Abstract
In early lactation (EL), stressor salience modulates neuroendocrine stress responses, but it is unclear whether this persists throughout lactation and which neural structures are implicated. We hypothesized that this process is specific to EL and that the infralimbic (IL) medial prefrontal cortex (mPFC) might provide a critical link between assessment of threat and activation of the hypothalamo-pituitary-adrenal (HPA) axis in EL. We measured neuroendocrine responses and neuronal Fos induction to a salient (predator odor) or non-salient (tail pinch) psychogenic stressor in EL and late lactation (LL) females. We found that EL females exhibited a large response to predator stress only in the presence of pups, while responses to tail pinch were reduced independently of pup presence. In LL, HPA axis responses were independent of pup presence for both stressors and only responses to tail pinch were modestly reduced compared to virgins. Intracerebral injection of the local anesthetic bupivacaine (BUP) (0.75%; 0.5 µl/side) in the IL mPFC did not differentially affect neuroendocrine responses to predator odor in virgin and EL females, suggesting that lactation-induced changes in this structure might not regulate stressor salience for the HPA axis. However, the IL mPFC displayed morphological changes in lactation, with significant increases in dendritic spine numbers and density in EL compared to LL and virgin females. EL females also showed improved performance in the attention set-shifting task (AST), which could reflect early plasticity in the IL mPFC at a time when rapid adaptation of the maternal brain is necessary for pup survival.
Introduction
Many of the manipulations designed to produce stress in the fetus and/or neonate rely on maternal stress exposure during gestation and/or lactation. This approach assumes that the mother is able to mount significant behavioral and neuroendocrine responses to stress throughout these periods, and that these result in changes in the environment of the offspring. However, this assumption is only partially correct because late pregnant and lactating females have been shown to display blunted neuroendocrine responses to a variety of stressors (Woodside, Citation2016). In spite of their reduced neuroendocrine responsiveness, however, lactating females do respond behaviorally to stressors (Windle, Wood, Kershaw, Lightman, & Ingram, Citation2013). Blunted neuroendocrine responses have also been documented in nursing women (Meinlschmidt, Martin, Neumann, & Heinrichs, Citation2010; Tu, Lupien, & Walker, Citation2005) although these reduced hormonal responses appear to be stressor dependent (Altemus et al., Citation2001) and sometimes more prominent in multiparous than primiparous mothers (Tu, Lupien, & Walker, Citation2006).
The mechanisms underlying stress hyporesponsivity also vary as a function of the stage of lactation because dams during early lactation (EL, 3–5 days postpartum) mount a stress response comparable to that of virgin females when the stressor applied threatens their pups (Deschamps, Woodside, & Walker, Citation2003) whereas late lactating (LL) females fail to show this response. These results suggest that in EL the capacity of the hypothalamo-pituitary-adrenal (HPA) axis to adequately respond to a stressor remains, but that assessment of stressor salience modulates the activity of inputs to the hypothalamic corticotropin releasing hormone (CRH)-containing parvocellular paraventricular nucleus (pPVN) neurons. Evidence that presence of pups in situations of threat does indeed change maternal brain activity comes from a previous fMRI study showing that pup presence modifies the activation of the temporal cortex, nucleus accumbens (NAcc), basolateral amygdala and other regions in response to a male intruder (Nephew, Caffrey, Felix‐Ortiz, Ferris, & Febo, Citation2009). We consider that such changes in brain activity in the presence of pups are responsible for the rapid reversal of stress hyporesponsiveness that we have observed in early lactation. In LL females, the greater maturity of the young might influence maternal assessment of threat from external stressors and it is, therefore, possible that pup presence is less relevant at this stage.
The medial prefrontal cortex (mPFC) is critical for evaluation of stressor salience (Herman et al., Citation2016) and in particular its ventral parts, the prelimbic (PL) and infralimbic (IL) portions, which display opposing roles in the regulation of the stress axis (Choi et al., Citation2008; Ulrich-Lai & Herman, Citation2009). The PL region is inhibitory to pPVN neurons via projections to the peri-PVN and posterior bed nucleus of the stria terminalis (BNST) (Herman, Citation2012; Radley, Gosselink, & Sawchenko, Citation2009) while the IL is excitatory to pPVN neurons via indirect projections to the nucleus of the tractus solitaries (NTS) and anterior BNST (Herman, Citation2012; Vertes, Citation2004). Significant activity of the PL mPFC area has been observed during electroencephalographic (EEG) recordings in lactating rats after presentation of pup-associated odors in early to middle lactation (Hernandez-Gonzalez, Navarro-Meza, Prieto-Beracoechea, & Guevara, Citation2005; Hernandez-Gonzalez, Prieto-Beracoechea, et al. Citation2005), or in response to a variety of visual and audio infant stimuli in fMRI studies in humans (Kim et al., Citation2011; Ranote et al., Citation2004), suggesting that pup associated cues could significantly modify mPFC activity. Whether this persists throughout lactation is unknown. The mPFC is also part of the maternal circuitry (Afonso, Sison, Lovic, & Fleming, Citation2007) and there is evidence that the IL contributes to maternal responsiveness during the early stages of lactation, but that its role wanes later in lactation when the influence of the PL becomes predominant (Pereira & Morrell, Citation2011). It is therefore possible that there is a similar pattern of influence of the IL and PL on the stress response across lactation.
In addition to functional changes, morphological increases in the number of dendritic spines, dendritic length and branching, have been observed in mPFC neurons of females that have been weaned from their pups on postpartum day (PPD)24 (Leuner & Gould, Citation2010). It has been suggested that such changes as well as those observed in other brain areas support the enhanced demands on attention and cognitive flexibility in lactation (Slattery & Hillerer, Citation2016). However, it is currently unknown whether these morphological changes are also observed in early lactation and whether they would contribute to changes in neuroendocrine regulation and/or the ability to shift attention from one modality to another that might be essential in evaluating stressor salience. The attention set shifting task (AST) can be used to assess whether the formation, maintenance and shifting of attention, that is critical for maternal care is modified as a function of stage of lactation.
In these studies, we tested the hypotheses that (1) the stage of lactation and the presence of the pups at the time of stress exposure would influence the neuroendocrine response to either a salient (exposure to predator odor) or non-salient (tail pinch) psychogenic stressor, (2) the IL mPFC would be implicated, at least in part, in mediating stressor salience on stress responses in EL, and (3) lactation-induced morphological changes in the mPFC would allow for enhanced maternal attentional flexibility necessary for caring of the young.
