in the IL and VO (Experiment 2). Thus, the ventral subregions of the PFC, the IL and VO, exhibited some incidences of stress-induced changes in clock gene expression that extended beyond Per1. Within the PFC, there are dorsal (AC, PL) versus ventral (IL, VO) differences in both function and neural connectivity (Heidbreder & Groenewegen, Citation2003), which could contribute to the differences seen in stress-induced Per2 and Bmal1 mRNA expression.
Chronic or repeated stress alters the phase and amplitude of rhythmic Per1, Per2, and Bmal1 expression in brain and peripheral tissues (Logan et al., Citation2015; Razzoli, Karsten, Yoder, Bartolomucci, & Engeland, Citation2014; Tahara et al., Citation2015; Takahashi et al., Citation2013). The robust induction of Per1 mRNA by acute stress may mediate subsequent alterations of other components of the molecular clock under conditions of chronic stress. This ability of Per1 to act as a liaison between salient stimuli in the environment and extra-SCN molecular clocks indicates that it may also have non-circadian functions that assist an organism in adapting to its changing environment.
Intercellular signals mediating stress-induced Per1 expression
Stress-increased Per1 expression in peripheral and brain tissue was initially considered to be due primarily to an increase in corticosterone (Balsalobre et al., Citation2000; Takahashi et al., Citation2001; Yamamoto et al., Citation2005). There is a functional GRE within the promoter region of Per1, and glucocorticoids rapidly induce Per1 expression in some cell lines, liver and hippocampus (Balsalobre et al., Citation2000; Reddy, Gertz, Crawford, Garabedian, & Myers, Citation2012; Yamamoto et al., Citation2005). In addition, acute stress increases in Per1 expression in the hippocampus are accompanied by increased GR binding to a GRE associated with the Per1 gene (Mifsud & Reul, Citation2016).
Despite compelling evidence for a functional GRE associated with the mammalian Per1 gene, the second experiment showed that adrenalectomy did not attenuate Per1 mRNA in the medial subregions of the PFC. Recent studies reported that adrenalectomy or GR antagonist treatment failed to prevent acute stress-induced Per1 expression in other particular rodent brain regions (Al-Safadi et al., Citation2014; Bohacek et al., Citation2015), also indicating non-GR mechanisms involved in acute stress-induced Per1 expression. However, in the PVN and the VO, adrenalectomy significantly blunted stress-induced Per1 mRNA expression. Thus, stress-induced Per1 mRNA in the medial PFC, but not the PVN, or VO, appears to be largely independent of endogenous corticosterone. In addition to the GRE, the Per1 gene also contains a functional cAMP response element (CRE) within its promoter region. In the SCN, rapid light-induced Per1 expression during the subjective night depends on cAMP response element binding protein (CREB) activation and its binding to the Per1-associated CRE (Tischkau, Mitchell, Tyan, Buchanan, & Gillette, Citation2003). It is possible that this CRE-dependent process is responsible for stress-induced Per1 expression in extra-SCN tissue.
This CRE-dependent possibility is supported by the largely parallel induction of Per1 and cFos expression observed in this study. cFos is an IEG rapidly induced throughout most neuronal populations by neuronal excitation (Hughes & Dragunow, Citation1995; Kovács, Citation1998). Acute stress increased both Per1 and cFos mRNA throughout the PFC and the PVN, but to a much less extent in the RAI (small effect only in Experiment 1). Thus, the Per1 gene seems to operate much like an IEG. One dissociation that we observed between Per1 and cFos expression changes was the attenuation of stress-induced Per1 mRNA levels in the PVN of adrenalectomized rats at ZT4. The lack of a parallel effect of adrenalectomy on cFos mRNA is consistent with the finding that the cFos gene is not associated with a GRE and is not directly regulated by acute increases in corticosterone (Ginsberg, Campeau, Day, & Spencer, Citation2003). Nevertheless, in the PL and IL there was some attenuation of the stress-induced cFos mRNA by adrenalectomy. It should be noted that rats in Experiment 2 were adrenalectomized for 7 days, which can cause long-term changes in monoamine, neuropeptide, and corticotropin-releasing hormone signaling (Dunn, Citation1988; Moghaddam, Citation2002; Savontaus, Conwell, & Wardlaw, Citation2002). Consequently, the adrenal-dependent effects that we observed on stress-induced Per1 expression may reflect not only the absence of a surge of corticosterone during acute stress but also the absence of the tonic influence of corticosterone on various aspects of the neural stress response. The attenuated stress-induced cFos mRNA expression that we observed in some PFC subregions of adrenalectomized rats may be a reflection of that process.
