Abstract
Experience of adversity early in life and dysregulation of hypothalamus–pituitary–adrenocortical (HPA) axis activity are risk factors often independently associated with the development of psychopathological disorders, including depression, PTSD and pathological aggression. Additional evidence suggests that in combination these factors may interact to shape the development and expression of psychopathology differentially, though little is known about underlying mechanisms. Here, we studied the long-term consequences of early life stress exposure on individuals with differential constitutive glucocorticoid responsiveness to repeated stressor exposure, assessing both socio-affective behaviors and brain activity in regions sensitive to pathological alterations following stress. Two rat lines, genetically selected for either low or high glucocorticoid responsiveness to repeated stress were exposed to a series of unpredictable, fear-inducing stressors on intermittent days during the peripuberty period. Results obtained at adulthood indicated that having high glucocorticoid responses to repeated stress and having experience of peripuberty stress independently enhanced levels of psychopathology-like behaviors, as well as increasing basal activity in several prefrontal and limbic brain regions in a manner associated with enhanced behavioral inhibition. Interestingly, peripuberty stress had a differential impact on aggression in the two rat lines, enhancing aggression in the low-responsive line but not in the already high-aggressive, high-responsive rats. Taken together, these findings indicate that aberrant HPA axis activity around puberty, a key period in the development of social repertoire in both rats and humans, may alter behavior such that it becomes anti-social in nature.
Introduction
Experience of adversity in childhood and adolescence is a major risk factor in the development of psychopathological disorders later in life (Caspi & Moffitt, Citation2006; Heim & Binder, Citation2012; Nugent, Tyrka, Carpenter, & Price, Citation2011; Widom & Maxfield, Citation1996). However, not all individuals experiencing early adversity go on to develop psychopathology and increased understanding of the factors underlying this variability would assist in the identification and treatment of vulnerable individuals. A growing number of findings indicate that having a differential physiological sensitivity to stress may be a factor that modulates the outcome of early life stress exposure (Bevilacqua et al., Citation2012; Binder et al., Citation2008; Bryushkova et al., Citation2016; Klengel et al., Citation2013).
Exposure of an individual to a stressful challenge is characterized by a response that includes metabolic, behavioral and physiological components, and involves the activation and interaction of several neuro-physiological systems (McEwen, Citation1998). The consequences of the stress response are generally adaptive in the short term, restoring homeostasis and mediating adaptation following cessation of the stressor. However, repeated, prolonged or inadequate stress responses may eventually lead to physiological damage, thought to form the pathological basis of stress-related disorders (McEwen, Citation2007).
Glucocorticoids exert a multitude of peripheral and central effects, both genomic and non-genomic, via their actions upon mineralocorticoid (MR) and glucocorticoid (GR) receptors (de Kloet, Citation2014; Joels, Pasricha, & Karst, Citation2013). Activation of GR in several brain areas acts to inhibit continuation of the stress response, with this negative feedback acting via both fast and slow mechanisms (Joels et al., Citation2013). A common adaptation of the hypothalamus–pituitary–adrenocortical (HPA) axis in response to repeated exposure to the same stressor in humans is a reduction of the glucocorticoid response across repeated stress exposures (Federenko, Nagamine, Hellhammer, Wadhwa, & Wust, Citation2004; Gerra et al., Citation2001), thought to minimize the impact of frequently experienced stressors. Rats also demonstrate this adaptation (though see (Rabasa et al., Citation2015)), as well as individual differences in the degree of adaptation (Walker, Zanoletti, Guillot de Suduiraut, & Sandi, Citation2017).
In outbred rats, previous studies found that exposure to peripuberty stress increases the expression of anxiety-like, depression-like and aggressive behaviors, in concert with behaviorally-consequent shifts in activation of brain regions important in socio-emotional function (Marquez et al., Citation2013; Tzanoulinou, Garcia-Mompo, et al., Citation2014; Tzanoulinou, Riccio, et al., Citation2014). Here, we have used two genetically-selected lines of rats showing differential glucocorticoid responsiveness (i.e. low or high) to repeated exposure to stressors (Walker et al., Citation2017) to assess whether exposure to stress during the peripuberty period would have a different neurobehavioral outcome in the two lines. At the behavioral level, we assessed anxiety-like, depression-like, and aggressive behaviors at adulthood. At the neurobiological level, we examined basal activity in different brain areas, including subregions of the prefrontal cortex, amygdala, and hippocampus. The selection of these brain regions was motivated by (i) their ongoing development during adolescence (Casey, Jones, & Hare, Citation2008; Spear, Citation2000); and previous work indicating that (ii) they show enhanced activity in peripubertally stressed rats (Marquez et al., Citation2013), as well as (iii) altered resting-state activation in stress-related psychopathologies (Koch et al., Citation2016; New et al., Citation2009).
Materials and methods
Subjects
Experimental animals were male offspring from the sixth generation of recently developed lines of differentially stress reactive rats (Walker et al., Citation2017). Male and female Wistar Han rats, acting as intruders and as cohabitating partners, respectively, were purchased from a commercial breeder (Janvier, Le Genest-Saint-Isle, France).
Experimental animals were derived from 10 to 12 litters. At weaning on postnatal day (p) 21, pairs of male rats from different litters were matched according to weight and mixed among home cages. Animals assigned to the same experimental group were housed together. The stress protocol was applied over seven intermittent days between p28 and p42. Rats then remained undisturbed, except for weekly cage changes, until experimental procedures began at adulthood (designated as p90). Rats were handled three times before the first behavioral test.
Rats were maintained on a 12-h light–dark cycle (lights on at 07:00 h), in a temperature- and humidity-controlled environment (21 ± 1 °C; 55% humidity ±5%), with ad libitum access to laboratory chow and water. Experiments were performed between 08:00 and 12:00 h, except where otherwise stated, with at least one week between each test. All procedures were conducted in accordance with the Swiss National Institutional Guidelines on Animal Experimentation and approved by a license from the Swiss Cantonal Veterinary Office Committee for Animal Experimentation.