Methods
Animals
Male and female Sprague-Dawley rats (Charles River, Quebec, Canada; 250–280 g) were housed in groups of three in standard polycarbonate rat cages and allowed to habituate to the animal facility conditions for at least seven days before mating. Females were mated (three females/male); and pregnancy was verified by the presence of sperm in the vaginal smears, which was designated as gestation day (GD) 0). All rats were returned to group cages (cage size 55 × 35 × 20 cm) until GD15 when females were singly housed with environmental enrichment within the cage prior to delivery of their pups. The day of birth was considered to be PPD 0. On PPD1, the number of pups in the litter and the average birth weight were determined, and then all litters were culled to 10 pups to ensure comparable conditions across all dams. All rats were kept under standard laboratory conditions of light (12-h light/dark cycle, lights on at 08:00 h, off at 20:00 h), temperature (22 °C ± 1 °C) and humidity (60 ± 5% humidity) and had free access to rat chow and water. All experimental procedures were performed during the light phase of the light–dark cycle, between 09:00 h and 12:00 h. A total of 152 females and, for mating, 20 males were used for these experiments. All animal procedures were reviewed and approved by the Animal Care Committee at McGill University, following guidelines from the Canadian Council on Animal Care (CCAC).
Surgical procedures
Intravenous cannula implantation
In order to determine basal and stress-induced plasma ACTH and corticosterone concentrations, a jugular-vein catheter was implanted under isoflurane anesthesia in early lactating (EL) females on PPD2 (n = 25) and virgin females (n = 20). Briefly, the jugular vein was exposed by a skin incision and blunt dissection and a silicone catheter (ID:0.64 mm, OD: 1.19 mm) was inserted approximately 3 cm into the jugular vein towards the right atrium, secured to the vein by silk thread and exteriorized between the scapulae. Each catheter was filled with heparinized (Hep) 0.9% saline (50 U/ml) and flushed the day after surgery with Hep-saline. Females were left undisturbed for 48 h prior to the stress experiment.
Intracerebral cannula implantation
For intra-mPFC IL infusions in EL and virgin (V) females, a 26-G bilateral guide cannula (7.00 mm below pedestal) was stereotaxically implanted 0.5 mm above both the left and right IL cortex (+3.2 mm bregma, −0.75 or +0.75 mm lateral, 4.00 below surface of the skull) (Paxinos & Watson, Citation1998) under isoflurane anesthesia in pregnant females on GD15 and virgin females. The guide cannula was closed using a dummy cannula approximately 1 mm longer than the guide cannula. All rats were handled daily (stroking, holding, cleaning of the cannula dummy) for at least one week following surgery to minimize nonspecific stress-induced activation of the mPFC on the day of the experiment.
Experimental procedures
All general experimental procedures and cohorts are depicted in Supplementary Figure 1.
Stress testing
All stress tests were performed in the morning within 2–3 hours after lights on and all females were habituated to handling at least two days prior to the stress test to avoid nonspecific stress due to manipulation (either cannula connection or tail vein sampling). Virgin, early lactation (PPD4) and late lactation (PPD15–16, pups normally weaned on PPD21) females were transferred to the experimental room two hours before stress onset and placed in black experimental enclosures with an open lid (71 cm × 66 cm × 41 cm) (2 rat cages/box; random assignment of status and treatment). Pups were either removed from the dam two hours prior to stress onset and transferred to another room, or stayed with the dam during the testing procedure. Pups remaining with the mother were observed to be away from the mother or to suckle intermittently during stress exposure. Females were exposed to 30 min of predator odor or tail pinch stress in separate experimental batches. Predator odor stress consisted of attaching two, 5 cm × 5 cm. squares of cotton towel that had been exposed to ferret urine, hair and skin under the lid of the cage for 30 min according to the method of Campeau, Nyhuis, Sasse, Day, and Masini (Citation2008). Towels were obtained from a separate facility and had been kept in the cage of male ferrets for one month and, once collected, were cut into small square pieces and kept in a freezer prior to the experiment. On the test day, towel pieces were thawed and dampened with water just prior to being placed in the rat cages. Once the towels were placed in the cage, dim lights were turned on in the enclosure box and the lid was rapidly closed in order to limit spread of the odor to adjacent cages. After 30 min, towels were quickly removed from the cage and from the experimental room, enclosure box lights turned off and cage lids re-opened. Tail pinch stress consisted of attaching a wooden or plastic cloth pin (or clothes peg) to the base of the tail for 30 min. Blood samples (0.2 ml) were collected before stress onset (0 min), and at 30 and 60 min after the onset of stress. When venous blood samples were collected, each sample was immediately replaced with the same amount of sterile 0.9% saline. All blood samples were kept on ice prior to plasma separation and freezing.
Stress testing in EL females
For rats tested on PPD4 (EL), the venous catheter was attached to a PE50 polypropylene extension tubing connected to a 1-ml plastic syringe filled with sterilized 0.9% saline–heparin (50 IU/ml) immediately on placing the rat in the experimental chamber (2 h prior to baseline collection). At the same time, the home cage lids were replaced by experimental lids, to allow flexible moving of the catheter system and attaching predator towels for subsequent stress-exposure. Rats were left undisturbed for 2 h before withdrawal of a basal blood sample of 0.2 ml and stress onset.
Stress testing in LL females
Due to the presence of older pups with LL females, we could not use blood collection via jugular catheters and used tail blood collection instead. Basal blood samples (0.2 ml) were collected after cutting <0.3 cm of the tail tip with a sharp blade and gently massaging the tail for less than 1 min to allow the blood to drip into the collection tube. Additional tail samples were collected 30 and 60 min after stress onset. Blood was collected in tubes containing EDTA (60 mg/ml) and kept on ice until centrifuged for plasma collection. Plasma was stored at −20 °C prior to assay.
Reversible inhibition of IL mPFC during stress in EL females
A separate cohort of virgin and EL rats was used for these experiments and procedures were similar to those described for EL females above, except that only predator odor stress was used and all EL females were tested in the presence of their pups. After collection of a basal blood sample, the intracerebral dummy cannula was removed and a bilateral injector was inserted to deliver either a single infusion of bupivacaine (BUP 0.5 µl/side; Marcaine 0.75%, Hospira Healthcare Cooperation, Saint-Laurent, Canada) or vehicle (0.5 µl/side; dextrose (82.5 mg/ml) in isotonic saline) directly to the left and right mPFC IL over 2 min. Bupivacaine is used to induce reversible inactivation and is preferred over lidocaine or muscimol treatment as it blocks action potentials regardless of neuronal phenotype and has a longer duration of action (30–50 min) than lidocaine (Fozzard, Lee, & Lipkind, Citation2005; Hsu & Packard, Citation2008). After the infusion, the cannula was kept in place for 2 min to allow for complete diffusion of the drug, while the test animal was allowed to move freely. Ten minutes after the end of the infusion, rats were exposed to the stress of predator odor as described above and blood samples were collected 15, 30, and 60 min after stress onset. Ninety min after stress onset, rats were deeply anesthetized with i.p. injection of a mixture (100 μl/100 g BW) of ketamine (50 mg/ml)/xylazine (5 mg/ml)/acepromazine (1 mg/ml) diluted in 0.9% NaCl and perfused intra-cardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4. Immediately after perfusion, brains were post-fixed in 4% PFA at 4 °C overnight, before transfer to 30% sucrose in 0.1 M sodium phosphate solution (pH 7.4) for at least one week. Brains were then frozen on dry ice and kept at −80 °C prior to slicing for cannula placement. To verify cannula placement in the mPFC IL, 25 µm coronal sections were cut, mounted directly onto slides and stained with Cresyl violet. Images were obtained at 10 × magnification and correct cannula placement was checked by visual inspection of the cannula tracks. Only rats with correct cannula placement in the mPFC IL were included in the data analysis (see ).