Experiments 1 and 2, somewhat unexpectedly, demonstrated stress-induced cFos mRNA in the SCN at ZT4. There is evidence for non-photic cues to alter SCN clock phase, but primarily only when administered during the rats’ inactive phase (Dibner et al., Citation2010; Edelstein & Amir, Citation1995). Consistent with those observations, in Experiment 3, stress did not induce cFos mRNA in the SCN at ZT16. It should be noted that regardless of time of day, stress had no acute effect on clock gene expression in the SCN.
Sex comparison
While female rats had higher stress-induced plasma corticosterone concentrations than male rats, female rats did not have higher stress-induced Per1 mRNA expression compared to male rats. This result further indicates that stress-induced Per1 mRNA can be independent of acute elevations in corticosterone. Alternatively, Droste, de Groote, Lightman, Reul, and Linthorst (Citation2009) found that restraint stress increased free corticosterone within the hippocampus equally between male and female rats. Thus, it is possible that despite the finding that there was a sex difference in plasma corticosterone concentrations, there may not have been a sex difference in restraint-induced free corticosterone within the brain. Another study observed greater increases in hippocampal Per1 mRNA in female mice compared to male mice after swim stress, but in that study, neither sex showed a change in Per1 mRNA expression after restraint stress (Bohacek et al., Citation2015). That study also found that pretreatment with a GR antagonist did not block increases in Per1 mRNA after swim stress, again pointing to a corticosterone independent mechanism.
In the PL and SCN (ZT4 only), male rats had greater stress-induced cFos mRNA compared to females. Other studies have found greater stress-induced cFos expression in males, particularly in the PFC (CitationFigueiredo, Dolgas, & Herman, 2002). Another study also observed higher stress-induced cFos mRNA levels in the PL of male rats compared to female rats, and that sex difference was most pronounced when female rats were stressed during proestrus (Bland et al., Citation2005). The sex difference in stress-induced cFos mRNA may be related to gonadal hormones. There was also a sex-specific effect of stress on clock gene expression. Stress increased Per2 mRNA in the PFC and PVN only in female rats, indicating a role of gonadal hormones in stress-induced Per2 mRNA expression in extra-SCN brain regions. The sex-specific Per2 mRNA induction may be corticosterone-mediated (unlike Per1), as females had greater stress-induced plasma corticosterone. Further studies examining sex differences in how molecular clock rhythms respond to acute and chronic stress are necessary.
Concluding comments
Acute restraint stress rapidly (within 30 min) increased Per1 mRNA in the PVN and PFC of male and female rats. Thus, altered Per1 expression may be the manner by which stress can subsequently alter the phase and amplitude of extra-SCN molecular clocks. However, further studies examining an extended time-course of the effect of acute stress on subsequent clock gene expression are necessary. Stress-induced Per1 mRNA in the medial PFC was largely corticosterone-independent and may be CRE-mediated. Daily acute induction of Per1 expression and subsequent alterations to the molecular clock in extra-SCN tissue may be important for phase adjustment of extra-SCN molecular clocks to non-photic environmental changes, as further evidenced by changes in the phase of extra-SCN clock gene expression by temporal shifts in daily food access or repeated stress (Tahara et al., Citation2015). Temporally unpredictable recurring alterations of Per1 expression by environmental factors, such as chronic unpredictable stress (Logan et al., Citation2015), may result in disrupted profiles of extra-SCN molecular clocks, contributing to some of the adverse neurobiological effects of chronic stress.
