Abstract
Obesity and chronic stress are considered independent risk factors for the development of cardiovascular diseases and changes in autonomic system activity. However, the cardiovascular consequences induced by the association between high-fat diet (HFD) and chronic stress are not fully understood. We hypothesized that the association between HFD and exposure to a chronic variable stress (CVS) protocol for four weeks might exacerbate the cardiovascular and metabolic disturbances in rats when compared to these factors singly. To test this hypothesis, male Wistar rats were divided into four groups: control-standard chow diet (SD; n = 8); control-HFD (n = 8); CVS-SD (n = 8); and CVS-HFD (n = 8). The CVS consisted of repeated exposure of the rats to different inescapable and unpredictable stressors (restraint tress; damp sawdust, cold, swim stress and light cycle inversion). We evaluated cardiovascular function, autonomic activity, dietary intake, adiposity and metabolism. The HFD increased body weight, adiposity and blood glucose concentration (∼15%) in both control and CVS rats. The CVS–HFD rats showed decreased insulin sensitivity (25%) compared to CVS–SD rats. The control-HFD and CVS–HFD rats presented increased intrinsic heart rate (HR) values (∼8%). CVS increased cardiac sympathetic activity (∼65%) in both SD- and HFD-fed rats. The HFD increased basal HR (∼10%). Blood pressure and baroreflex analyzes showed no differences among the experimental groups. In conclusion, the present data indicate absence of interaction on autonomic imbalance evoked by either CVS or HFD. Additionally, HFD increased HR and evoked metabolic disruptions which are independent of stress exposure.
Introduction
Obesity and chronic stress are considered independent risk factors for the development of cardiovascular diseases (Ford et al., Citation1998; Haines, Imeson, & Meade, Citation1987; Inoue, Citation2014; Kawachi, Sparrow, Vokonas, & Weiss, Citation1994; Knox & Uvnas-Moberg, Citation1998; Poirier et al., Citation2006; Sgoifo, Carnevali, & Grippo, Citation2014; Steptoe & Kivimaki, Citation2012). Several studies have correlated psychosocial conditions, such as stress and psychiatric disorders (e.g. anxiety and depression), with the development of cardiovascular diseases (Crestani, Citation2016; Knox & Uvnas-Moberg, Citation1998; Rozanski, Blumenthal, & Kaplan, Citation1999; Sgoifo et al., Citation2014; Steptoe & Kivimaki, Citation2012). Epidemiological studies have also pointed out a positive correlation between stress levels and the incidence of cardiovascular diseases (Ford et al., Citation1998; Haines et al., Citation1987; Kawachi et al., Citation1994; Knox & Uvnas-Moberg, Citation1998). Similarly, the excess of adipose tissue, especially in the abdominal region, is directly associated with the etiology of chronic diseases, such as cardiovascular diseases (Despres & Lemieux, Citation2006). The positive correlation between obesity and hypertension is strong, consistent, and confirmed by genetic and clinical studies (Dorresteijn, Visseren, & Spiering, Citation2012). It is estimated that each 10% increase of body mass index is associated with an elevation of 3.9 mmHg in the systolic blood pressure (Timpson et al., Citation2009).
The modern lifestyle in industrialized societies is characterized by increased intake of processed foods, high in fat and sugar, as well as by continual exposure to stressful situations (Kyrou & Tsigos, Citation2008). Stress has been associated with changes in food preferences and the amount of food intake (Ulrich-Lai, Fulton, Wilson, Petrovich, & Rinaman, Citation2015). For instance, it has been reported that stress favors a higher caloric intake of palatable foods, such as foods rich in sugar and fat (Kim, Yang, Kim, & Lim, Citation2013; Laugero, Falcon, & Tucker, Citation2011). This altered selection of foods is referred to as “'comfort-food”' – a term that reflects the idea that eating palatable foods reduces stress responses, thus providing a potential means to increase the sense of well-being and, consequently, relieve stress (Ulrich-Lai et al., Citation2015). However, pre-clinical studies have demonstrated “anhedonic behavior” in mice and rats exposed to chronic stressors, which was evidenced by decreased intake and/or preference for sweet fluids and decreased intake of palatable food in brief feeding tests (Willner, Citation2005).