Neuronal Fos activation after predator odor stress
Separate naïve cohorts of V, EL, and LL females were exposed to predator odor as described above, but were neither surgically prepared nor sampled before and after stress exposure. EL and LL females were tested either with or without their pups. Ninety min after stress onset, rats were deeply anesthetized and perfused with saline, followed by 4% PFA as described above. Brains were collected and processed for determination of Fos immunoreactivity.
Immunohistochemistry for c-Fos
Coronal brain sections were prepared using a cryostat. To analyze c-Fos activity in the PVN, the BNST, and the mPFC (IL and PL regions), respective brain regions were cut in 40 µm coronal sections and subsequently stored at 4 °C in a cryoprotection solution (glycerol, ethylene glycol, and 0.1 M phosphate buffer, pH 7.4, at a ratio of 1:1:2 by volume). Immunostaining for c-Fos was performed on free-floating sections using the diaminobenzidine (DAB) peroxidase method as previously described (McLaughlin, Verlezza, Gray, Hill, & Walker, Citation2016). Briefly, brain sections were treated with 0.6% H2O2 in Tris-buffered saline (TBS: 0.15 M NaCl, 0.1 M Tris–HCl, pH 7.5) for 30 min and rinsed for 3 × 5 min in PBS. Thereafter, sections were incubated in blocking solution (0.04% Triton x-100 in 1× PBS; 1% BSA) for 1 h followed by incubation with the primary rabbit c-Fos antibody (1:3000, #PC38, Calbiochem, San Diego, CA) in blocking solution overnight at 4 °C. The next day, the sections were incubated with biotinylated secondary goat anti-rabbit antibody (1:500, Vectastain elite ABC kit; Vector Laboratories, Burlingame, CA), followed by the avidin–biotin-peroxidase complex reaction (1 h; Vectastain elite ABC kit; Vector Laboratories, Burlingame, CA). Immunostaining was revealed using DAB (20 mg/ml in PBS with 0.01% H2O2, 0.04% NiCl2) staining. Stained sections were mounted on microscope slides and cover-slipped with PerMount (ThermoFisher, Montreal, QC). To determine the number of c-Fos-positive cells, every fourth section (160 µm interval) throughout the PVN, the BNST and mPFC was examined for c-Fos-positive cells. A 5X objective was used to trace the respective brain region and images for further analysis were taken at 10X magnification. Using the polygon selection measurement in ImageJ, a defined area was determined in which c-Fos-positive cells were counted regardless of shape or size. The defined area was used to calculate the number of c-Fos-positive cells per µm2 of the respective brain region. A minimum of 4–14 sections for each rat (n = 3/group) and brain region were counted and averaged.
Golgi staining of IL mPFC neurons
Separate cohorts of virgins (V, n = 6), EL (PPD4, n = 4) and LL (PPD15-16, n = 5) females were used for the morphological analysis of IL mPFC neurons. Rats were anesthetized with a cocktail of ketamine/xylazine/acepromazine as described above and transcardially perfused with ice cold 0.9% saline for 5 min. Brains were collected and placed in Golgi Cox solution (35 mM K2Cr2O7; 38 mM HgCl2, and 43 mM K2CrO4 in dH2O) in opaque vials to prevent exposure to light, and left at room temperature. The Golgi Cox solution in the vials was replaced after six hours, and again after another 12 hours. After 14 days, Golgi Cox solution was replaced with 30% sucrose for another four or five days at 4 °C. Brains were sliced at 200 µm using a Leica VT1200 vibrating-blade microtome at 26 mm/s. Slices were collected onto gelatin-coated slides, and kept in a damp and dark environment for four to five days at 4 °C. Slides were developed in the dark in ammonium hydroxide for 40 minutes, followed by incubation in Kodak film fixing solution for 40 minutes, and then in a dehydrating series of ethanol and xylene washes. Slides were coverslipped with Permount, and left to dry for two weeks before tracing. Neuronal tracing was performed using the Neurolucida software program (MBF Inc, Burlington, VT). Pyramidal neurons were selected from layers II and III of the right IL mPFC based on size, clarity of the stain, and isolation from other dendritic processes. A total of four to seven neurons per animal were traced and the total number of spines and total dendritic length were averaged for each animal.
Attention set shifting task
Virgin (n = 20, AST completed: n = 9), EL (PPD 3–5, AST completed n = 10), and LL (PPD 14–16, n = 12, AST completed: n = 9) female rats were kept in a reversed 12 h light/dark cycle (lights off at 08:00 h) and food restricted 7–10 days prior to the onset of training (virgin = 7 g food daily, EL = 15 g, LL = 27 g) to reach 80–90% of their original body weight. Training and testing was conducted during the early portion of the dark phase in a dimly lit room as described previously (Afonso et al., Citation2007). Two rats from different experimental groups were tested simultaneously. The testing apparatus consisted of a large box (width 40 cm × length 60 cm × height 30 cm) divided into three chambers; a starting chamber into which the animal was introduced, and two identical feeding chambers, each containing a digging bowl (7 cm, depth 4 cm). A removable panel separated the starting chamber from the feeding chambers, which were themselves divided by a stationary central panel. This ensured that the rat could access the bowls only at the experimenters’ discretion. The digging bowls in the digging chambers differed in one or two dimensions; odor and/or texture, depending on the stage of testing. For each stage of the test, the surface of either bowl was covered with one of six textures and filled with bedding material scented with one of six odors (described in Supplementary Table 1). This provided a sufficient number of exemplars such that the bowls varied in novel odor and texture combinations for each stage of the experiment.
Habituation (days 1 and 2)
On day 1, rats were trained to dig for a reward (half a Kellogg’s Froot Loop) in two untextured digging bowls filled with unscented bedding. On day 2, the rats were trained to discriminate between two bowls based on exemplars of one dimension at a time; odor then texture, only one exemplar in either dimension being associated with the reward. This represented the simple discrimination (SD) stage of training.