Experimental design
A 2 × 2 factorial design was used: two genetically-selected breeding lines (low and high corticosterone in response to repeated stress, hereafter named low- and high-line, respectively) were exposed to one of two treatment conditions (peripubertal stress [PPS] or control-handling [control]), resulting in four experimental groups. Assessment of anxiety-like behavior on the elevated plus maze (EPM) was performed (n = 22/group). Subsequently, n = 8/group went forward for measurement of depression-like behavior, followed by measurement of brain energy metabolism using 2-deoxyglucose (2-DG) autoradiography. An additional n = 12/group was assessed for aggression using the resident–intruder test.
Peripuberty stress protocol
The stress protocol was performed as previously described (Marquez et al., Citation2013). Following exposure to an open field (50 × 50 × 30 cm) for five minutes on p28, the stress protocol consisted of the presentation of two different stressors, each one lasting 25 minutes (see ). Stressors were: exposure to the synthetic fox odor trimethylthiazoline (TMT; Phero Tech Inc., Delta, Canada) or exposure to an elevated platform (EP). Rats were exposed to TMT via a scent-charged cloth in a brightly lit (250 lx) plastic box (38 × 27.5 × 31 cm). The EP (12 × 12 cm, elevated 95 cm from the ground) was also located under direct bright light (500 lx).
Figure 1. Experimental design. Animals were weaned at post-natal day (p)21 and assigned to control or peripuberty stress groups. The stress protocol consisted of exposure to an open field (OF) and elevated platform (EP) on p28, followed by intermittent, variable exposure to an EP and predator odor (trimethylthiazoline, TMT) until p42. Control animals were handled briefly on the days on which their experimental counterparts were exposed to stress. Behavioral testing started at p90, with a minimum delay of one week imposed between tests. 2-DG: 2-deoxyglucose.
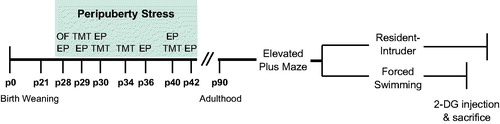
The stressors were applied during peripuberty, on seven intermittent days between p28 and p42, following a variable schedule. To assess the effect of stress exposure on HPA axis activity, blood samples were taken on p28, p30, and p42, once at the offset of stress (response sample) and again 30 minutes later (recovery sample). Blood samples were collected by tail nick. Briefly, rats were wrapped in a cloth and, within 1 min, up to 100 μl of blood was collected from a small incision made in one of the tail arteries. Same-day samples were collected within a short interval and the second collection was therefore obtained from the same incision. Control rats underwent brief handling on stress days, no blood samples were taken.
All blood samples were collected into ice-cold heparin-coated capillary tubes (Sarstedt, Sevelen, Switzerland) and chilled until centrifugation (10,000 rpm at 4 °C for 4 min). Plasma was collected into new tubes and stored at −20 °C until subsequent analysis.
Behavioral procedures
Elevated plus maze
Anxiety-like behavior was evaluated using the EPM test. The apparatus consisted of two opposing open arms (50 × 10 cm) perpendicular to two enclosed arms (50 × 10 × 50 cm), extending from a central platform (10 × 10 cm) and elevated 65 cm above the floor. Light levels were maintained at 14–16 lx on the open arms and 5–7 lx on the closed arms. At the start of the test, the rat was placed on the central platform facing a closed arm and allowed to explore the maze for five minutes. In between animals, the apparatus was cleaned with 5% ethanol solution and dried. Behavior was monitored using a ceiling-mounted video camera and analyzed with a computerized tracking system (Ethovision 9; Noldus IT, Wageningen, Netherlands). The time spent in the open and closed arms, and distance moved, were recorded. Two rats fell from the maze during the testing period and were therefore excluded from the analysis (one “Low PPS” rat and one “High Control” rat).
Forced-swimming test
Rats were submitted to a forced-swimming test (FST) to evaluate depression-like behavior. Animals were placed in a plastic beaker (25 cm diameter × 46 cm depth) containing 30 cm of water (25 °C) for 15 minutes. A second session was performed 24 h later for five minutes. Both sessions were recorded using a ceiling mounted video camera, and the time spent immobile (making only those movements necessary to keep the snout above the water), swimming or climbing was quantified manually with the aid of in-house software (Clicker; EPFL, Lausanne, Switzerland) by an experimenter blind to experimental group.
Resident–intruder test
Prior to the night of the resident–intruder test, experimental rats cohabited with a female partner for 10 days to encourage territoriality. The female was removed 30 minutes before the onset of the test, and replaced following the end of the test. The test was performed during the beginning of the dark cycle (between 19:00 and 22:00 h). The resident was exposed in its home cage to a smaller (5–10% lighter), unfamiliar male intruder of the same strain for 30 minutes. Each intruder was used only once. Encounters were video-recorded and scored offline by an experimenter blind to the experimental group, assisted by Observer software (Noldus IT, Wageningen, Netherlands). The following parameters were quantified in terms of frequency and duration: offensive upright, lateral threat, keeping down, attack, biting, social investigation, nonsocial investigation and auto-grooming. The cumulative duration of the first four behaviors was summed to provide a measure of total offensive behavior. Latency to the first offensive event initiated by the resident was also recorded. To determine holistically the aggressiveness of each rat, aggression z scores were calculated from raw scores of variables described above. Specifically, total offensive behavior, frequency of attacks, and latency to offend were integrated to derive a single aggression score. This approach has been validated in other studies aimed at differentiation of individual differences in behavioral response to traumatic stress (Ritov, Boltyansky, & Richter-Levin, Citation2016).
Rats are a highly social species and, as such, have developed stereotyped patterns of social interaction, such as intention signaling and submissive posturing, that allow for the resolution of territorial and hierarchical conflicts with minimal injury to both parties (Koolhaas et al., Citation2013). Here, forms of attack performed by the resident which fell outside of species-specific norms of interaction were noted during scoring and collated. Specifically, the targeting of bites to vulnerable body parts (i.e. the head, genitals or underbelly), absence of signaling of intent to attack, and persistence of an attack in the face of submission by the intruder were all considered to engender “abnormal” forms of aggression (Haller, Citation2013).
Brain energy metabolism
One week after testing in the FST, animals (n = 8) were injected intraperitoneally with 14C-2-deoxy-d-glucose (165 μCi/kg; Hartmann Analytic, Braunschweig, Germany) 45 minutes before being sacrificed via decapitation in order to evaluate brain glucose metabolism under basal conditions. Brains were removed and flash frozen in isopentane chilled to −45 °C, and subsequently stored at −80 °C until further processing.
