Abstract
Inconsistent associations have been reported between perceived stress and C-reactive protein (CRP), a marker of systemic inflammation. We previously observed a male-specific inverse relationship between perceived stress and CRP in a cross-sectional study. In the present study, we examined the longitudinal association between changes in perceived stress and CRP, and further analyzed whether changes in coping strategies and social support modify this association. This study included 8454 participants in both a baseline survey and a follow-up survey 5 years later. Psychosocial measures (i.e. perceived stress, coping strategies, and social support) and CRP concentrations were measured by identical means in both surveys. Consistent with our previous findings, increased perceived stress was significantly associated with lower CRP in men (ptrend = .037), but not in women. Increased “emotional expression,” a coping strategy, was also associated with lower CRP in women (ptrend = .024). Furthermore, interactions between perceived stress and a coping strategy (positive reappraisal) or social support on CRP were found in men (pinteraction = .007 and .038, respectively); the above inverse association between stress and CRP was not detected for participants with diminished positive reappraisal or social support. In conclusion, increases in perceived stress during a 5-year period were associated with decreases in CRP among healthy men, and the observed association was possibly modified by coping strategy or social support.
Introduction
Various psychosocial factors have been linked to the risk of cardiovascular disease (CVD) (Brotman, Golden, & Wittstein, Citation2007; Iso et al., Citation2002; Rosengren et al., Citation2004). It has been postulated that one of the possible mechanisms for this linkage is that these factors deteriorate or ameliorate low-grade systemic inflammation, thus affecting future CVD events (Ridker, Cushman, Stampfer, Tracy, & Hennekens, Citation1998). In this context, psychosocial factors, including mental stress, have been examined in association with circulating C-reactive protein (CRP) (Gimeno et al., Citation2007; Johnson, Abbasi, & Master, Citation2013; Shimanoe et al., Citation2014; Shivpuri, Gallo, Crouse, & Allison, Citation2012), a marker of systemic inflammation. Accumulated data concerning the association between psychosocial stress and CRP are conflicting (Johnson et al., Citation2013), in contrast with close to consistent positive associations between depression or distress and CRP (Gimeno et al., Citation2009; Wium-Andersen, Ørsted, Nielsen, & Nordestgaard, Citation2013).
Published large cross-sectional studies on the association between perceived stress and CRP have reported mixed results, variously showing a positive (Shivpuri et al., Citation2012), null (Ranjit et al., Citation2007), or inverse association (Shimanoe et al., Citation2014). As for the positive association between stress and CRP, there are postulated biological mechanisms; stress can promote inflammatory responses through sympathetic and para-sympathetic nervous systems (Raison, Capuron, & Miller, Citation2006), and chronic stress over extended periods of time can decrease the abilities of stress-related hormones (e.g. cortisol) to regulate immune system cells, leading to a state of sustained/chronic low-grade inflammation (Gu, Tang, & Yang, Citation2012). However, more epidemiological data on stress and CRP are required before inferring further possible mechanisms.
Prior work examining associations between perceived stress and CRP in humans has been largely cross-sectional, which compare groups of participants with different exposures and outcomes at a single point in time. Meanwhile, longitudinal studies examine real changes in exposure or outcome over a certain period of time within the same individuals, and these properties are considered superior to those of cross-sectional studies (Ruspini, Citation2002). To the best of our knowledge, a few extant longitudinal studies with 100–400 participants have found either a positive (Barbosa-Leiker et al., Citation2014; McDade, Hawkley, & Cacioppo, Citation2006) or null association (Miller, Rohleder, & Cole, Citation2009). Furthermore, two recent studies using a national dataset indicated a positive longitudinal association only in particular subgroups of participants (those in mid-adulthood [n = 599] and those with high levels of mastery at baseline [n < 2400 according to the authors' estimation]) (Elliot, Mooney, Infurna, & Chapman, Citation2017; Yang et al., Citation2016). Additional large longitudinal studies are clearly needed.
Interestingly, the Framingham Heart Study suggested that changes in CRP over 5 years were more strongly associated with changes in body mass index (BMI) or stopping hormone replacement therapy during follow-up than BMI or hormone replacement therapy at baseline (Fontes et al., Citation2013), which may be attributable to the fact that CRP is an acute-phase protein (Pepys, Citation1981). Likewise, changes in perceived stress, rather than baseline levels, may be more relevant to changes in CRP during follow-up; however, no longitudinal studies to date have examined associations between simultaneous changes in perceived stress and CRP.
Additionally, it is important to consider the possible reasons for the inconsistencies among studies of psychosocial stress and CRP. Possible reasons include different means of measuring psychosocial stress (Johnson et al., Citation2013), residual confounding (Douglas, Taylor, & O'Malley, Citation2004; Ranjit et al., Citation2007), reverse causation by underlying inflammation-related diseases (Bjerkeset, Romild, Smith, & Hveem, Citation2011), the presence or absence of some possible protective factors (Shimanoe et al., Citation2014; von Känel et al., Citation2012) such as coping strategy (Lazarus, Citation1985) or emotional social support (Hogan, Linden, & Najarian, Citation2002). In different cohorts, these factors may modify the association between psychosocial stress and CRP. In this context, since social support and coping strategy may “buffer” the harmful influence of stress-induced CVD reactivity (Cohen & Wills, Citation1985; Uchino, Citation2006; von Känel et al., Citation2012), additional longitudinal studies need to explore how coping strategies and social support affect the association between perceived stress and CRP.