Testing (day 3)
Following another SD test with novel exemplars, rats were subject to a compound discrimination (CD) test for which a second dimension was introduced (the initial correct exemplar and relevant dimension being the same as in the previous stage). For the intradimensional (ID) and extradimensional (ED) shift, new texture and odor exemplars were presented to the rat. In the ID shift, the relevant dimension was reinforced. In the ED shift, however, the reward was associated with the previous irrelevant dimension. Thus, to successfully predict reward in the ED shift, the rat was required to shift their attention set, i.e. shift their attention from one dimension to another. There were two possible shifts the rat could perform; odor to texture, or texture to odor. For each stage, rats were also tested on reversal learning (R1, 2, and 3, respectively) for which the exemplars and relevant dimension remained the same but the previous negative exemplar was instead associated with the reward. The first four trials of each stage were discovery trials for which the rats were allowed to dig in either bowl. For all subsequent trials, the trial was terminated if they first dug in the unbaited bowl, and an error was recorded. The number of trials and errors needed to reach success criterion (six consecutive correct trials) constituted the main variable measured. Once the rats completed all discriminations they were returned to their home cages, placed back on the normal light/dark schedule, and given ad libitum access to food and water.
Hormone assays
Plasma ACTH and corticosterone concentrations were quantified by radioimmunoassay using commercially available kits according to methods previously described (Naef, Gratton, & Walker, Citation2013). For determination of ACTH, we used a kit from Diasorin (Diasorin, Minneapolis, MN, Indianapolis, MN). However, because of technical difficulties, we lost a small proportion of our samples collected under Heparin conditions (EL female group) resulting in low number of rats in some of our EL female groups. Total and nonspecific binding ranged between 28–37% and 2.5–4.1%, respectively. Intra and inter-assay variability for ACTH determinations was 4.2% and 11.6%, respectively. For determination of plasma corticosterone concentrations, we used a kit from MP Biomedicals (Santa Ana, CA) as previously described (McLaughlin et al., Citation2016). Total and nonspecific binding ranged between 44–49% and 2.2–2.8%, respectively. Intra- and inter-assay variability corticosterone determinations were 1.5% and 3.44%, respectively. The limit of detection for ACTH and corticosterone concentrations was 15 pg/ml and 0.31 μg/dl, respectively.
Statistical analysis
To compare stress-induced hormone release between groups, hormone concentrations at baseline were first compared using one-way ANOVA. For each rat, the value from each time point was normalized relative to its own baseline (0 min) value and the area under the curve (AUC) was calculated using three time points, 0, 30, and 60 min post stress for each animal (AUC = ½ × (0 min + 2 × 30 min + 60 min)). The results were compared between groups using one-way ANOVA. Fos counts were analyzed using two-way ANOVA with stage of lactation and presence/absence of pups as between subjects variables. Golgi data were analyzed using one-way ANOVA. Alpha level was set at p < .05, for all analyses and, where appropriate, post hoc comparisons were made using Fisher’s LSD or the Newman–Keuls post hoc test. Behavioral data were analyzed using two-way ANOVA with trials as the repeated measure and with reproductive status (virgin vs EL vs LL) as the between groups measure. Where appropriate simple main effects and pairwise comparisons were used to further investigate effects using the Tukey HSD test. Statistical analyses were performed using PASW 18 for Mac. All numerical data are expressed as the mean ± SEM.
Results
Effects of pup presence and stressor salience on hormonal stress responses in EL and LL females
In this set of experiments, we used two psychological stressors and considered the predator odor as more salient than tail pinch. To ease comparison, data are presented as normalized AUC for both ACTH and corticosterone concentrations and raw data can be found in Supplementary Figures 2 and 3. In the EL groups and as expected, baseline plasma corticosterone concentrations were higher in EL + females than in virgins for both stressors (V = 5.44 ± 1.74 µg/dl, EL+ = 9.34 ± 1.25 µg/dl; p < .05). In the EL females and consistent with earlier reports (Deschamps et al., Citation2003), we found that the response to predator stress was larger when the pups were present in the cage. There were close to significant effects of group for both ACTH (F(2,10) = 3.20, p = .084, ) and corticosterone (F(2,10) = 4.17, p = .052, ) in the predator odor stress condition. In the tail pinch stress condition, however, the AUC for ACTH responses was significantly lower in both EL groups than virgins (significant effect of group F(2,11) = 7.67, p = .008), but although the pattern of results for corticosterone was similar there was no significant effect of group on this parameter.
Figure 1. Plasma ACTH (A, C) and corticosterone (CORT, B, D) integrated response (normalized area under the curve, AUC) to either exposure to predator (ferret) odor (left) or tail pinch stress (right) for 30 min in early lactating (EL) females on postpartum day 4 (panels A and B) and late lactating (LL) females on postpartum day 16 (panels C and D). The response of virgin females (V, n = 8) was compared to that of EL females in the presence (EL+, n = 3) or the absence (EL−, n = 3) of their pups during testing (A, B) using intravenous sampling. The response of virgin females (V, n = 8–12) was compared to that of LL females in the presence (LL+, n = 5) or the absence (LL−, n = 5) of their pups during testing (C, D) using tail vein sampling. In the EL group, ANOVA showed no effect of group for predator odor in either ACTH or corticosterone. In the tail pinch condition, ANOVA showed a significant group effect for ACTH responses (p < .01). In the LL group, there was no group effect for either ACTH or corticosterone responses to either stressors. Values represent the mean ± SEM and the number of virgin females in each group is indicated inside the bars (see more details about group sizes in Supplementary Figure 1). **p < .01.
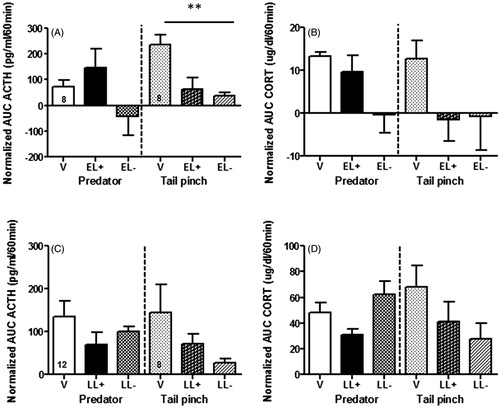
In the LL group, plasma corticosterone concentrations at baseline were still elevated compared to virgins (V = 5.8 ± 1.22 µg/dl, LL+ =20.7 ± 3.77 µg/dl, p < .01) and the pattern of response to stress was not significantly affected by the presence of pups in either stress condition. In the tail pinch stress, there was a trend for LL females to show reduced ACTH () and corticosterone () responses compared to virgin females, but this effect was not significant. Overall, there was no group effect for either ACTH or corticosterone responses to either stressor in LL females.
We did not directly compare EL and LL females because we used a different blood sampling method for each group, iv cannula for the EL group and tail vein sampling for the LL females. However, in order to assess the consequences of blood sampling differences, we compared ACTH and corticosterone responses to predator odor in virgin females used in the experiments with the EL and LL females. ANOVA showed no significant effect of sampling method for ACTH secretion (F(1,18) = 0.91, p = .35), but a significant effect for corticosterone secretion (F(1,18) = 10.06, p = .005), with no effects being seen at 0 min and most of the effect being observed at the 30 min (p < .01) and some at the 60 min time point (p < .05).