Coronal sections (20 μm thick) were sampled on a cryostat. One out of six sections within several pre-determined regions of interest was collected on Superfrost slides. The selected ROIs fulfilled the following criteria: (i) subject to ongoing development during adolescence (Casey et al., Citation2008; Spear, Citation2000); (ii) previously found to show metabolic changes following PPS (Marquez et al., Citation2013); (iii) found to show altered resting-state activation in stress-related psychopathologies (Koch et al., Citation2016; New et al., Citation2009). Prefrontal cortex, hippocampus, and amygdala met all these criteria.
Slides were processed for autoradiography along with a calibrated 14C‐microscale on XAR‐5 Kodak Biomax MR autoradiography film (Sigma‐Aldrich, Buchs, Switzerland) for three days. After developing the films, the slides were counterstained with 0.2% cresyl violet acetate (Sigma-Aldrich, Buchs, Switzerland), dehydrated through increasing concentrations of ethanol, cleared in xylene, and coverslipped with DPX (Sigma, Buchs, Switzerland) to provide a histological control.
Images from the 2‐DG XAR films were obtained with a digital camera and aligned with the corresponding Nissl‐stained images to allow for structure identification using MCID Core TM 7.0 software (MCID, Swanscombe, UK). Uptake of 2-DG was assessed via densitometric analysis of the XAR films. Briefly, images were calibrated with 14C standard curves, the regions of interest were delineated manually by an assistant blind to the experimental group and the optical densities were calculated for these regions. The 2‐DG uptake was normalized to that of the whole slice, which did not vary between the experimental groups, to control for potential differences in film exposure. Data were averaged from three sections, including both hemispheres, per region of interest. Analyses of 2-DG expression in the various regions of interest were conducted by another researcher, also blind to the experimental condition.
Corticosterone measurement
Measurements of total corticosterone were obtained from all blood plasma samples (dilution 1/40), via use of an enzymatic immunoassay kit performed according to manufacturer’s instructions (Enzo Life Sciences, Lausen, Switzerland). Levels were calculated using a standard curve method. Specificity of the assay, as indexed by cross reactivity, was 100% for corticosterone, 28.6% for deoxycorticosterone, 1.7% for progesterone, and less than 0.3% for all other steroids measured. The intra- and inter-assay coefficients of variation were both below 10%.
Statistics
Data were analyzed using SPSS 17.0 (Chicago, IL). Results are presented as the mean ± SEM. A mixed three-way repeated measures ANOVA was used to analyze measurements of corticosterone concentration obtained from plasma taken across the stress protocol, with selection line as the between-subjects factor and postnatal day and sample timepoint as the within-subjects factors. Significant three-way interaction was broken down by sample timepoint, and mixed two-way repeated measures ANOVAs were performed. Significant two-way interactions were broken down by postnatal day and differences between selection lines explored using independent samples t-tests. To control for familywise error rate, Bonferroni’s correction was applied. For within-subjects ANOVA, where sphericity assumptions were violated, the Greenhouse–Geisser correction was applied.
All other variables were analyzed using two-way ANOVA, with selection line and treatment as between-subjects factors. To permit this, data transformations (shown in brackets) were performed on the following behavioral parameters to produce a normal distribution of data: offensive behavior % (arcsine); attack frequency and latency to offend (log10). Significant interactions were broken down by line and explored using independent samples t-tests, with treatment group as the between-subjects factor. Behavioral variables were correlated with basal brain activity using Pearson’s correlation. Statistical significance level was set at p < .05.
Results
Constitutive differences in stress responsiveness are reflected in differential corticosterone response across repeated exposures to stress during the peripubertal period
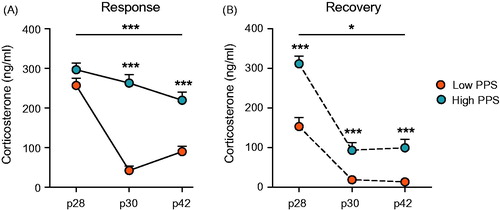
Analysis of plasma corticosterone concentration from samples obtained at various timepoints (i.e. at 0 and 30 min after stress exposure on p28, p30, and p42) across the PPS protocol revealed a significant interaction between selection line, postnatal day and sampling timepoint (three-way RM ANOVA: F(2,82) = 22.38, p < .001). When broken down by sampling timepoint, analysis revealed that corticosterone response to stress differed between lines across days not only in the response sample (i.e. taken immediately after exposure to stressors; two-way RM ANOVA: F(2,84) = 17.61, p < .001) but also in the recovery sample (i.e. taken 30 min after exposure to stressors; two-way RM ANOVA: F(2, 82) = 3.76, p = .036, ε = 0.83). Lines were originally selected according to corticosterone response adaptation to repeated stress rather than magnitude of response at first stressor (Walker et al., Citation2017). As expected therefore, low- and high-line rats did not differ in their corticosterone response upon first stressor exposure (p28: t(42) = −1.58, p = .122) but did differ when sampled at the offset of stress on the third (p30: t(42) = −9.31, p < .001) and final days of the protocol (p42: t(42) = −5.35, p < .001). Low-line rats demonstrated significantly lower corticosterone levels than high-line rats in response to both of these later stress exposures. Similar differences between lines were observed in the recovery plasma sample, though in this case low-line rats also had significantly lower corticosterone levels (i.e. quicker recovery) at this timepoint on the first day of stress exposure (p28: t(41) = −5.31, p < .001), as well as on the other days sampled (p30: t(42) = −3.78, p < .001; p42: t(42) = −4.11, p < .001).
Figure 2. Corticosterone response to repeated stressor exposure across days during the peripubertal period differed in accordance with selection line. Low- and high-line rats did not differ in their response to a first exposure to stress on p28. Thereafter, low-line rats showed habituation of the corticosterone response to stressors on p30 and p42, an effect attenuated in high-line rats (A). These differences were reflected by corticosterone concentration in a second plasma sample, taken 30 minutes after the offset of stress (B). In addition to strong habituation across stressors, low-line rats showed accelerated recovery of the HPA axis following the first stress session on postnatal (p) day 28 (two-way RM ANOVA: interaction is represented by an uncapped line; independent sample t-test results are above each timepoint; *p < .05, ***p < .001; see text for details).