The purpose of this study was to draw on an ongoing cohort study of the general population of Japan to explore the association between changes in perceived stress and CRP in a large longitudinal study. Furthermore, when any association between changes in perceived stress and CRP was identified, we evaluated whether that association was modified by changes in any coping strategy or social support.
Methods
Design/setting and participants
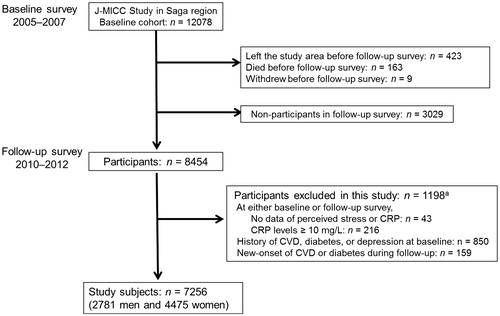
This longitudinal study is based on the Japan Multi-Institutional Collaborative Cohort Study in the Saga region. Its details have been described elsewhere (Hara et al., Citation2010; Nanri et al., Citation2011). In brief, the baseline survey between 2005 and 2007 recruited 12,078 men and women who were residents of Saga city and aged from 40 to 69 years. Of these participants, 8454 (70%) also participated in a follow-up survey about 5 years later, between 2010 and 2012 (Hara et al., Citation2015). In both baseline and follow-up surveys, we performed on-site assessments using a self-report questionnaire on psychosocial and lifestyle factors, collected anthropometric measurements, and sampled venous blood. For the present study, 7256 participants remained after applying the exclusion criteria by O’Connor et al. (Citation2009) (). The study, including all methods described herein, was approved by the Ethics Committees of both Saga University Faculty of Medicine (Approved date: 16 September 2005, IRB approval no. 17-11) and Nagoya University Graduate School of Medicine (Approved date: 20 July 2005, IRB approval no. 253), with reference to relevant ethical guidelines for medical research in Japan. Informed consent was obtained from all individual participants included in the study.
Measurement of CRP concentrations
Venous blood (21 mL) was drawn from each participant, and serum, plasma, and buffy coat were separated and stored at −80 °C until tested. At the baseline survey, serum CRP was measured using a high-sensitivity latex-enhanced immunonephelometric assay on a BN II analyzer (Dade Behring, Marburg, Germany) at an external laboratory (SRL, Hachioji, Japan). In this laboratory, intra- and inter-assay coefficients of variation for this assay were 3.1 and 1.3%, respectively. At the follow-up survey, CRP was measured immediately after blood collection and separation using the same assay by the same laboratory. The detection limit of this assay of 0.05 mg/L was assigned to values below that limit (n = 173 at baseline and 211 at follow-up). To examine survey-to-survey variation across assays, 11 control specimens stored at −80 °C were measured at the time of both surveys, similarly to the participants’ specimens. The between-survey coefficient of variation was 3.9%, and the corresponding intra-class correlation coefficient was 0.997.
Psychosocial factors
Psychosocial factors were evaluated identically in both baseline and follow-up surveys. A self-administered questionnaire on psychosocial factors as well as lifestyle factors, medication use, and histories of lifestyle-related diseases was sent to the participants beforehand, and they were instructed to bring their completed questionnaires to a survey location. Missing or inconsistent answers were checked with a face-to-face interview by a research nurse. The following single question was used to evaluate the level of perceived stress (Iso et al., Citation2002; Shimanoe et al., Citation2014): “How much stress did you feel during the last year?” There were four possible responses: “I felt no stress at all,” “I felt little stress,” “I felt moderate stress,” and “I felt much stress,” with assigned scores of 1–4, respectively. This question has been found to be associated with cardiovascular risk or mortality in previous studies (Iso et al., Citation2002; Nielsen, Kristensen, Schnohr, & Grønbaek, Citation2008) and also had an inverse association with CRP in our cross-sectional study (Shimanoe et al., Citation2014).
Four of the coping strategy items were selected from the subscales of a dispositional version of the General Coping Questionnaire because they showed the largest factor loadings of the questionnaire items (Sasaki, Kitaoka-Higashiguchi, Morikawa, & Nakagawa, Citation2009; Sasaki & Yamasaki, Citation2002). One additional item was taken from the brief COPE (Carver, Citation1997). In response to the query “How do you cope with various problems and unpleasant events you experience in daily life?” participants were asked to report the frequency (four response categories: “seldom,” “sometimes,” “often,” and “very often,” with assigned scores of 1–4, respectively) of each of the following coping strategies: (i) “I express my negative feelings and thoughts”; (ii) “I consult with someone close and ask for encouragement”; (iii) “I try to interpret the problem in a favorable way”; (iv) “I try hard to solve the problem”; and (v) “I let the problem take its own course.” These five strategies were termed “emotional expression,” “emotional support seeking,” “positive reappraisal,” “problem solving,” and “disengagement,” respectively. In our previous cross-sectional study, disengagement was inversely associated with CRP (Shimanoe et al., Citation2014).
A four-item scale (Cronbach’s ɑ = 0.81) (Shimanoe et al., Citation2014) was used to examine emotional social support from family and friends (Fukahori et al., Citation2007), the scores of which correlate with scores of the General Health Questionnaire-28 (Goldberg & Hillier, Citation1979). Participants were asked about the frequency (“seldom,” “sometimes,” “often,” and “very often”) of each of the following types of support usually provided for them by their family and friends: (1) care in times of poor health, (2) consultation regarding personal distress, (3) encouragement when having to work hard, and (4) help with their job or housework. These items were each scored on a four-point scale, giving a sum score ranging from 4 to 16. Higher scores represent higher levels of social support.