Disclosure statement
The authors have no conflicts of interest to disclose.
Additional information
Funding
References
- Al-Safadi, S., Al-Safadi, A., Branchaud, M., Rutherford, S., Dayanandan, A., Robinson, B., … Yamazaki, S. (2014). Stress-induced changes in the expression of the clock protein PERIOD1 in the rat limbic forebrain and hypothalamus: Role of stress type, time of day, and predictability. PLoS One, 9, e111166. doi: 10.1371/journal.pone.0111166
- Arnsten, A.F.T., & Rubia, K. (2012). Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 356–367. doi: 10.1016/j.jaac.2012.01.008
- Babb, J.A., Masini, C.V., Day, H.E.W., & Campeau, S. (2014). Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress, 17, 224–234. doi: 10.3109/10253890.2014.905534
- Bae, K., Jin, X., Maywood, E.S., Hastings, M.H., Reppert, S.M., & Weaver, D.R. (2001). Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron, 30, 525–536. doi: 10.1016/S0896-6273(01)00302-6
- Balsalobre, A., Brown, S.A., Marcacci, L., Tronche, F., Kellendonk, C., Reichardt, H.M., … Schibler, U. (2000). Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science (New York, N.Y.), 289, 2344–2347. doi: 10.1126/science.289.5488.2344
- Bland, S.T., Schmid, M.J., Der-Avakian, A., Watkins, L.R., Spencer, R.L., & Maier, S.F. (2005). Expression of c-Fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Research, 1051, 90–99. doi: 10.1016/j.brainres.2005.05.065
- Bohacek, J., Manuella, F., Roszkowski, M., & Mansuy, I.M. (2015). Hippocampal gene expression induced by cold swim stress depends on sex and handling. Psychoneuroendocrinology, 52, 1–12. doi: 10.1016/j.psyneuen.2014.10.026
- Buijs, R.M., Markman, M., Nunes-Cardoso, B., Hou, Y.X., & Shinn, S. (1993). Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: A light and electron microscopic study. The Journal of Comparative Neurology, 335, 42–54. doi: 10.1002/cne.903350104
- Buijs, R.M., La Fleur, S.E., Wortel, J., Van Heyningen, C., Zuiddam, L., Mettenleiter, T.C., … Niijima, A. (2003). The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. The Journal of Comparative Neurology, 464, 36–48. doi: 10.1002/cne.10765
- Bunger, M.K., Wilsbacher, L.D., Moran, S.M., Clendenin, C., Radcliffe, L.A., Hogenesch, J.B., … Bradfield, C.A. (2000). Mop3 is an essential component of the master circadian pacemaker in mammals. Cell, 103, 1009–1017. doi: 10.1016/S0092-8674(00)00205-1
- Bunney, B.G., Li, J.Z., Walsh, D.M., Stein, R., Vawter, M.P., Cartagena, P., … Bunney, W.E. (2015). Circadian dysregulation of clock genes: Clues to rapid treatments in major depressive disorder. Molecular Psychiatry, 20, 48–55. doi: 10.1038/mp.2014.138
- Burioka, N., Takata, M., Okano, Y., Ohdo, S., Fukuoka, Y., Miyata, M., … Shimizu, E. (2005). Dexamethasone influences human clock gene expression in bronchial epithelium and peripheral blood mononuclear cells in vitro. Chronobiology International, 22, 585–590. doi: 10.1081/CBI-200062416
- Cheon, S., Park, N., Cho, S., & Kim, K. (2013). Glucocorticoid-mediated period 2 induction delays the phase of circadian rhythm. Nucleic Acids Research, 41, 6161–6174. doi: 10.1093/nar/gkt307
- Chun, L.E., Woodruff, E.R., Morton, S., Hinds, L.R., & Spencer, R.L. (2015). Variations in phase and amplitude of rhythmic clock gene expression across prefrontal cortex, hippocampus, amygdala, and hypothalamic paraventricular and suprachiasmatic nuclei of male and female rats. Journal of Biological Rhythms, 30, 417–436. doi: 10.1177/0748730415598608
- Conway-Campbell, B.L., Sarabdjitsingh, R.A., McKenna, M.A., Pooley, J.R., Kershaw, Y.M., Meijer, O.C., … Lightman, S.L. (2010). Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. Journal of Neuroendocrinology, 22, 1093–1100. doi: 10.1111/j.1365-2826.2010.02051.x