Exposure to peripuberty stress gives rise to dissociable alterations in psychopathology-like behaviors between the selected lines
Elevated plus maze
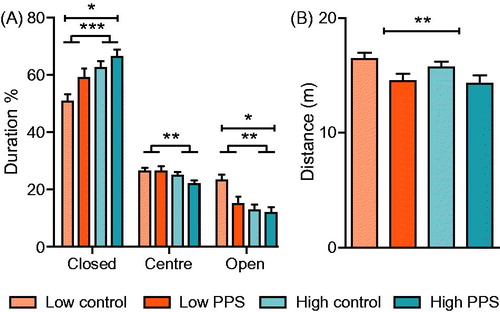
We first evaluated whether rats exposed to peripuberty stress demonstrated differential behavioral responses to the EPM when compared to their respective control groups. Irrespective of stress experience, high-line rats spent more time in the protected, closed arm of the maze than low-line rats ( main effect of line, closed arm: F(1,82) = 11.83, p < .001). Accordingly, they also spent less time on the open arms (main effect of line: F(1,82) = 9.48, p = .003) and less time in the center square (main effect of line, center: F(1,82) = 3.96, p = .005) when compared to low-line rats. Exposure to peripuberty stress led to enhanced anxiety-like behavior, irrespective of line. Specifically, stressed rats spent more time in the closed arms (main effect of treatment: F(1,82) = 4.88, p = .030), and less time on the open arms of the maze (main effect of treatment: F(1,82) = 4.52, p = .037). The interaction between line and peripuberty stress exposure on the time spent in the open arms did not reach statistical significance (line*treatment: F(1,82) = 2.71, p = .104). In order to account for potential type II errors due to low sample size, we performed an analysis of simple effects. Results indicated that time spent in the open arms of the EPM was increased in low-line rats exposed to peripuberty stress (F(1,82) = 7.11, p = .009) but not in high-line rats exposed to the same stress (F(1,82) = 0.12, p = .735). In addition to influence on the time spent exploring certain zones of the maze, peripubertally stressed rats showed a reduction in the distance traveled on the maze (main effect of treatment: F(1,82) = 7.66, p = .007).
Figure 3. Anxiety-like behavior on the elevated plus maze. Low-line rats spent less time in the closed arm of the maze, and more time on the open arm when compared to high-line rats (A). Following exposure to peripuberty stress (PPS), rats spent more time in the closed arm and less time in the open arm of the maze. Additionally, stressed rats showed a decrement in locomotion in terms of distance covered on the maze (B) in comparison to non-stressed controls (two-way ANOVA: main effect of treatment is represented by a capped line; main effect of line is represented by a line with feet; *p < .05; **p < .01; ***p < .001; see text for details).
Forced-swimming test
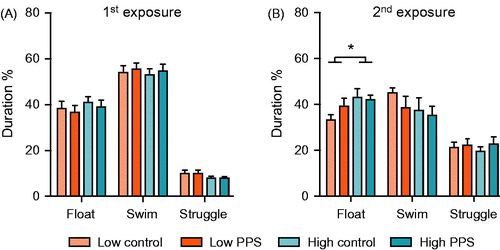
To assess animals’ coping (i.e. depression-like) behaviors, we exposed a subset of rats (n = 8/group) to a two-day forced-swimming procedure. Lines did not differ in behavioral response to the first episode of forced swimming (). Irrespective of stress experience, high-line rats spent more time floating on the second exposure to the water than low-line rats ( main effect of line: F(1,28) = 4.27, p = .048). No differences between lines were found in amount of time swimming or struggling (main effect of line, swimming: F(1,28) = 1.60, p = .205; main effect of line, struggling: F(1,28) = 0.51, p = .768). Peripuberty stress experience did not independently influence any aspect of behavioral response, to forced swimming (main effect of treatment, floating: F(1,28) = 0.64, p = .430; main effect of treatment, swimming: F(1,28) = 0.99, p = .329; main effect of treatment, struggling: F(1,28) = 0.64, p = .430), nor did it appear to interact with selection line in terms of floating (line*treatment: F(1,28) = 1.27, p = .270), swimming (line*treatment: F(1,28) = 0.24, p = .630) or struggling behavior (line*treatment: F(1,28) = 0.17, p = .683). In order to account for potential type II errors due to low sample size, we performed an analysis of simple effects. Results indicated that time spent in floating in the FST was not increased in low- or high-line rats following exposure to peripuberty stress (low: F(1,28) = 1.86, p = .184; high: F(1,28) = 0.05, p = .821).
Figure 4. Depression-like behavior in the forced-swimming test. Neither selection line, nor prior exposure to peripuberty stress (PPS), influenced behavioral coping response to the first exposure to forced-swimming (A). When re-exposed to this stressor on the following day, compared with low-line rats, high-line rats spent significantly more time engaged in passive coping, as indexed by time spent floating (B) (two-way ANOVA: main effect of line; *p < .05; see text for further details).
Resident–intruder test
In a second subset of rats (n = 12/group), we evaluated the influence of line and peripuberty stress on aggression in the resident–intruder test. In terms of total duration spent engaged in offensive behavior, we found higher levels of aggression in high-line rats relative to low-line rats ( main effect of line: F(1,44) = 8.90, p = .005). A similar effect was found in the relative frequency of attacks, whereby high-line rats attacked the intruder more times than low-line rats ( main effect of line: F(1,44) = 4.98, p = .031). In terms of readiness to perform a first offensive action, we found a variable influence of selection line and stress exposure on behavior ( line*treatment: F(1,44) = 4.39, p = .042). Experience of peripuberty stress did not alter the already short latency of high-line rats to initiate attacks (t-test: t(22) = −1.00, p = .328), whereas it tended to shorten the time taken for low-line rats to do so (t-test: t(22) = 1.84, p = .080).