Covariates
Employment status, body mass index (BMI, kg/m2), drinking (ethanol; g/day), smoking (cigarettes/day), physical activity level (total energy expenditure divided by basal metabolic rate, measured with a single-axis accelerometer (Kenz Lifecorder EX; Suzuken, Nagoya, Japan), sleeping hours, medication (use of analgesics or lipid-lowering drugs), and menopausal status were measured identically at both baseline and follow-up surveys, as previously described (Nanri et al., Citation2011; Shimanoe et al., Citation2014).
Statistical analysis
Because sex differences in the relationship between perceived stress and CRP were identified in our previous cross-sectional study (Shimanoe et al., Citation2014), analyses were performed separately for men and women, using the SAS statistical software package (Ver. 9.3 for Windows; SAS Institute, Cary, NC). Associations between changes in each psychosocial factor and changes in CRP during follow-up were evaluated by multiple linear regression analysis. Baseline and follow-up measurements of CRP were log-transformed because they were skewed to the right; the difference between the two log-transformed values was used as a dependent variable. For each of perceived stress, coping strategies, and social support, the difference between two scores at baseline and follow-up (i.e. the score at baseline subtracted from the score at follow-up) was included in the model as a main independent variable. The adjusted percent change in CRP for a one-point increase in score for each of perceived stress, coping strategies, and social support was calculated by converting the obtained regression coefficient (β) to (exp[β] − 1) × 100.
Four different models were run to adjust for the following factors: (i) age at baseline (years); (ii) socioeconomic factors (changes in employment status [three categories: constant, retired or unemployed, or employed]) and lifestyle factors (changes in BMI [kg/m2], drinking [g/day], smoking [cigarettes/day], physical activity level [continuous], and hours of sleep [per day]); (iii) psychosocial factors (changes in scores of perceived stress, each coping strategy, and social support); and (iv) medication (four categories: no/no, no/yes, yes/no, and yes/yes at baseline/follow-up). The first to fourth models included the above covariates of (i), (i) + (ii), (i) + (ii) + (iii), and (i) + (ii) + (iii) + (iv), respectively, in addition to the main psychosocial variable. For women, an additional analysis was performed with further adjustment for changes in menopausal status (constant or menopause) or status of hormone replacement therapy (constant, initiation, or discontinuation) (Fontes et al., Citation2013).
Next, because possible differences in the association between perceived stress and CRP according to different baseline CRP or age category had previously been observed by our team (Shimanoe et al., Citation2014), whether the association between changes in perceived stress and CRP varied by baseline CRP concentrations (<1, 1 to <3, and ≥3 mg/L) or baseline age category (<50, 50–59, and ≥60 years) were examined in stratified analyses. Similarly, stratified analyses were performed for each coping strategy or level of social support that might moderate the association between perceived stress and CRP, as described in the “Introduction” section. The corresponding interactions were tested by including in the multivariate models an additional interaction term between the change in score of perceived stress and each stratification variable as a continuous variable without categorization. Two-tailed p values < .05 were considered statistically significant.
Results
shows the assessed characteristics of the participants at baseline and follow-up surveys. Values for CRP at baseline were significantly positively correlated with those at follow-up (Spearman's rank correlation coefficient =0.59, p < .001). The geometric mean of CRP decreased significantly between the two surveys in both sexes. During follow-up, the prevalence of very high levels of perceived stress and use of some coping strategies (namely, emotional support seeking, positive reappraisal, and problem solving) decreased significantly in both men and women. Social support, drinking, smoking, and physical activity level also declined significantly during follow-up in both sexes. At follow-up, women used emotional expression more frequently, and were more likely to be post-menopausal than at baseline. Analgesics or lipid-lowering drugs were used more frequently at follow-up than at baseline.
Table 1. Characteristics of study participants at baseline and follow-up after 5 years.
The correlations between changes in perceived stress and changes in other psychosocial factors and covariates by sex are shown in . In both men and women, changes in perceived stress were positively associated with changes in emotional expression, and emotional support seeking, whereas they were inversely associated with changes in CRP and BMI. In men, changes in perceived stress were inversely related to retirement during follow-up and changes in physical activity. In women, changes in perceived stress showed positive associations with changes in positive reappraisal, problem solving, and menopausal status, but had an inverse association with changes in sleeping hours.
Table 2. Correlations of changes in perceived stress with changes in other psychosocial factors and covariates during follow-up by sex.
presents results from multiple regression analyses on the association between changes in each psychosocial factor and CRP by sex. After adjustment for age at baseline, changes in perceived stress were inversely associated with changes in CRP in men (−7% change for one category increase of perceived stress, ptrend = .007), but not in women. The inverse association in men remained statistically significant even after controlling additionally for all covariates (−5% change, ptrend = .037, Model 3), and also remained robust after adjustment for perceived stress at baseline survey (data not shown). In women, further adjustment for menopausal status or hormone replacement therapy gave almost identical non-significant results (data not shown). In the associations between changes in CRP and coping strategies, an inverse association was detected with emotional expression in women (−5% change, ptrend = .024, Model 3 in ), but changes in social support were not related to changes in CRP.
Table 3. Multiple regression analysis on association between changes in each psychosocial factor and C-reactive protein (CRP) by sex.