- Dibner, C., Schibler, U., & Albrecht, U. (2010). The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annual Review of Physiology, 72, 517–549. doi: 10.1146/annurev-physiol-021909-135821
- Dickmeis, T. (2009). Glucocorticoids and the circadian clock. The Journal of Endocrinology, 200, 3–22. doi: 10.1677/JOE-08-0415
- Droste, S.K., de Groote, L., Lightman, S.L., Reul, J.M.H.M., & Linthorst, A.C.E. (2009). The ultradian and circadian rhythms of free corticosterone in the brain are not affected by gender: An in vivo microdialysis study in Wistar rats. Journal of Neuroendocrinology, 21, 132–140. doi: 10.1111/j.1365-2826.2008.01811.x
- Dunn, A.J. (1988). Stress-related changes in cerebral catecholamine and indoleamine metabolism: Lack of effect of adrenalectomy and corticosterone. Journal of Neurochemistry, 51, 406–412. doi: 10.1111/j.1471-4159.1988.tb01053.x
- Edelstein, K., & Amir, S. (1995). Non-photic manipulations induce expression of Fos protein in the suprachiasmatic nucleus and intergeniculate leaflet in the rat. Brain Research, 690, 254–258. doi: 10.1016/0006-8993(95)00736-A
- Figueiredo, H.F., Dolgas, C.M., & Herman, J.P. (2002). Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology, 143, 2534–2540. doi: 10.1210/endo.143.7.8888
- Ginsberg, A.B., Campeau, S., Day, H.E., & Spencer, R.L. (2003). Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-Fos mRNA or Fos protein expression in the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology, 15, 1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x
- Harbour, V.L., Weigl, Y., Robinson, B., Amir, S., & Bartell, P.A. (2014). Phase differences in expression of circadian clock genes in the central nucleus of the amygdala, dentate gyrus, and suprachiasmatic nucleus in the rat. PLoS One, 9, e103309. doi: 10.1371/journal.pone.0103309
- Hastings, M., O’Neill, J.S., & Maywood, E.S. (2007). Circadian clocks: Regulators of endocrine and metabolic rhythms. The Journal of Endocrinology, 195, 187–198. doi: 10.1677/JOE-07-0378
- Heidbreder, C.A., & Groenewegen, H.J. (2003). The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and Biobehavioral Reviews, 27, 555–579. doi: 10.1016/j.neubiorev.2003.09.003
- Hughes, P., & Dragunow, M. (1995). Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacological Reviews, 47, 133–178.
- Kalsbeek, A., Liu, J., Lei, J., Timmermans, L., Foppen, E., Cailotto, C., & Fliers, E. (2012a). Differential involvement of the suprachiasmatic nucleus in lipopolysaccharide-induced plasma glucose and corticosterone responses. Chronobiology International, 29, 835–849. doi: 10.3109/07420528.2012.699123
- Kalsbeek, A., van der Spek, R., Lei, J., Endert, E., Buijs, R.M., & Fliers, E. (2012b). Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Molecular and Cellular Endocrinology, 349, 20–29. doi: 10.1016/j.mce.2011.06.042
- Kessler, R. (2003). Epidemiology of women and depression. Journal of Affective Disorders, 74, 5–13. https://doi.org/10.1016/S0165-0327(02)00426-3
- Kovács, K.J. (1998). Invited review c-Fos as a transcription factor: A stressful (re)view from a functional map. Neurochemistry International, 33, 287–297. https://doi.org/10.1016/S0197-0186(98)00023-0
- Li, J.Z., Bunney, B.G., Meng, F., Hagenauer, M.H., Walsh, D.M., Vawter, M.P., … Bunney, W.E. (2013). Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America, 110, 9950–9955. doi: 10.1073/pnas.1305814110
- Logan, R.W., Edgar, N., Gillman, A.G., Hoffman, D., Zhu, X., & McClung, C.A. (2015). Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biological Psychiatry, 78, 249–258. doi: 10.1016/j.biopsych.2015.01.011
- Mayberg, H.S., Liotti, M., Brannan, S.K., McGinnis, S., Mahurin, R.K., Jerabek, P.A., … Fox, P.T. (1999). Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. The American Journal of Psychiatry, 156, 675–682.