Figure 5. Aggression in the resident–intruder test. Compared to low-line rats, high-line rats spent a greater duration of the test engaged in offensive behaviors (A) and attacked more frequently (C). There was an interaction between selection line and experience of peripuberty stress (PPS) in terms of readiness to engage in offensive behavior (C), with low-line PPS rats tending to be quicker to attack than low-line controls. When these three measures were combined (D), an interaction between selection line and treatment revealed that PPS exposure increased the overall aggressiveness of low-line rats, while not altering relative aggressiveness of high-line rats. Low-line rats were more likely to perform abnormal forms of aggression (E; diagonally striped bars), the proportion of rats performing “normal” forms of attack was similar between groups (solid bars) (two-way ANOVA: main effect of line is represented by a line with feet; post hoc t-tests by a narrow, uncapped line; *p < .05; **p < .01; see text for further details).
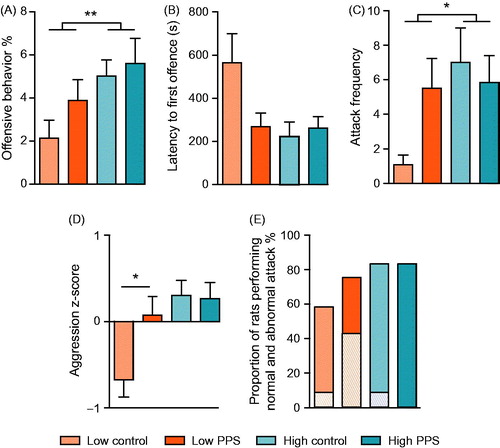
To take a more holistic view of the aggressiveness of each rat, we derived a composite z score from the variables outlined above. Analysis of the aggression score showed that, irrespective of early life experience, high-line rats were more aggressive than low-line rats ( main effect of line: F(1,44) = 9.13, p = .004). Interestingly, exposure to peripuberty stress had differential effects on the aggressiveness of the different selection lines (line*treatment: F(1,44) = 4.10, p = .049). Peripuberty stress did not significantly alter the already pronounced levels of aggression in high-line rats (t-test: t(22) = 0.14, p = .890) but significantly enhanced the aggressiveness of the non-aggressive low-line rats (t-test: t(22) = −2.57, p = .018).
Additionally, we assessed the propensity of animals from each experimental group to perform abnormal forms of aggression including: targeting of bites to vulnerable body parts, failure to signal intent to attack, and continued attack despite clear signaling of submission by the intruder. The percentages of rats from each group performing any attack were as follows: Low control: 58%; Low PPS: 75%; High control: 83%; High PPS: 83%. The percentages of rats from each group performing aberrant forms of attack were as follows: Low control: 8%; Low PPS: 42%; High control: 8%; High PPS: 0% (). Low-line rats exposed to peripuberty stress were thus more likely to engage in this atypical form of behavior than animals from any other experimental group.
Selection line and peripuberty stress exposure are associated with differences in brain metabolism under basal conditions
To interrogate potential neurobiological correlates of variation in psychopathology-like behaviors, basal brain energy metabolism was studied via 2-DG autoradiography (). We focused our analyses on several predefined regions of interest, specifically: prefrontal cortex, dorsal hippocampus and amygdala.
Figure 6. Quantification of brain activity under basal conditions, as indexed by uptake of 2-deoxyglucose (2-DG), in stress-sensitive limbic brain regions. In ventral and medial subdivisions of the orbitofrontal cortex (A), relative to low-line rats, high-line rats had enhanced 2-DG uptake. Moreover, PPS exposed rats showed enhanced 2-DG uptake relative to controls in those same regions. This pattern was mirrored in the prelimbic division of the medial prefrontal cortex (B) but not in other subregions. In hippocampus (C), high-line rats again had higher 2-DG uptake than low-line rats. Levels of prelimbic cortex activity under basal conditions were associated with locomotion on the EPM (D–F) (two-way ANOVA: main effect of treatment is represented by a capped line, main effect of line by a line with feet; *p < .05; ***p < .001; see text for details). FST: forced-swimming test; EPM: elevated plus maze; vOFC: ventral orbitofrontal cortex; mOFC: medial orbitofrontal cortex; PLCx: prelimbic cortex; BLA: basolateral amygdala; CeA: central nucleus of the amygdala; MeA: medial nucleus of the amygdala.
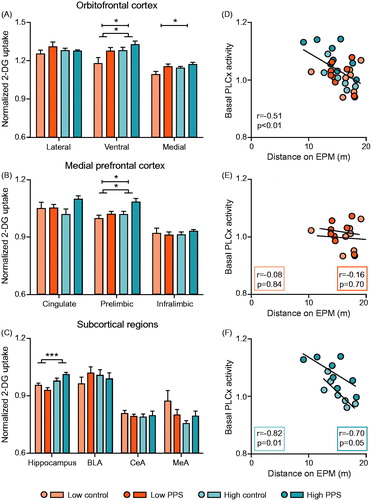
In the ventral subdivision of the orbitofrontal cortex, there was an effect of both selection line and stress experience in brain activity. Specifically, in comparison to low-line rats, high-line rats showed greater uptake of 2-DG ( main effect of line: F(1,28) = 5.36, p = .028). Additionally, groups exposed to stress during peripuberty showed increased uptake of 2-DG relative to control groups (main effect of treatment: F(1,28) = 4.86, p = .036), though interaction between selection line and stress was not evident (line*treatment: F(1,28) = 0.60, p = .445). A similar pattern of findings was observed in the medial subdivision of orbitofrontal cortex (main effect of line: F(1,28) = 2.93, p = .098; main effect of treatment: F(1,28) = 5.76, p = .023; line*treatment: F(1,28) = 0.76, p = .390) but not in the lateral subdivision (data not shown). Caudally, in medial prefrontal subregions, the same pattern of differential 2-DG uptake was repeated in the prelimbic cortex ( main effect of line: F(1,28) = 6.64, p = .016; main effect of treatment: F(1,28) = 6.83, p = .014; line*treatment: F(1,28) = 1.65, p = .209). No additional effects were found either dorsally, in anterior cingulate cortex, or ventrally, in infralimbic cortex (data not shown).
In subcortical regions (), relative to low-line rats, high-line rats had enhanced 2-DG uptake in dorsal hippocampus (main effect of line: F(1,27) = 15.82, p < .001). Interestingly, there was evidence indicating differential modulation of basal metabolism in dorsal hippocampus in the lines following experience of peripuberty stress (line*treatment: F(1,27) = 5.46, p = .027). However, further analyses revealed the differences between control and stress groups to be non-significant (t-test: low-line: t(14) = 1.52, p = .150; high-line: t(13) = −1.76, p = .101). No group differences were found in subnuclei of the amygdala (data not shown).