Differential Fos activation in EL and LL after predator odor stress
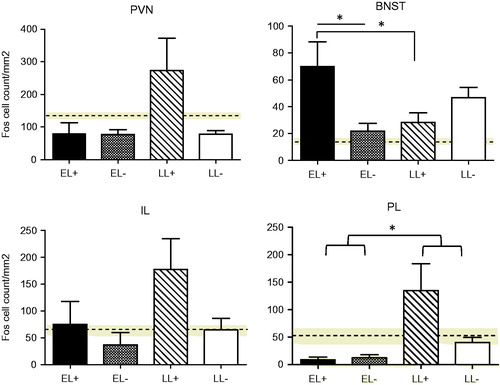
Here, we investigated whether the differential effect of pup presence on neuroendocrine responses to predator odor, between EL and LL females, would be reflected in differences in activation of Fos protein within particular structures in the extended stress circuitry (). Neither stage of lactation nor pup presence affected Fos induction in the PVN after predator stress exposure, but there was a differential effect of pup presence in EL and LL groups in the BNST (significant group × pup interaction (F(1,8) = 8.977, p = .017). In the BNST, there was greater Fos activation in the EL females in the presence of their pups than when pups were absent (p = .015) whereas in LL the presence or absence of the pups did not influence Fos expression (p = .27). Fos expression in EL when the pups were present was significantly higher than expression in LL with pups present (p = .03), but no differences in lactational stage were observed when pups were absent (p = .15). In the mPFC, there was a trend for pup presence to increase Fos expression in the IL portion in both EL and LL groups (F(1,8) = 3.652, p = .092). In the PL area, no significant pup effect was observed for either EL or LL females. Fos induction in this area was greater in the LL than the EL group (F(1,7) = 6.58, p = .037).
Figure 2. Regional differences in the density of c-Fos immunoreactive cells in response to the predator odor in early lactating (EL) or late lactating (LL) females in the presence (+) or absence (−) of their pups 2 h prior to and during stress testing. Density of c-Fos-immunoreactivity (IR) in virgin females is shown as the horizontal dotted line across the graph and SEM for this group is indicated as the gray zone centered on the broken line. No significant group effects were found in the paraventricular nucleus of the hypothalamus (PVN), but a significant mother × pup interaction was found for the bed nucleus of the stria terminalis (BNST) (p < .05).For the prelimbic (PL) medial prefrontal cortex (mPFC), there was a significant mother effect (p < .05). A minimum of 4–14 sections for each rat (n = 3/group) and brain region were counted and averaged. Values represent the mean ± SEM. *p < .05.
Reversible IL mPFC inhibition in EL females
Administration of BUP over the IL mPFC did not modify the ACTH response to predator odor in either virgin or EL + females, although the peak of ACTH response (15 min) was lower in EL + females injected with BUP (). There was a significant effect of time (F(3,123) = 4.826, p = .003), but no effect of drug treatment or interaction between drug × time on this measure. Plasma corticosterone responses also displayed a significant time effect for both virgins and EL + groups (F(3,123) = 28.71, p < .001), but there were no significant effects of either drug or of a drug × time interaction ().
Figure 3. Plasma ACTH response to 30 min exposure to predator odor in virgin (A) or early lactating (EL) females in the presence (+) of their pups (EL+). (B) Females pretreated with either vehicle (VEH, broken line, V: n = 10/group, EL: n = 12) or bupivacaine (BUP 0.75% solution in 0.5 µl/side, full line, V: n = 10, EL: n = 13) over the infralimbic (IL) portion of the medial prefrontal cortex (mPFC) 10 min prior to stress onset. Injection time is indicated by the arrow and stress exposure is indicated by the dotted rectangle on top of the X-axis. There was a significant effect of time for EL+ (p < .01), but no effect of drug treatment or interaction between drug × time. Values represent the mean ± SEM. The right panel depicts a composite of placement of IL cannulas for IL drug injection in our experimental rats according to the atlas of Paxinos (Paxinos, Watson, Pennisi, & Topple, Citation1985) and using levels between 3.0 and 3.72 mm anterior from bregma. PL: prelimbic medial prefrontal cortex.
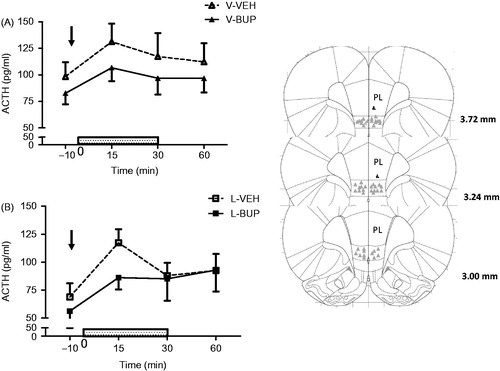
Table 1. Plasma corticosterone concentrations (μg/dl) after reversible IL mPFC lesions with bupivacaine.
Morphological changes in IL mPFC in lactating females
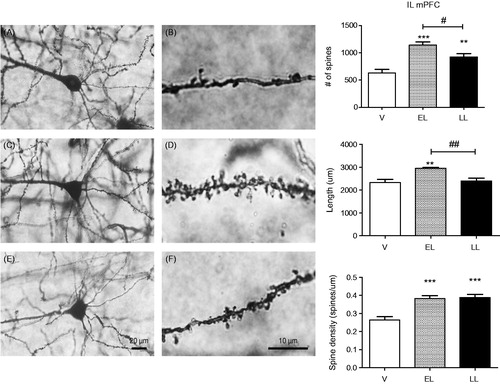
As the mPFC is involved in stress regulation, attention and also several aspects of maternal behavior, we determined whether differential responses to stressors as a function of stage of lactation might be associated with significant changes in dendritic morphology in this region. We focused on the IL portion of the mPFC because of its stimulatory inputs to the BNST and PVN (Ulrich-Lai & Herman, Citation2009). As shown in , there were significant group effects for spine number (F(2,12) = 15.40, p = .0005), length (F(2,12) = 7.69, p = .007), and spine density (F(2,12) = 17.47, p = .0003). EL and LL females displayed a significant increase in both the number of spines and spine density in layers 2 and 3 of the IL compared to virgins (p < .01 or .001) but only EL females had greater dendritic length than virgins. EL values for the total number of spines (p < .05) and dendritic length (p < .01) were higher than those in LL females.
Figure 4. Left: representative illustration of infralimbic (IL) medial prefrontal cortex (mPFC) Golgi-stained neurons in virgin (V, (A, B)), early lactating (EL, (C, D)), and late lactating (LL, (E, F)) females. Pictures were taken with Olympus BX63 at 40X(A, C, E) and oil-immersion 100X(B, D, F). Bars represent 20 µm (40X) and 10 µm (100X). Right: morphological analysis of IL mPFC neurons in virgin (V, n = 6 rats, 3–4 neurons/rat), EL (PPD4, n = 4; 4–7 neurons/rat), and LL (PPD16, n = 5; 4–5 neurons/rat) females by Golgi Cox staining. Total number of spines (top panel), dendritic length (middle panel), and spine density (bottom panel) were measured in layers 2–3 of the IL mPFC using the Neurolucida software (MBF Inc, Warwick, RI). A total of 3–7 neurons per rat were traced and averaged. Values represent the mean ± SEM. **p < .01; ***p < .001 compared to virgins (Student–Newman–Keuls test); #p < .05; ##p < .01 compared to LL females (Student–Newman–Keuls test).