- McCarthy, M.J., & Welsh, D.K. (2012). Cellular circadian clocks in mood disorders. Journal of Biological Rhythms, 27, 339–352. doi: 10.1177/0748730412456367
- Mifsud, K.R., & Reul, J.M.H.M. (2016). Acute stress enhances heterodimerization and binding of corticosteroid receptors at glucocorticoid target genes in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 113, 11336–11341. doi: 10.1073/pnas.1605246113
- Moghaddam, B. (2002). Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biological Psychiatry, 51, 775–787. doi: 10.1016/S0006-3223(01)01362-2
- Mukherjee, S., Coque, L., Cao, J.L., Kumar, J., Chakravarty, S., Asaithamby, A., … McClung, C.A. (2010). Knockdown of clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biological Psychiatry, 68, 503–511. doi: 10.1016/j.biopsych.2010.04.031
- Oishi, K., Sakamoto, K., Okada, T., Nagase, T., & Ishida, N. (1998). Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochemical and Biophysical Research Communications, 253, 199–203.
- Oster, H., Werner, C., Magnone, M.C., Mayser, H., Feil, R., Seeliger, M.W., … Albrecht, U. (2003). cGMP-dependent protein kinase II modulates mPer1 and mPer2 gene induction and influences phase shifts of the circadian clock. Current Biology, 13, 725–733. doi: 10.1016/S0960-9822(03)00252-5
- Paxinos, G., & Watson, C. (1998). The rat brain in stereotaxic coordinates (4th ed.). San Diego (CA): Academic Press.
- Rath, M.F., Rohde, K., Fahrenkrug, J., & Møller, M. (2013). Circadian clock components in the rat neocortex: Daily dynamics, localization and regulation. Brain Structure and Function, 218, 551–562. doi: 10.1007/s00429-012-0415-4
- Razzoli, M., Karsten, C., Yoder, J.M., Bartolomucci, A., & Engeland, W.C. (2014). Chronic subordination stress phase advances adrenal and anterior pituitary clock gene rhythms. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 307, R198–R205. doi: 10.1152/ajpregu.00101.2014
- Reddy, A.B., Maywood, E.S., Karp, N.A., King, V.M., Inoue, Y., Gonzalez, F.J., … Hastings, M.H. (2007). Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology (Baltimore, Md.), 45, 1478–1488. doi: 10.1002/hep.21571
- Reddy, T.E., Gertz, J., Crawford, G.E., Garabedian, M.J., & Myers, R.M. (2012). The hypersensitive glucocorticoid response specifically regulates period 1 and expression of circadian genes. Molecular and Cellular Biology, 32, 3756–3767. doi: 10.1128/MCB.00062-12
- Reppert, S.M., & Weaver, D.R. (2002). Coordination of circadian timing in mammals. Nature, 418, 935–941. doi: 10.1038/nature00965
- Savontaus, E., Conwell, I.M., & Wardlaw, S.L. (2002). Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Research, 958, 130–138. https://doi.org/10.1016/S0006-8993(02)03674-0
- Shearman, L.P., Zylka, M.J., Weaver, D.R., Kolakowski, L.F., & Reppert, S.M. (1997). Two period homologs: Circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron, 19, 1261–1269. doi: 10.1016/S0896-6273(00)80417-1
- Shigeyoshi, Y., Taguchi, K., Yamamoto, S., Takekida, S., Yan, L., Tei, H., … Okamura, H. (1997). Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell, 91, 1043–1053. doi: 10.1016/S0092-8674(00)80494-8
- So, A.Y.L., Bernal, T.U., Pillsbury, M.L., Yamamoto, K.R., & Feldman, B.J. (2009). Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America, 106, 17582–17587. doi: 10.1073/pnas.0909733106
- Spencer, R.L., & Deak, T. (2017). A users guide to HPA axis research. Physiology & Behavior, 178, 43–65. doi: 10.1016/j.physbeh.2016.11.014
- Spencer, S., Falcon, E., Kumar, J., Krishnan, V., Mukherjee, S., Birnbaum, S.G., & McClung, C.A. (2013). Circadian genes period 1 and period 2 in the nucleus accumbens regulate anxiety-related behavior. European Journal of Neuroscience, 37, 242–250. doi: 10.1111/ejn.12010
- Spiga, F., Walker, J.J., Terry, J.R., & Lightman, S.L. (2014). HPA axis – rhythms (Vol. 4, pp. 1273–1298). Hoboken (NJ): John Wiley & Sons, Inc.