Basal activity within prelimbic cortex was associated with psychopathology-like behavioral tendencies
In brain regions where both constitutive differences in stress responsiveness and stress experience were found to influence resting activity, as indexed by 2-DG uptake, we performed correlations with outcome measures derived from the EPM and FST. Overall, we found basal activation of the prelimbic cortex to be negatively correlated with distance traveled on the EPM ( r = −0.51, p = .003). When analyzing the subgroups independently, basal activation of the prelimbic cortex was negatively correlated with distance in the High control (r = −0.82, p = .012) and High PPS (r = −0.70, p = .053) groups () but not in the Low control (r = −0.08, p = .843) or Low PPS (r = −0.16, p = .704) groups (). No other meaningful correlations were found.
Discussion
Here, we explored the interaction of two key risk factors for psychiatric disorders, constitutive differences in glucocorticoid responsiveness to stressors and early life stress exposure, in the expression of psychopathology-like behaviors when rats are tested at adulthood. Both risk factors were found to influence the expression of psychopathology-like behavior. Specifically, in the absence of early stress exposure, rats that show impaired habituation to repeated stressors (i.e. the high-line) displayed increased anxiety-like, depression-like and aggressive behaviors relative to low-line rats. Exposure to peripuberty stress enhanced anxiety-like and aggressive behavior in its own right, as previously shown (Marquez et al., Citation2013; Tzanoulinou, Garcia-Mompo, et al., Citation2014; Tzanoulinou, Riccio, et al., Citation2014). However, we did not observe an unequivocal synergy between the two factors, as might have been expected (Clinton, Watson, & Akil, Citation2014; McIlwrick et al., Citation2016; Stedenfeld et al., Citation2011).
In the case of aggression, and to a lesser extent anxiety-like behavior, peripuberty stress experience appeared to affect the lines differentially. For the most part, stress-induced alterations were expressed by the low-line across behavioral measures, a finding in accordance with similar studies (Cohen et al., Citation2006). Whether this finding indicates that high-line rats were insensitive to peripuberty stress cannot be clarified without further investigation. However, it appears that a ceiling may have been reached in aggressive and anxiety-like behavior among high-line rats such that it could not be further enhanced by peripuberty stress exposure. Interestingly, a similar lack of enhancement in psychopathology-like behavior was found following adolescent stress exposure in high-anxious, stress-sensitive, “low-responder” rats, adding weight to this speculation (Rana et al., Citation2016).
Analyses of corticosterone responses during peripuberty stress sessions validated the selection criterion in the cohort included in this study (Walker et al., Citation2017). From a similar initial corticosterone response, low-line rats showed a marked reduction in the response across repeated stress sessions, an effect strongly attenuated in high-line rats, in line with previous findings (Walker et al., Citation2017). In addition, low-line rats demonstrated robust negative feedback inhibition of the corticosterone response, as indexed by the recovery sample taken 30 minutes after the cessation of stress, particularly following the first stress exposure. Enhanced negative feedback of the HPA axis, via increased GR sensitivity, is considered one of the key neuroendocrine alterations found in individuals with PTSD (Yehuda, Boisoneau, Lowy, & Giller, Citation1995). Interestingly, in addition to trauma specific symptoms, PTSD sufferers frequently present other comorbidities including affective perturbations and enhanced anger (Contractor et al., Citation2015; Durham, Byllesby, Armour, Forbes, & Elhai, Citation2016).
No differences in Nr3c1 (encoding GR) expression were previously observed in HPA axis regions of low- and high-line rats, suggesting another basis for negative feedback differences observed between lines here (Walker et al., Citation2017). Interestingly, in the same study, reduced expression of Fkbp5 in paraventricular nucleus of the hypothalamus (PVN) of low-line rats was found. FKBP5 regulates the sensitivity of the GR to its ligand by altering its binding affinity (Binder, Citation2009), and a reduction in its expression in the PVN could conceivably result in enhanced negative feedback in low-line animals. In support of this notion, several studies have found the development of psychopathology following early life stress to be modulated by genetic variation in FKBP5 expression (Bevilacqua et al., Citation2012; Binder et al., Citation2008; Bryushkova et al., Citation2016; Klengel et al., Citation2013).
Low stress reactivity, potentially occurring as a result of high GR sensitivity, has been described in psychopathological disorders characterized by pathological aggression, including conduct disorder, anti-social personality disorder, and psychopathy (McBurnett, Lahey, Rathouz, & Loeber, Citation2000; O’Leary, Loney, & Eckel, Citation2007; Raine, Citation1996; Walker, Papilloud, Huzard, & Sandi, Citation2016). Moreover, early life stress has also been implicated as a risk factor for the development of pathological aggression (Beach et al., Citation2010; Caspi & Moffitt, Citation2006; Weder et al., Citation2009; Widom & Maxfield, Citation1996). Our findings here that peripuberty stress selectively enhances aggression in the low-line, especially in light of increased incidence of abnormal forms of aggression in this group, suggest that the low-line may provide a useful animal model within which to explore the mechanisms underlying development of abnormal aggression following adverse early life experience.
We additionally sought to investigate the interaction between constitutive stress-sensitivity and peripuberty stress experience on the activity of brain regions associated with altered resting-state function in psychopathological conditions (Koch et al., Citation2016; New et al., Citation2009). As indexed by 2-DG uptake, we found enhanced basal activity in subregions of the prefrontal cortex in high-line rats relative to low-line. Moreover, peripuberty stress experience was found to increase activity in the same regions. The two risk factors interacted to influence basal brain activity in a complex fashion. In accordance with behavioral findings, peripuberty stress appeared to bring 2-DG uptake in low-line rats closer to levels observed in both groups of high-line rats in ventral and medial orbitofrontal cortex and medial amygdala. However, as a result of low statistical power, the overarching pattern of results is difficult to interpret. In relating brain activity measures to behavioral findings, we found that basal activity in the prelimbic cortex to be negatively associated with locomotion on the EPM in high-line but not low-line rats. These findings suggest that propensity toward higher activity in the prefrontal cortex at rest is associated with the strength of anxiety-like behavioral inhibition in high-line rats under test conditions.