Effect of reproductive status on attention set shifting capacity
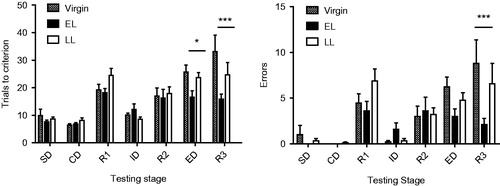
Given the observed morphological changes in the mPFC and the neuroendocrine responses that vary as a function of pup presence, stressor salience, and lactational stage, we similarly investigated whether attentional abilities were altered as a function of stage of lactation using the AST. The number of trials to criterion and errors in the AST are depicted in . For trials to criterion, there was a significant effect of reproductive state (F(2,25) = 4.69, p = .019), test (F(6,150) = 26.9, p < .0001) and a significant test × reproductive state interaction (F(12,150) = 2.50, p = .005). The main effects of reproduction were observed in the extradimensional shift (ED, p = .018) and in the third reversal (R3, p < .0001) where EL females took fewer trials to criterion than either virgins or LL females. LL females had a performance intermediate between EL and V females. For the number of errors, the main effect of reproduction was not significant (p = .085), but there was a significant test effect (F(6,150) = 16.70, p < .0001) and a significant test × reproductive state interaction (F(12,150) = 2.29, p = .010). EL females made fewer errors in the R3 condition than the other two groups (p < .0001). Together, the results of both number of trials to criterion and error rate analyses suggest a higher attentional capacity in EL females than in LL or virgin females.
Figure 5. Number of trials to criterion (six consecutive correct responses, left panel) and number of errors (right panel) recorded in the various testing stages of the attention set shifting task (AST) for virgin (n = 9), early lactating (EL, n = 10), and late lactating (LL, n = 9) females. Bars represent mean values ± SEM. Significant effects of reproductive status were observed in the third reversal (R3) stage for both number of trials (p < .001) and number of errors (p < .001). Number of trials were also significantly different in the extradimensional shift (ED) (p < .05). Missing bars for error number indicate that the value was zero in the SD and CD tests. SD: simple discrimination, CD: compound discrimination; R1: first reversal; ID: intradimensional shift; R2: second reversal; ED: extradimensional shift; R3: third reversal. Values represent the mean ± SEM. See Supplemental for more details. *p < .05; ***p < .001 (Tukey HSD test).
Discussion
Earlier studies have shown that the neuroendocrine hyporesponsiveness to stress in lactation is context dependent, and in some situations depends on the presence of the pups during stress exposure (Deschamps et al., Citation2003). This observation suggests that the same stimulus induces a different level of activation of the extended stress circuitry as a function of the presence of pups. In this study, our aim was to determine whether the effect of stressor salience on the activation of the HPA axis was observed throughout lactation and to test the hypothesis that specific neuronal structures in the limbic-HPA axis and in particular the IL mPFC are critical mediators of the effect of pup presence on neuroendocrine activation.
We observed a quite different pattern of ACTH and corticosterone responses to stress between early and late lactating females both in terms of the type of stressor and effect of presence of pups. In early lactation, mothers exhibited ACTH and corticosterone hyporesponsiveness to a non-salient stressor such as tail pinch stress, but the neuroendocrine responses to predator odor remained similar to responses in virgins when pups were present at the time of stress exposure. When pups were not present, both ACTH and corticosterone responses to the salient stressor were blunted, supporting previous findings with exposure to fox odor or male intruder stress (Deschamps et al., Citation2003). In late lactation, the pattern of neuroendocrine response was different, showing less hyporesponsiveness and no evidence of a modulatory role of pup presence for the salient stimulus. However, ACTH and corticosterone responses to the non-salient stressor still appeared somewhat reduced in LL females, although the effect did not reach statistical significance. These results extend previous findings showing a partial recovery of the HPA axis activity in LL females in some conditions such as exposure to a salient stressor, but not others (Walker, Trottier, Rochford, & Lavallee, Citation1995). While both predator odor and tail pinch are considered to represent psychogenic stressors, they are not considered to be equivalent in terms of relevance to pup safety or somatosensory stimulation. Tail pinch has a somatosensory component, but there was no pain component to the application of this stressor as observed behaviorally. This additional component might also have contributed to stressor-related differences in EL and LL females, although it did not increase the magnitude of the hormonal responses in either reproductive group.
One potential limitation of our comparison of neuroendocrine stress responses in early and late lactating rats results from the different sampling methods used in these groups that were necessitated by experimental constraints. Comparison of virgin groups showed that while tail vein sampling did not affect ACTH responses, it increased corticosterone secretion compared to sampling by iv cannulation. However, for the purpose of our neuroendocrine study, we only performed within-age comparison between stressor types and pup presence, thus eliminating the confounding effect of sampling method on our results.
Consistent with the observation of a greater neuroendocrine response in EL females in the presence of pups, we found that c-Fos activation following predator odor exposure was higher in the BNST when pups were present (EL+) than in their absence (EL−). The BNST is a critical hub integrating multiple afferents that modulate pPVN activity, with primarily GABAergic projections to the PVN neurons through the posterior BNST and stimulatory projections from the anteroventral BNST (Herman, Citation2012). Although we did not differentiate between the BNST subregions, we found that compared to virgins, activation of this structure was enhanced in all lactating groups, but that a differential effect of pup presence was only observed in early lactation. As there was no significant effect of pup presence in EL females on PVN c-Fos activation, which was still lower than levels observed in virgins, we suggest that pup presence might have activated primarily GABAergic inputs to PVN neurons in these females. In contrast, in late lactation, the presence of pups increased c-Fos activation in the PVN, IL, and PL mPFC, whereas there was no effect of pup presence on c-Fos induction in the BNST. In none of these areas did increased c-Fos activation correspond with larger neuroendocrine responses in late lactation. The reason for this dissociation between c-Fos activation and hormonal measures is unclear. There are at least three pathways through which the IL mPFC might stimulate activity in the pPVN (McKlveen, Myers, & Herman, Citation2015): one of these is through the anterior BNST (Vertes, Citation2004) and although we only examined c-Fos expression in the BNST as a whole, the lack of changes in this area suggests that this pathway is not involved. Alternatively, activation of pPVN neurons in LL females with their pups might result from indirect stimulatory projections from the IL mPFC through the posterior hypothalamus or NTS region (Herman et al., Citation2016; Myers et al., Citation2016; Myers, Mark Dolgas, Kasckow, Cullinan, & Herman, Citation2014; Myers, Scheimann, Franco-Villanueva, & Herman, Citation2017). In contrast, we also observed increased c-Fos in the PL mPFC. Outputs from this area might stimulate GABAergic inputs from the posterior BNST and enhance inhibition on pPVN neurons. Interestingly, earlier studies using single unit recordings in the PL area of the mPFC in early lactation have demonstrated that brief pup contact is sufficient to elicit increased neuronal firing activity in this region (Febo, Citation2012). The lower temporal resolution of Fos activation in our study compared to electrophysiological recordings might have prevented us from observing pup-related activation in the mPFC of EL females, or this activation might be transient and not sustained as in LL females. Here, we focused on activation of selected areas in the extended stress circuitry but others have documented a greater BOLD signal in periaqueductal gray, mediodorsal thalamus, temporal cortex, and NAcc in lactating females stressed in the presence of their pups (Nephew et al., Citation2009), suggesting that pup presence might also influence other neuronal pathways not directly linked to stress responsiveness.