Next, we examined whether the association between changes in perceived stress and CRP in men differed by baseline CRP or baseline age category (). Participants with CRP concentrations ≥3 mg/mL accounted for 5.0% of men at baseline. The inverse association between perceived stress and CRP during follow-up was not significantly modified by CRP at baseline (). According to age-stratified analysis (), there was a significant inverse association between changes in perceived stress and CRP in men in their 60 s (8% decrease with ptrend = .042) but not in younger men. However, the corresponding interaction was not significant (pinteraction = .41).
Figure 2. Associations between changes in perceived stress and C-reactive protein (CRP) stratified by baseline CRP and age in men. The p values for trends were estimated by multiple linear regression analysis with inclusion in the model of the change in score of perceived stress as a main independent variable. The p values for interactions were tested by including in the multivariate models an additional interaction term between the change in score of perceived stress and each stratification variable as a continuous variable. Adjustments were made for age at baseline, socioeconomic factors (changes in employment status), lifestyle factors (changes in body mass index, drinking, smoking, physical activity level, and hours of sleep), psychosocial factors (changes in coping strategies and social support), and medication. The adjusted % change in CRP for a one-point increase in score for perceived stress was calculated by converting the obtained regression coefficient (β) to (exp[β] − 1) × 100.
![Figure 2. Associations between changes in perceived stress and C-reactive protein (CRP) stratified by baseline CRP and age in men. The p values for trends were estimated by multiple linear regression analysis with inclusion in the model of the change in score of perceived stress as a main independent variable. The p values for interactions were tested by including in the multivariate models an additional interaction term between the change in score of perceived stress and each stratification variable as a continuous variable. Adjustments were made for age at baseline, socioeconomic factors (changes in employment status), lifestyle factors (changes in body mass index, drinking, smoking, physical activity level, and hours of sleep), psychosocial factors (changes in coping strategies and social support), and medication. The adjusted % change in CRP for a one-point increase in score for perceived stress was calculated by converting the obtained regression coefficient (β) to (exp[β] − 1) × 100.](/cms/asset/a4b62289-69fc-48eb-a51f-f93e34b07e72/ists_a_1435638_f0002_b.jpg)
Finally, we tested whether any coping strategy or social support moderated the association between changes in perceived stress and CRP. We found significant interactions between perceived stress and either positive reappraisal (pinteraction = .007) or social support on CRP (pinteraction = .038) in men. The p for interaction between perceived stress and positive reappraisal remained significant even after Bonferroni's correction for multiple tests on coping strategies (0.007 × 5 = 0.035). In stratified analyses by the two factors, the inverse association between perceived stress and CRP in men was not evident for decreases during follow-up in positive reappraisal or social support ().
Figure 3. Associations between changes in perceived stress and C-reactive protein (CRP) stratified by changes in positive reappraisal or social support in men. The p values for trends were estimated by multiple linear regression analysis with inclusion in the model of the change in score of perceived stress as a main independent variable. The p values for interactions were tested by including in the multivariate models an additional interaction term between the change in score of perceived stress and each stratification variable as a continuous variable. Adjustments were made for age at baseline, socioeconomic factors (changes in employment status), lifestyle factors (changes in body mass index, drinking, smoking, physical activity level, and hours of sleep), psychosocial factors (changes in coping strategies and social support), and medication excluding the corresponding stratification variable. The adjusted % change in CRP for a one-point increase in score for perceived stress was calculated by converting the obtained regression coefficient (β) to (exp[β] − 1) × 100.
![Figure 3. Associations between changes in perceived stress and C-reactive protein (CRP) stratified by changes in positive reappraisal or social support in men. The p values for trends were estimated by multiple linear regression analysis with inclusion in the model of the change in score of perceived stress as a main independent variable. The p values for interactions were tested by including in the multivariate models an additional interaction term between the change in score of perceived stress and each stratification variable as a continuous variable. Adjustments were made for age at baseline, socioeconomic factors (changes in employment status), lifestyle factors (changes in body mass index, drinking, smoking, physical activity level, and hours of sleep), psychosocial factors (changes in coping strategies and social support), and medication excluding the corresponding stratification variable. The adjusted % change in CRP for a one-point increase in score for perceived stress was calculated by converting the obtained regression coefficient (β) to (exp[β] − 1) × 100.](/cms/asset/14b179bb-f6f1-4781-888b-231719d4c014/ists_a_1435638_f0003_b.jpg)
Discussion
In the present study, a one-point increase in the score of perceived stress was associated with about a 5% decrease in CRP after adjusting for covariates in men, as compared with almost no change (0.8% increase) in women. These results support our previous finding in a cross-sectional study that perceived stress was inversely associated with CRP in men (Shimanoe et al., Citation2014). It is noteworthy that this male-specific feature of the identified associations is similar to results from our cross-sectional study. In women, a one-point increase in “emotional expression,” a coping strategy, was associated with a 5% decrease in CRP. Moreover, the inverse association between perceived stress and CRP in men was significantly modified by changes in “positive reappraisal” or social support.