A limitation of the study relates to the quality of the information provided by the 2-DG method. The method provides an index of the metabolic demand for glucose within the cells of each region but does not provide any information regarding the nature of those cells, nor the signals they propagate. Previous studies employing the PPS model in rats reported reductions of GAD protein, an enzyme involved in the activity-dependent synthesis of GABA, in the same regions in which we report enhanced 2-DG uptake, namely: ventral and medial orbitofrontal cortex; and medial prefrontal cortex (Tzanoulinou et al., Citation2016). We therefore speculate that alterations in 2-DG uptake found under resting conditions here may reflect enhanced excitatory drive via reduced inhibition. In agreement with this idea, increases in anxiety-like behavior and alterations in affective and social motivation have been found under conditions of tonically increased activation of prefrontal cortex (Ferenczi et al., Citation2016; Yizhar et al., Citation2011).
In summary, we report a lack of synergism between the risk factors studied here, constitutive stress sensitivity and early life stress exposure, in the development of psychopathology-like behavior. Rats with high glucocorticoid response to repeated stressors had enhanced expression of psychopathology-like behaviors, which were unaltered by experience of peripuberty stress. In contrast, rats with constitutively low glucocorticoid response to repeated stressors appeared more vulnerable to the effects of peripuberty stress, particularly with regard to development of aggression. The behavioral findings reported here, considered in conjunction with neuroendocrine alterations, implicate the low line as a useful model with which to further explore mechanisms related to the development of abnormal aggression following early life adversity.
Acknowledgements
We thank Dr. Aurélie Papilloud and Damien Huzard for assistance in the performance of the stress protocol. We are additionally grateful to Dr. Alexandre Bacq and Yann Dubois for their assistance in digitalizing autoradiography images.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Beach, S.R., Brody, G.H., Gunter, T.D., Packer, H., Wernett, P., & Philibert, R.A. (2010). Child maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorder. Journal of Family Psychology, 24, 12–20. doi:10.1037/a0018074
- Bevilacqua, L., Carli, V., Sarchiapone, M., George, D.K., Goldman, D., Roy, A., & Enoch, M.A. (2012). Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Archives of General Psychiatry, 69, 62–70. doi:10.1001/archgenpsychiatry.2011.152
- Binder, E.B. (2009). The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology, 34 Suppl. 1, S186–S195. doi:10.1016/j.psyneuen.2009.05.021
- Binder, E.B., Bradley, R.G., Liu, W., Epstein, M.P., Deveau, T.C., Mercer, K.B., … Ressler, K.J. (2008). Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA, 299, 1291–1305. doi:10.1001/jama.299.11.1291
- Bryushkova, L., Zai, C., Chen, S., Pappa, I., Mileva, V., Tiemeier, H., … Beitchman, J.H. (2016). FKBP5 interacts with maltreatment in children with extreme, pervasive, and persistent aggression. Psychiatry Research, 242, 277–280. doi:10.1016/j.psychres.2015.09.052
- Casey, B.J., Jones, R.M., & Hare, T.A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126. doi:10.1196/annals.1440.010
- Caspi, A., & Moffitt, T.E. (2006). Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews Neuroscience, 7, 583–590. doi:10.1038/nrn1925
- Clinton, S.M., Watson, S.J., & Akil, H. (2014). High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress, 17, 97–107. doi:10.3109/10253890.2013.850670
- Cohen, H., Zohar, J., Gidron, Y., Matar, M.A., Belkind, D., Loewenthal, U., … Kaplan, Z. (2006). Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biological Psychiatry, 59, 1208–1218. doi:10.1016/j.biopsych.2005.12.003
- Contractor, A.A., Elhai, J.D., Fine, T.H., Tamburrino, M.B., Cohen, G., Shirley, E., … Calabrese, J.R. (2015). Latent profile analyses of posttraumatic stress disorder, depression and generalized anxiety disorder symptoms in trauma-exposed soldiers. Journal of Psychiatric Research, 68, 19–26. doi:10.1016/j.jpsychires.2015.05.014
- de Kloet, E.R. (2014). From receptor balance to rational glucocorticoid therapy. Endocrinology, 155, 2754–2769. doi:10.1210/en.2014-1048
- Durham, T.A., Byllesby, B.M., Armour, C., Forbes, D., & Elhai, J.D. (2016). Relations between anger and DSM-5 posttraumatic stress disorder symptoms. Psychiatry Research, 244, 403–409. doi:10.1016/j.psychres.2016.08.004
- Federenko, I.S., Nagamine, M., Hellhammer, D.H., Wadhwa, P.D., & Wust, S. (2004). The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. Journal of Clinical Endocrinology & Metabolism, 89, 6244–6250. doi:10.1210/jc.2004-0981
- Ferenczi, E.A., Zalocusky, K.A., Liston, C., Grosenick, L., Warden, M.R., Amatya, D., … Deisseroth, K. (2016). Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science, 351, aac9698. doi:10.1126/science.aac9698
- Gerra, G., Zaimovic, A., Mascetti, G.G., Gardini, S., Zambelli, U., Timpano, M., … Brambilla, F. (2001). Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuro-endocrinology, 26, 91–107. doi:10.1016/S0306-4530(00)00046-9
- Haller, J. (2013). The neurobiology of abnormal manifestations of aggression – A review of hypothalamic mechanisms in cats, rodents, and humans. Brain Research Bulletin, 93, 97–109. doi:10.1016/j.brainresbull.2012.10.003
- Heim, C., & Binder, E.B. (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology, 233, 102–111. doi:10.1016/j.expneurol.2011.10.032
- Joels, M., Pasricha, N., & Karst, H. (2013). The interplay between rapid and slow corticosteroid actions in brain. European Journal of Pharmacology, 719, 44–52. doi:10.1016/j.ejphar.2013.07.01