The effect of the IL mPFC inactivation experiment with BUP fails to support a role for the IL mPFC in the mediation of pup effects on the stress response in early lactation. The data suggest that this manipulation had little effect on stress-induced hormone release, consistent with the finding that pup presence only marginally increased c-Fos activation in the IL mPFC in both early and late lactation. Although we found that the IL mPFC apparently plays no role in the modulation of the stress response in early lactation, we were able to show that this area exhibits important morphological changes as early as days 4–5 of lactation. At this time, spine number, dendrite length, and spine density were all increased. The increase in spine density was maintained into late lactation, but dendrite length was not. These data are similar to previous reports of morphological changes in brain areas such as the hippocampus (Kinsley et al., Citation2006; Pawluski, Lambert, & Kinsley, Citation2016), NAcc, medial amygdala (Slattery & Hillerer, Citation2016), dorsal raphe (Holschbach & Lonstein, Citation2017), and cingulate cortex (Salmaso, Quinlan, Brake, & Woodside, Citation2011) in early lactation. Similarly, plastic changes were observed in the mPFC of females that have undergone a reproductive episode (Leuner & Gould, Citation2010). Increases in gray matter volume of the PFC and other brain regions have also been reported in nursing mothers between 2–3 weeks and 3–4 months postpartum (Kim et al., Citation2010). It has been argued that the sensory experience provided by the pups is important in maintaining increases in dendritic spine density in several brain areas (Kinsley et al., Citation2006) as well as behaviors, such as increased spatial working memory.
The increased spine density observed in the IL mPFC in early lactation is associated with a dramatic increase in attentional set shifting abilities in EL compared to LL or to virgin females. Attentional set shifting represents one of several attentional modalities and provides information on the ability to recognize relevant information and adjust attention accordingly (Lovic & Fleming, Citation2004). Lower errors and number of trials to criterion in the AST have been reported in LL females (PPD24) compared to virgins (Leuner & Gould, Citation2010), but here, we extend these data to document that attentional abilities are even greater in early lactation. Although higher spine density in the IL mPFC was maintained in late lactation females compared to virgins, their performance in the AST was lower than EL rats and closer to that of virgins, suggesting that dendritic remodeling in other regions of the mPFC might also be critical to regulate attention shifting processes during lactation.
In summary, our present studies demonstrate the critical role of pup presence on neuroendocrine activation to a salient stressor in early lactation and the related activation of upstream targets such as the BNST, although the source of the inputs producing this enhanced response is not clear. Subsequent data suggest that it does not result from a simple increase in direct excitatory output from IL mPFC to BNST (Myers et al., Citation2016), but rather that other structures like the posterior hypothalamus or NTS might be involved in pup-induced increases in HPA responses in early lactation. Interestingly, the continuous stimulation provided by suckling pups produced significant morphological changes in the IL mPFC that were associated with increased attentional set shifting abilities in early lactation. These data emphasize the rapid adaptation of the maternal brain to the needs of the developing pups. In the early postpartum period, when pups are totally dependent upon their mother for survival, their presence has a profound effect on her ability to mount an adequate stress response while at the same time enhancing her attentional capabilities. The greater independence of older pups is associated with a reduction of both effects. These data also emphasize the need for additional network analysis studies taking into account stimulus salience and the presence of pups as significant factors in the regulation of larger neural circuits associated with the extended stress circuitry, fear processing, cognition, and reward.
Claire-Dominique_Walker_et_al_supplemental_content.zip
Download Zip (228.1 KB)Disclosure statement
The authors have no financial interest in, or conflict of interest with the subject and materials discussed in the present manuscript.
Additional information
Funding
References
- Afonso, V.M., Sison, M., Lovic, V., & Fleming, A.S. (2007). Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behavioral Neuroscience, 121, 515–526. doi:10.1037/0735-7044.121.3.515
- Altemus, M., Redwine, L.S., Leong, Y.M., Frye, C.A., Porges, S.W., & Carter, C.S. (2001). Responses to laboratory psychosocial stress in postpartum women. Psychosomatic Medicine, 63, 814–821. doi:10.1097/00006842-200109000-00015
- Campeau, S., Nyhuis, T.J., Sasse, S.K., Day, H.E., & Masini, C.V. (2008). Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neuroscience & Biobehavioral Reviews, 32, 1277–1286. doi:10.1016/j.neubiorev.2008.05.014
- Choi, D.C., Evanson, N.K., Furay, A.R., Ulrich-Lai, Y.M., Ostrander, M.M., & Herman, J.P. (2008). The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology, 149, 818–826. doi:10.1210/en.2007-0883
- Deschamps, S., Woodside, B., & Walker, C.D. (2003). Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. Journal of Neuroendocrinology, 15, 486–497. doi:10.1046/j.1365-2826.2003.01022.x
- Febo, M. (2012). Firing patterns of maternal rat prelimbic neurons during spontaneous contact with pups. Brain Research Bulletin, 88, 534–542. doi:10.1016/j.brainresbull.2012.05.012
- Fozzard, H.A., Lee, P.J., & Lipkind, G.M. (2005). Mechanism of local anesthetic drug action on voltage-gated sodium channels. Current Pharmaceutical Design, 11, 2671–2686. doi:10.2174/1381612054546833
- Herman, J.P. (2012). Neural pathways of stress integration: Relevance to alcohol abuse. Alcohol Research, 34, 441–447. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23584110
- Herman, J.P., McKlveen, J.M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., … Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6, 603–621. doi:10.1002/cphy.c150015
- Hernandez-Gonzalez, M., Navarro-Meza, M., Prieto-Beracoechea, C.A., & Guevara, M.A. (2005). Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behavioural Processes, 70, 132–143. doi:10.1016/j.beproc.2005.06.002