The results of cross-sectional studies on the association between perceived stress and CRP are mixed, and most of the limited number of longitudinal studies has used small samples. According to published longitudinal data, changes in CRP indicated no association with chronic interpersonal stress at baseline in 103 women (Miller et al., Citation2009) and 211 men (Barbosa-Leiker et al., Citation2014), whereas counter-stress (a negatively worded question in the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, Citation1983)) in 172 women (Barbosa-Leiker et al., Citation2014) at baseline had a positive association. In another longitudinal study, current CRP was positively associated with changes in perceived stress during the previous year in 188 men and women (McDade et al., Citation2006). Additionally, as noted above, two recent studies using a national dataset revealed positive longitudinal associations only in subgroups of participants (Elliot et al., Citation2017; Yang et al., Citation2016). Specifically, in Yang et al. (Citation2016), high social strain at baseline was associated with a higher CRP at follow-up among those in mid-adulthood (n = 599), but not among those in late adulthood (n = 812). In Elliot et al. (Citation2017), chronic stress at baseline was associated with an increase in CRP at follow-up among those with high levels of mastery at baseline or with decreased levels of mastery at follow-up; however, the overall longitudinal association was not significant.
These studies employed two different analytical designs. One design examined whether stress at baseline, one point in time, was associated with later changes in CRP (Barbosa-Leiker et al., Citation2014; Cohen et al., Citation1983; Elliot et al., Citation2017; Miller et al., Citation2009). This design is likely to be valid when the effect(s) of a cause take a long time (e.g. one or more years) to manifest and has the advantage of being able to determine the time sequence of exposure and outcome. The other design evaluated whether past levels of stress at a single point in time or past changes in stress over a certain period predicted current CRP (i.e. at one point in time) (McDade et al., Citation2006; Yang et al., Citation2016); however, the validity of this approach seems uncertain because of the lack of information about past CRP. None of these longitudinal studies examined both changes in perceived stress and CRP as we did in this study, which may partly explain the difference between their findings and those of the present study.
In contrast, the longitudinal design we employed is unable to establish the time sequence of exposure and outcome (e.g. changes in perceived stress and changes in CRP, in this study) because such a design examines simultaneous changes of both measures. Although the best analytical design for longitudinal data on psychosocial stress and CRP is still being debated, the Framingham heart study reported that, for each covariate (e.g. BMI), changes during follow-up are more predictive, than baseline measurements, of changes in CRP during follow-up (Fontes et al., Citation2013). Similarly, we observed that changes in BMI were more strongly associated with changes in CRP, than was baseline BMI (data not shown). It thus seems that changes in perceived stress may be more relevant, than perceived stress at baseline, to changes in CRP. In fact, we did not detect any significant association between perceived stress and CRP in the same analyses as those used in previous longitudinal studies (data not shown).
Other possible reasons for the above mentioned differences across studies include differences in the following: the methods for ascertaining perceived stress, exclusion criteria, adjustment factors, and levels of moderators. As for exclusion criteria, we removed participants with new onset of inflammation-related disease (e.g. CVD and diabetes) to avoid the influence of “reverse causation” because the development of such diseases increases CRP and may also increase perceived stress levels. The difference in adjustment factors is particularly relevant when discussing BMI, which was one of the strongest determinants of CRP. We adjusted for BMI in our study, but Yang et al. (Citation2016) did not. We found that perceived stress was inversely associated with BMI (), whereas the study by Yang et al. (Citation2016) showed a positive association between social strain and BMI. Thus, adjusting or not adjusting for BMI might have a large impact on the observed associations. In the US study (Yang et al., Citation2016), obesity was believed to mediate the positive association between stress and CRP. This suggests that certain ethnic/cultural differences in eating behaviors in response to stress (e.g. under-eating in Japan versus over-eating in the US) might underlie the difference between the two studies. Finally, the levels of particular moderators such as mastery (Infurna & Mayer, Citation2015) might account for the above differences if they differ substantially across studies. While mastery was found to moderate the association between chronic stress and CRP in a US study (Elliot et al., Citation2017), we were unable to confirm this in our results due to the lack of relevant data.
The relationship between perceived stress and CRP may vary according to baseline CRP (Wium-Andersen et al., Citation2013). In our cross-sectional study (Shimanoe et al., Citation2014), an inverse association between perceived stress and CRP was observed at baseline CRP of <3 mg/L, but not at concentrations of ≥3 mg/L. We consider that this may indicate either that CRP moderates the influence of perceived stress or that higher CRP more strongly influences reverse causation; however, we were unable to establish the clinical significance of the inverse association observed at CRP of <3 mg/L. In this longitudinal study in which reverse causation by baseline CRP was unlikely to exert a large influence, similar inverse associations between perceived stress and CRP were found for baseline CRP of <3 and 3–10 mg/L (); however, the associations were not statistically significant at the latter concentrations, probably because there were so few study participants with those concentrations. Thus, the inverse association now appears to hold true at subclinical CRP of up to 10 mg/L in participants with no clinically manifested inflammation-related disease.
A possible mechanism for the link between perceived stress and CRP is mediation by lifestyle and psychosocial factors that are correlated with CRP, such as obesity (Ranjit et al., Citation2007), smoking and physical activity (Adams et al., Citation2015; McDade et al., Citation2006), socioeconomic position (Gimeno et al., Citation2007), and coping strategies (Shimanoe et al., Citation2014). However, the male-specific inverse association remained robust after full adjustment (Model 3 in ), suggesting that other mechanisms are responsible. Although changes in concentrations of stress hormones (e.g. cortisol), which have anti-inflammatory properties (Saldanha, Tougas, & Grace, Citation1986) could play a role in the inverse association between perceived stress and CRP observed in this study, repeated elevations of cortisol are implicated in sustained/chronic systemic inflammation by the pathways through which those elevations contribute to glucocorticoid receptor resistance (Silverman & Sternberg, Citation2012). Thus, there is no known mechanism through which perceived stress could contribute to lower CRP at present. Further studies are needed to determine the biological mechanisms underlying our findings.