- Klengel, T., Mehta, D., Anacker, C., Rex-Haffner, M., Pruessner, J.C., Pariante, C.M., … Binder, E.B. (2013). Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience, 16, 33–41. doi:10.1038/nn.3275
- Koch, S.B., van Zuiden, M., Nawijn, L., Frijling, J.L., Veltman, D.J., & Olff, M. (2016). Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depress Anxiety, 33, 592–605. doi:10.1002/da.22478
- Koolhaas, J.M., Coppens, C.M., de Boer, S.F., Buwalda, B., Meerlo, P., & Timmermans, P.J. (2013). The resident–intruder paradigm: A standardized test for aggression, violence and social stress. Journal of Visualized Experiments, e4367. doi:10.3791/4367
- Marquez, C., Poirier, G.L., Cordero, M.I., Larsen, M.H., Groner, A., Marquis, J., … Sandi, C. (2013). Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Translational Psychiatry, 3, e216. doi:10.1038/tp.2012.144
- McBurnett, K., Lahey, B.B., Rathouz, P.J., & Loeber, R. (2000). Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry, 57, 38–43. doi:10.1001/archpsyc.57.1.38
- McEwen, B.S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. doi:10.1111/j.1749-6632.1998.tb09546.x
- McEwen, B.S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87, 873–904. doi:10.1152/physrev.00041.2006
- McIlwrick, S., Rechenberg, A., Matthes, M., Burgstaller, J., Schwarzbauer, T., Chen, A., & Touma, C. (2016). Genetic predisposition for high stress reactivity amplifies effects of early-life adversity. Psychoneuroendocrino-logy, 70, 85–97. doi:10.1016/j.psyneuen.2016.04.023
- New, A.S., Hazlett, E.A., Newmark, R.E., Zhang, J., Triebwasser, J., Meyerson, D., … Buchsbaum, M.S. (2009). Laboratory induced aggression: A positron emission tomography study of aggressive individuals with borderline personality disorder. Biological Psychiatry, 66, 1107–1114. doi:10.1016/j.biopsych.2009.07.015
- Nugent, N.R., Tyrka, A.R., Carpenter, L.L., & Price, L.H. (2011). Gene-environment interactions: Early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berl), 214, 175–196. doi:10.1007/s00213-010-2151-x
- O'Leary, M.M., Loney, B.R., & Eckel, L.A. (2007). Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology, 32, 183–191. doi:10.1016/j.psyneuen.2006.12.004
- Rabasa, C., Gagliano, H., Pastor-Ciurana, J., Fuentes, S., Belda, X., Nadal, R., & Armario, A. (2015). Adaptation of the hypothalamus–pituitary–adrenal axis to daily repeated stress does not follow the rules of habituation: A new perspective. Neuroscience & Biobehavioral Reviews, 56, 35–49. doi:10.1016/j.neubiorev.2015.06.013
- Raine, A. (1996). Autonomic nervous system factors underlying disinhibited, antisocial, and violent behavior. Biosocial perspectives and treatment implications. Annals of the New York Academy of Sciences, 794, 46–59. doi:10.1111/j.1749-6632.1996.tb32508.x
- Rana, S., Nam, H., Glover, M.E., Akil, H., Watson, S.J., Clinton, S.M., & Kerman, I.A. (2016). Protective effects of chronic mild stress during adolescence in the low-novelty responder rat. Stress, 19, 133–138. doi:10.3109/10253890.2015.1108304
- Ritov, G., Boltyansky, B., & Richter-Levin, G. (2016). A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction. Molecular Psychiatry, 21, 630–641. doi:10.1038/mp.2015.169
- Spear, L.P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 24, 417–463. doi:10.1016/S0149-7634(00)00014-2
- Stedenfeld, K.A., Clinton, S.M., Kerman, I.A., Akil, H., Watson, S.J., & Sved, A.F. (2011). Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiology & Behavior, 103, 210–216. doi:10.1016/j.physbeh.2011.02.001
- Tzanoulinou, S., Garcia-Mompo, C., Castillo-Gomez, E., Veenit, V., Nacher, J., & Sandi, C. (2014). Long-term behavioral programming induced by peripuberty stress in rats is accompanied by GABAergic-related alterations in the Amygdala. PLoS One, 9, e94666. doi:10.1371/journal.pone.0094666
- Tzanoulinou, S., Garcia-Mompo, C., Riccio, O., Grosse, J., Zanoletti, O., Dedousis, P., … Sandi, C. (2016). Neuroligin-2 expression in the prefrontal cortex is involved in attention deficits induced by peripubertal stress. Neuropsychopharmacology, 41, 751–761. doi:10.1038/npp.2015.200
- Tzanoulinou, S., Riccio, O., de Boer, M.W., & Sandi, C. (2014). Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Translational Psychiatry, 4, e410. doi:10.1038/tp.2014.54
- Walker, S.E., Papilloud, A., Huzard, D., & Sandi, C. (2016). The link between aberrant hypothalamic-pituitary-adrenal axis activity during development and the emergence of aggression-animal studies. Neuroscience & Biobehavioral Reviews. doi:10.1016/j.neubiorev.2016.10.008
- Walker, S.E., Zanoletti, O., Guillot de Suduiraut, I., & Sandi, C. (2017). Constitutive differences in glucocorticoid responsiveness to stress are related to variation in aggression and anxiety-related behaviors. Psychoneuroendocrinology, 84, 1–10. doi:10.1016/j.psyneuen.2017.06.011
- Weder, N., Yang, B.Z., Douglas-Palumberi, H., Massey, J., Krystal, J.H., Gelernter, J., & Kaufman, J. (2009). MAOA genotype, maltreatment, and aggressive behavior: The changing impact of genotype at varying levels of trauma. Biological Psychiatry, 65, 417–424. doi:10.1016/j.biopsych.2008.09.013
- Widom, C.S., & Maxfield, M.G. (1996). A prospective examination of risk for violence among abused and neglected children. Annals of the New York Academy of Sciences, 794, 224–237. doi:10.1111/j.1749-6632.1996.tb32523.x
- Yehuda, R., Boisoneau, D., Lowy, M.T., & Giller, E.L. Jr. (1995). Dose–response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Archives of General Psychiatry, 52, 583–593. doi:10.1001/archpsyc.1995.03950190065010
- Yizhar, O., Fenno, L.E., Prigge, M., Schneider, F., Davidson, T.J., O'Shea, D.J., … Deisseroth, K. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature, 477, 171–178. doi:10.1038/nature10360