- Hernandez-Gonzalez, M., Prieto-Beracoechea, C., Navarro-Meza, M., Ramos-Guevara, J.P., Reyes-Cortes, R., & Guevara, M.A. (2005). Prefrontal and tegmental electrical activity during olfactory stimulation in virgin and lactating rats. Physiology & Behavior, 83, 749–758. doi:10.1016/j.physbeh.2004.09.013
- Holschbach, M.A., & Lonstein, J.S. (2017). Motherhood and infant contact regulate neuroplasticity in the serotonergic midbrain dorsal raphe. Psychoneuroendocrinology, 76, 97–106. doi:10.1016/j.psyneuen.2016.10.023
- Hsu, E., & Packard, M.G. (2008). Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiology of Learning and Memory, 89, 504–512. doi:10.1016/j.nlm.2007.08.006
- Kim, P., Feldman, R., Mayes, L.C., Eicher, V., Thompson, N., Leckman, J.F., & Swain, J.E. (2011). Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry, 52, 907–915. doi:10.1111/j.1469-7610.2011.02406.x
- Kim, P., Leckman, J.F., Mayes, L.C., Feldman, R., Wang, X., & Swain, J.E. (2010). The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience, 124, 695–700. doi:10.1037/a0020884
- Kinsley, C.H., Trainer, R., Stafisso-Sandoz, G., Quadros, P., Marcus, L.K., Hearon, C., … Lambert, K.G. (2006). Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Hormones and Behavior, 49, 131–142. doi:10.1016/j.yhbeh.2005.05.017
- Leuner, B., & Gould, E. (2010). Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. Journal of Neuroscience, 30, 13499–13503. doi:10.1523/JNEUROSCI.3388-10.2010
- Lovic, V., & Fleming, A.S. (2004). Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task: Reversal of effects with maternal-like licking stimulation. Behavioural Brain Research, 148, 209–219. doi:10.1016/S0166-4328(03)00206-7
- McKlveen, J.M., Myers, B., & Herman, J.P. (2015). The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine and behavioural responses to stress. Journal of Neuroendocrinology, 27, 446–456. doi:10.1111/jne.12272
- McLaughlin, R.J., Verlezza, S., Gray, J.M., Hill, M.N., & Walker, C.D. (2016). Inhibition of anandamide hydrolysis dampens the neuroendocrine response to stress in neonatal rats subjected to suboptimal rearing conditions. Stress, 19, 114–124. doi:10.3109/10253890.2015.1117448
- Meinlschmidt, G., Martin, C., Neumann, I.D., & Heinrichs, M. (2010). Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress, 13, 163–171. doi:10.3109/10253890903128632
- Myers, B., Carvalho-Netto, E., Wick-Carlson, D., Wu, C., Naser, S., Solomon, M.B., … Herman, J.P. (2016). GABAergic signaling within a limbic-hypothalamic circuit integrates social and anxiety-like behavior with stress reactivity. Neuropsychopharmacology, 41, 1530. doi:10.1038/npp.2015.311
- Myers, B., Mark Dolgas, C., Kasckow, J., Cullinan, W.E., & Herman, J.P. (2014). Central stress-integrative circuits: Forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Structure and Function, 219, 1287–1303. doi:10.1007/s00429-013-0566-y
- Myers, B., Scheimann, J.R., Franco-Villanueva, A., & Herman, J.P. (2017). Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neuroscience & Biobehavioral Reviews, 74, 366–375. doi:10.1016/j.neubiorev.2016.05.011
- Naef, L., Gratton, A., & Walker, C.D. (2013). Exposure to high fat during early development impairs adaptations in dopamine and neuroendocrine responses to repeated stress. Stress, 16, 540–548. doi:10.3109/10253890.2013.805321
- Nephew, B.C., Caffrey, M.K., Felix‐Ortiz, A.C., Ferris, C.F., & Febo, M. (2009). Blood oxygen level‐dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups. European Journal of Neuroscience, 30, 934–945.
- Pawluski, J.L., Lambert, K.G., & Kinsley, C.H. (2016). Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Hormones and Behavior, 77, 86–97. doi:10.1016/j.yhbeh.2015.06.004
- Paxinos, G., & Watson, C. (1998). A stereotaxic atlas of the rat brain. New York: Academic.
- Paxinos, G., Watson, C., Pennisi, M., & Topple, A. (1985). Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. Journal of Neuroscience Methods, 13, 139–143. doi:10.1016/0165-0270(85)90026-3
- Pereira, M., & Morrell, J.I. (2011). Functional mapping of the neural circuitry of rat maternal motivation: Effects of site-specific transient neural inactivation. Journal of Neuroendocrinology, 23, 1020–1035. doi:10.1111/j.1365-2826.2011.02200.x
- Radley, J.J., Gosselink, K.L., & Sawchenko, P.E. (2009). A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. Journal of Neuroscience, 29, 7330–7340. doi:10.1523/JNEUROSCI.5924-08.2009
- Ranote, S., Elliott, R., Abel, K.M., Mitchell, R., Deakin, J.F., & Appleby, L. (2004). The neural basis of maternal responsiveness to infants: An fMRI study. Neuroreport, 15, 1825–1829. doi:10.1097/01.wnr.0000137078.64128.6a
- Salmaso, N., Quinlan, M., Brake, W., & Woodside, B. (2011). Changes in dendritic spine density on layer 2/3 pyramidal cells within the cingulate cortex of late pregnant and postpartum rats. Hormones and Behavior, 60, 65–71. doi:10.1016/j.yhbeh.2011.03.002
- Slattery, D.A., & Hillerer, K.M. (2016). The maternal brain under stress: Consequences for adaptive peripartum plasticity and its potential functional implications. Frontiers in Neuroendocrinology, 41, 114–128. doi:10.1016/j.yfrne.2016.01.004
- Tu, M.T., Lupien, S.J., & Walker, C.D. (2005). Measuring stress responses in postpartum mothers: Perspectives from studies in human and animal populations. Stress, 8, 19–34. doi:10.1080/10253890500103806
- Tu, M.T., Lupien, S.J., & Walker, C.D. (2006). Multiparity reveals the blunting effect of breastfeeding on physiological reactivity to psychological stress. Journal of Neuroendocrinology, 18, 494–503. doi:10.1111/j.1365-2826.2006.01441.x
- Ulrich-Lai, Y.M., & Herman, J.P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews. Neuroscience, 10, 397–409. doi:10.1038/nrn2647
- Vertes, R.P. (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse (New York, NY), 51, 32–58. doi:10.1002/syn.10279
- Walker, C.D., Trottier, G., Rochford, J., & Lavallee, D. (1995). Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. Journal of Neuroendocrinology, 7, 615–622. doi:10.1111/j.1365-2826.1995.tb00799.x
- Windle, R.J., Wood, S.A., Kershaw, Y.M., Lightman, S.L., & Ingram, C.D. (2013). Adaptive changes in basal and stress-induced HPA activity in lactating and post-lactating female rats. Endocrinology, 154, 749–761. doi:10.1210/en.2012-1779
- Woodside, B. (2016). Mood, food, and fertility: Adaptations of the maternal brain. Comprehensive Physiology, 6, 1493–1518. doi:10.1002/cphy.c150036