In the associations between coping strategies and CRP, we found a significant inverse association between emotional expression and CRP in women. To our knowledge, this is the first report on such an association between coping strategy and CRP. Emotional suppression (i.e. the reverse of “emotional expression” in this study) was associated with increased sympathetic activation (Gross, Citation1998). Norepinephrine acts on immune system cells to activate nuclear factor-κB (NF-κB). NF-κB activation in turn leads to the production of proinflammatory cytokines such as interleukin-6, which stimulates the synthesis and release of CRP (Raison et al., Citation2006). Thus, such immunological responses may mediate the relation between changes in emotional expression and CRP.
It is notable that the male-specific inverse association between changes in perceived stress and CRP was modified by a reduction in one coping strategy (positive reappraisal) or social support (); the above inverse association was not detected at diminished positive reappraisal or social support. Although there is some cross-sectional evidence for interactions between psychosocial stress and coping strategies or social support seeking on CRP (Low, Matthews, & Hall, Citation2013; Shimanoe et al., Citation2014), only one longitudinal study, the Multi-Ethnic Study, has reported that high levels of emotional social support buffer the association between high stress and CRP in middle-aged women (Mezuk, Diez Roux, & Seeman, Citation2010). As we described earlier, consistent positive associations between depression and CRP have been reported in many studies (Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimäki, Citation2015; Howren, Lamkin, & Suls, Citation2009). Thus, our finding of attenuation of the inverse association between perceived stress and CRP due to diminished coping strategies and social support might explain how harmful psychosocial stress (e.g. distress) is generated (Aschbacher et al., Citation2013; Uchino, Citation2006).
Our investigation has many strengths, including its longitudinal design, careful adjustment for potential confounders, and stringent criteria for excluding study participants to avoid reverse causation. Meanwhile, it does have the following potential limitations. First, we were unable to establish the time sequence of exposure (i.e. changes in perceived stress) and outcome (i.e. changes in CRP) as described above. Therefore, reverse causation is still possible if subclinical elevation of CRP concentrations can cause decreases in perceived stress levels; however, we believe that this possibility is unlikely. Second, we used a single question about perceived stress without information on whether perceived stress in the past year referred to chronic stress (ongoing persistent situations, such as caregiving) (Gallo et al., Citation2014) or daily stressors (minor events in everyday living, such as work pressures) (Piazza, Almeida, Dmitrieva, & Klein, Citation2010). Our measure, however, has been related to CVD risk (Iso et al., Citation2002; Nielsen et al., Citation2008), has a fair 1-year repeatability (weighted kappa = 0.55) (Shimanoe et al., Citation2014), and showed some reasonable correlations with stress-related factors (coping strategies, working hours, employment status, hours of sleep, and BMI), as shown in . Third, this study measured only CRP as a marker of systemic inflammation. Future studies should investigate the associations of psychosocial factors with overall pre- and post-neuroimmune profiles by measuring a wide range of pro- and anti-inflammatory compounds. Fourth, we could not evaluate whether our findings would hold true during shorter or longer durations of follow-up (e.g. 1 month or 10 years) because the follow-up survey was conducted only once, 5 years after the baseline survey. Finally, we are currently unable to examine whether changes in psychosocial factors (e.g. reduced stress and increased social support) are associated with the onset of inflammatory diseases (e.g. CVD and diabetes), due to a limited number of new onsets of such diseases. We intend to analyze data from this new onset cohort when a sufficient number of endpoints have accrued.
Conclusion
This study provides longitudinal evidence that increases in perceived stress during a 5-year period are associated with decreases in CRP among older healthy men, and are possibly modified by the absence of coping strategy or social support. In addition, women had an inverse association between changes in emotional expression and CRP. The paucity of relevant data indicates a need for further longitudinal studies with varying follow-up periods that carefully consider analytical designs for identifying and quantifying a longitudinal association between psychosocial stress and inflammatory markers.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Adams, S.A., Wirth, M.D., Khan, S., Murphy, E.A., Heiney, S.P., Davis, L.C., … Hébert, J.R. (2015). The association of C-reactive protein and physical activity among a church-based population of African Americans. Preventive Medicine, 77, 137–140. doi:10.1016/j.ypmed.2015.05.010
- Aschbacher, K., O'Donovan, A., Wolkowitz, O.M., Dhabhar, F.S., Su, Y., & Epel, E. (2013). Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38, 1698–1708. doi:10.1016/j.psyneuen.2013.02.004