- Tahara, Y., Shiraishi, T., Kikuchi, Y., Haraguchi, A., Kuriki, D., Sasaki, H., … Shibata, S. (2015). Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Scientific Reports, 5, 11417. doi: 10.1038/srep11417
- Takahashi, K., Yamada, T., Tsukita, S., Kaneko, K., Shirai, Y., Munakata, Y., … Katagiri, H. (2013). Chronic mild stress alters circadian expressions of molecular clock genes in the liver. American Journal of Physiology. Endocrinology and Metabolism, 304, E301–E309. doi: 10.1152/ajpendo.00388.2012
- Takahashi, S., Yokota, S.-I., Hara, R., Kobayashi, T., Akiyama, M., Moriya, T., & Shibata, S. (2001). Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology, 142, 4910–4917. doi: 10.1210/endo.142.11.8487
- Tischkau, S.A., Mitchell, J.W., Tyan, S.-H., Buchanan, G.F., & Gillette, M.U. (2003). Ca2+/cAMP response element-binding Protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. Journal of Biological Chemistry, 278, 718–723. doi: 10.1074/jbc.M209241200
- Ulrich-Lai, Y.M., Arnhold, M.M., & Engeland, W.C. (2006). Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 290, R1128–R1135. doi: 10.1152/ajpregu.00042.2003
- Ulrich-Lai, Y.M., & Herman, J.P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews. Neuroscience, 10, 397–409. doi: 10.1038/nrn2647
- Viau, V., & Meaney, M.J. (1991). Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology, 129, 2503–2511. doi: 10.1210/endo-129-5-2503
- Watts, A.G., Swanson, L.W., & Sanchez-Watts, G. (1987). Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. The Journal of Comparative Neurology, 258, 204–229. doi: 10.1002/cne.902580204
- Welsh, D.K., Takahashi, J.S., & Kay, S.A. (2010). Suprachiasmatic nucleus: Cell autonomy and network properties. Annual Review of Physiology, 72, 551–577. doi: 10.1146/annurev-physiol-021909-135919
- Woodruff, E.R., Chun, L.E., Hinds, L.R., & Spencer, R.L. (2016). Diurnal corticosterone presence and phase modulate clock gene expression in the male rat prefrontal cortex. Endocrinology, 157, 1522–1534. doi: 10.1210/en.2015-1884
- Yamamoto, T., Nakahata, Y., Soma, H., Akashi, M., Mamine, T., & Takumi, T. (2004). Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Molecular Biology, 5, 18. doi: 10.1186/1471-2199-5-18
- Yamamoto, T., Nakahata, Y., Tanaka, M., Yoshida, M., Soma, H., Shinohara, K., … Takumi, T. (2005). Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. Journal of Biological Chemistry, 280, 42036–42043. doi: 10.1074/jbc.M509600200