- Barbosa-Leiker, C., Roper, V., McPherson, S., Lei, M., Wright, B., Hoekstra, T., & Kostick, M. (2014). Cross-sectional and longitudinal relationships between perceived stress and C-reactive protein in men and women. Stress and Health, 30, 158–165. doi:10.1002/smi.2507
- Bjerkeset, O., Romild, U., Smith, G.D., & Hveem, K. (2011). The associations of high levels of C-reactive protein with depression and myocardial infarction in 9258 women and men from the HUNT population study. Psychological Medicine, 41, 345–352. doi:10.1017/S0033291710000887
- Brotman, D.J., Golden, S.H., & Wittstein, I.S. (2007). The cardiovascular toll of stress. Lancet (London, England), 370, 1089–1100. doi:10.1016/S0140-6736(07)61305-1
- Carver, C.S. (1997). You want to measure coping but your protocol's too long: Consider the brief COPE. International Journal of Behavioral Medicine, 4, 92–100. doi:10.1207/s15327558ijbm0401_6
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. doi:10.2307/2136404
- Cohen, S., & Wills, T.A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98, 310–357. doi:10.1037/0033-2909.98.2.310
- Douglas, K.M., Taylor, A.J., & O’Malley, P.G. (2004). Relationship between depression and C-reactive protein in a screening population. Psychosomatic Medicine, 66, 679–683. doi:10.1097/01.psy.0000138132.66332.85
- Elliot, A.J., Mooney, C.J., Infurna, F.J., & Chapman, B.P. (2017). Associations of lifetime trauma and chronic stress with C-reactive protein in adults ages 50 years and older: Examining the moderating role of perceived control. Psychosomatic Medicine, 79, 622–630. doi:10.1097/PSY.0000000000000476
- Fontes, J.D., Yamamoto, J.F., Larson, M.G., Wang, N., Dallmeier, D., Rienstra, M., … Benjamin, E.J. (2013). Clinical correlates of change in inflammatory biomarkers: The Framingham heart study. Atherosclerosis, 228, 217–223. doi:10.1016/j.atherosclerosis.2013.01.019
- Fukahori, H., Matsui, N., Mizuno, Y., Yamamoto-Mitani, N., Sugai, Y., & Sugishita, C. (2007). Factors related to family visits to nursing home residents in Japan. Archives of Gerontology and Geriatrics, 45, 73–86. doi:10.1016/j.archger.2006.10.001
- Gallo, L.C., Roesch, S.C., Fortmann, A.L., Carnethon, M.R., Penedo, F.J., Perreira, K., … Isasi, C.R. (2014). Associations of chronic stress burden, perceived stress, and traumatic stress with cardiovascular disease prevalence and risk factors in the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. Psychosomatic Medicine, 76, 468–475. doi:10.1097/PSY.0000000000000069
- Gimeno, D., Brunner, E.J., Lowe, G.D., Rumley, A., Marmot, M.G., & Ferrie, J.E. (2007). Adult socioeconomic position, C-reactive protein and interleukin-6 in the Whitehall II prospective study. European Journal of Epidemiology, 22, 675–683. doi:10.1007/s10654-007-9171-9
- Gimeno, D., Kivimäki, M., Brunner, E.J., Elovainio, M., De Vogli, R., Steptoe, A., … Ferrie, J.E. (2009). Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-Year follow-up of the Whitehall II study. Psychological Medicine, 39, 413–423. doi:10.1017/S0033291708003723
- Goldberg, D.P., & Hillier, V.F. (1979). A scaled version of the General Health Questionnaire. Psychological Medicine, 9, 139–145. doi:10.1017/S0033291700021644
- Gross, J.J. (1998). Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74, 224–237. doi:10.1037/0022-3514.74.1.224
- Gu, H.-F., Tang, C.-K., & Yang, Y.-Z. (2012). Psychological stress, immune response, and atherosclerosis. Atherosclerosis, 223, 69–77. doi:10.1016/j.atherosclerosis.2012.01.021
- Haapakoski, R., Mathieu, J., Ebmeier, K.P., Alenius, H., & Kivimäki, M. (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity, 49, 206–215. doi:10.1016/j.bbi.2015.06.001
- Hara, M., Higaki, Y., Imaizumi, T., Taguchi, N., Nakamura, K., Nanri, H., … Tanaka, K. (2010). Factors influencing participation rate in a baseline survey of a genetic cohort in Japan. Journal of Epidemiology, 20, 40–45. doi:10.2188/jea.JE20090062
- Hara, M., Shimanoe, C., Otsuka, Y., Nishida, Y., Nanri, H., Horita, M., … Tanaka, K. (2015). Factors associated with non-participation in a face-to-face second survey conducted 5 years after the baseline survey. Journal of Epidemiology, 25, 117–125. doi:10.2188/jea.JE20140116
- Hogan, B.E., Linden, W., & Najarian, B. (2002). Social support interventions: Do they work? Clinical Psychology Review, 22, 381–440. doi:10.1016/S0272-7358(01)00102-7
- Howren, M.B., Lamkin, D.M., & Suls, J. (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine, 71, 171–186. doi:10.1097/PSY.0b013e3181907c1b
- Infurna, F.J., & Mayer, A. (2015). The effects of constraints and mastery on mental and physical health: Conceptual and methodological considerations. Psychology and Aging, 30, 432–448. doi:10.1037/a0039050
- Iso, H., Date, C., Yamamoto, A., Toyoshima, H., Tanabe, N., Kikuchi, S., … Ohno, Y. (2002). Perceived mental stress and mortality from cardiovascular disease among Japanese men and women: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk Sponsored by Monbusho (JACC Study). Circulation, 106, 1229–1236. doi:10.1161/01.CIR.0000028145.58654.41
- Johnson, T.V., Abbasi, A., & Master, V.A. (2013). Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Molecular Diagnosis & Therapy, 17, 147–164. doi:10.1007/s40291-013-0026-7
- Lazarus, R.S. (1985). The psychology of stress and coping. Issues in Mental Health Nursing, 7, 399–418. doi:10.3109/01612848509009463
- Low, C.A., Matthews, K.A., & Hall, M. (2013). Elevated C-reactive protein in adolescents: Roles of stress and coping. Psychosomatic Medicine, 75, 449–452. doi:10.1097/PSY.0b013e31828d3f1d
- McDade, T.W., Hawkley, L.C., & Cacioppo, J.T. (2006). Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago health, aging, and social relations study. Psychosomatic Medicine, 68, 376–381. doi:10.1097/01.psy.0000221371.43607.64
- Mezuk, B., Diez Roux, A.V., & Seeman, T. (2010). Evaluating the buffering vs. direct effects hypotheses of emotional social support on inflammatory markers: The multi-ethnic study of atherosclerosis. Brain, Behavior, and Immunity, 24, 1294–1300. doi:10.1016/j.bbi.2010.06.006
- Miller, G.E., Rohleder, N., & Cole, S.W. (2009). Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosomatic Medicine, 71, 57–62. doi:10.1097/PSY.0b013e318190d7de
- Nanri, H., Nakamura, K., Hara, M., Higaki, Y., Imaizumi, T., Taguchi, N., … Tanaka, K. (2011). Association between dietary pattern and serum C-reactive protein in Japanese men and women. Journal of Epidemiology, 21, 122–131. doi:10.2188/jea.JE20100110
- Nielsen, N.R., Kristensen, T.S., Schnohr, P., & Grønbaek, M. (2008). Perceived stress and cause-specific mortality among men and women: Results from a prospective cohort study. American Journal of Epidemiology, 168, 481–491. doi:10.1093/aje/kwn157
- O’Connor, M.-F., Bower, J.E., Cho, H.J., Creswell, J.D., Dimitrov, S., Hamby, M.E., … Irwin, M.R. (2009). To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity, 23, 887–897. doi:10.1016/j.bbi.2009.04.005
- Pepys, M.B. (1981). C-reactive protein fifty years on. Lancet (London, England), 1, 653–657.
- Piazza, J.R., Almeida, D.M., Dmitrieva, N.O., & Klein, L.C. (2010). Frontiers in the use of biomarkers of health in research on stress and aging. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 65, 513–525. doi:10.1093/geronb/gbq049
- Raison, C.L., Capuron, L., & Miller, A.H. (2006). Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology, 27, 24–31. doi:10.1016/j.it.2005.11.006
- Ranjit, N., Diez-Roux, A.V., Shea, S., Cushman, M., Seeman, T., Jackson, S.A., & Ni, H. (2007). Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Archives of Internal Medicine, 167, 174–181. doi:10.1001/archinte.167.2.174
- Ridker, P.M., Cushman, M., Stampfer, M.J., Tracy, R.P., & Hennekens, C.H. (1998). Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation, 97, 425–428. doi:10.1161/01.CIR.97.5.425
- Rosengren, A., Hawken, S., Ôunpuu, S., Sliwa, K., Zubaid, M., Almahmeed, W.A., … INTERHEART investigators. (2004). Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet, 364, 953–962. doi:10.1016/S0140-6736(04)17019-0
- Ruspini, E. (2002). Introduction to longitudinal research. London: Routledge.
- Saldanha, C., Tougas, G., & Grace, E. (1986). Evidence for anti-inflammatory effect of normal circulating plasma cortisol. Clinical and Experimental Rheumatology, 4, 365–366.
- Sasaki, M., Kitaoka-Higashiguchi, K., Morikawa, Y., & Nakagawa, H. (2009). Relationship between stress coping and burnout in Japanese hospital nurses. Journal of Nursing Management, 17, 359–365. doi:10.1111/j.1365-2834.2008.00960.x
- Sasaki, M., & Yamasaki, K. (2002). Development of a dispositional version of the General Coping Questionnaire (GCQ) and examination of its reliability and validity. Nihon Koshu Eisei Zasshi, 49, 399–408. [in Japanese].
- Shimanoe, C., Otsuka, Y., Hara, M., Nanri, H., Nishida, Y., Nakamura, K., … Tanaka, K. (2014). Gender-specific associations of perceived stress and coping strategies with C-reactive protein in middle-aged and older men and women. International Journal of Behavioral Medicine, 21, 821–832. doi:10.1007/s12529-013-9341-y
- Shivpuri, S., Gallo, L.C., Crouse, J.R., & Allison, M.A. (2012). The association between chronic stress type and C-reactive protein in the multi-ethnic study of atherosclerosis: Does gender make a difference? Journal of Behavioral Medicine, 35, 74–85. doi:10.1007/s10865-011-9345-5
- Silverman, M.N., & Sternberg, E.M. (2012). Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences, 1261, 55–63. doi:10.1111/j.1749-6632.2012.06633.x
- Uchino, B.N. (2006). Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29, 377–387. doi:10.1007/s10865-006-9056-5
- von Känel, R., Mausbach, B.T., Dimsdale, J.E., Mills, P.J., Patterson, T.L., Ancoli-Israel, S., … Grant, I. (2012). Ways of coping and biomarkers of an increased atherothrombotic cardiovascular disease risk in elderly individuals. Cardiovascular Psychiatry and Neurology, 2012, 1. doi: 10.1155/2012/875876.
- Wium-Andersen, M.K., Ørsted, D.D., Nielsen, S.F., & Nordestgaard, B.G. (2013). Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry, 70, 176–184. doi:10.1001/2013.jamapsychiatry.102
- Yang, Y.C., Boen, C., Gerken, K., Li, T., Schorpp, K., & Harris, K.M. (2016). Social relationships and physiological determinants of longevity across the human life span. Proceedings of the National Academy of Sciences of the United States of America, 113, 578–583. doi:10.1073/pnas.1511085112