Despite the evidence of interaction between chronic stress and obesity, in an extensive search of the literature no studies were found investigating the consequences of the association between high-fat diet (HFD) and exposure to chronic stressors on cardiovascular function and autonomic activity. Thus, the aim of this study was to evaluate the effects of the association between HFD and exposure to a protocol of chronic variable stress (CVS) on cardiovascular and autonomic function, dietary intake, adiposity, and metabolism in rats. We hypothesized that the association between HFD and CVS might exacerbate the cardiovascular and metabolic disturbances in rats when compared to the effects evoked by these factors individually.
Methods
Animals
Male Wistar rats (60–70 days old) were obtained from the animal breeding facility of the Federal University of Santa Catarina (UFSC) and were assigned to the Department of Physiological Sciences at least seven days before the start of the experimental procedures. The rats were maintained in collective polypropylene cages (three to four rats/cage) with food (see diet description below), and water provided ad libitum in a room with controlled temperature (23 ± 2 °C) and humidity (55 ± 10%). Lights were on from 07:00 h to 19:00 h. Housing conditions and experimental procedures were carried out following protocols approved by the Ethical Committee for the Use of Animal of the UFSC (protocol # 3587130516), which complies with Brazilian and international guidelines for animal use and welfare.
Experimental design
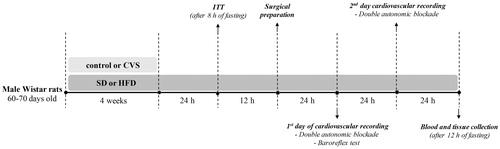
Rats were divided into four experimental groups: control and standard diet (control-SD; n = 8); control and HFD (control-HFD; n = 8); CVS and standard chow diet (CVS-SD; n = 8); and CVS and HFD (CVS-HFD; n = 8). The rats were submitted to CVS or remained undisturbed for four weeks. The SD or HFD diet was offered concurrently with CVS exposure (i.e. for four weeks). Twenty-four hours after the last stress stimulus, all the rats were submitted to 8 h of fasting to conduct an insulin tolerance test (ITT) and fasting blood glucose concentration measurement. On the following day, the rats were subjected to surgical preparation, and cardiovascular evaluations were performed 24 and 48 h later. All the cardiovascular recordings were made in the light phase of the light-dark cycle, when the rats tend to be less active. To avoid the influence of the acute stress of handling and transporting rats on cardiovascular recording days, rats were transferred to the experimental room in their home box and allowed 60 min to adapt to the experimental room conditions before starting cardiovascular recording. Moreover, during the cardiovascular recording the rats were not manipulated. To assure stable cardiovascular parameters, rats were subjected to a 20-min period of basal cardiovascular recording on two days of the experiments. On the first day, after the baseline recording, rats received an intravenous infusion of phenylephrine and sodium nitroprusside (SNP) in a random order. Then, rats received intravenous administration of methylatropine and propranolol in a random order. On the second day, after the baseline recording, rats were treated with methylatropine and propranolol in the opposite sequence to that on day 1. On the following day, after 12 h of fasting, the rats were euthanized with an overdose of inhalational anesthetic (isoflurane, 4–5% in 100% O2) and blood samples were collected by cardiac puncture for triglycerides (TGL) and total cholesterol analyzes. The retroperitoneal, epididymal and mesenteric adipose tissues and adrenals were removed and weighed. presents a schematic representation of the complete protocol.
Diet
The experimental groups received either SD (Biobase, Águas Frias, SC, Brazil) or HFD for four weeks. The SD diet contained 22 g of protein, 48 g of carbohydrates, 4 g of total fat, 8 g of fiber and 200 mg of sodium per 100 g of diet. The HFD was composed of standard rat chow plus peanuts, milk chocolate, and sweet biscuits in a proportion of 3:2:2:1, as previously described (Speretta et al., Citation2012), and contained 13 g of protein, 40 g of carbohydrate, 19 g of total fat, 4 g of fiber and 73 mg of sodium per 100 g of diet (Speretta et al., Citation2016). The caloric values of the diets were approximately: 3.16 kcal/g for SD and 3.82 kcal/g for HFD (Speretta et al., Citation2016). Food intake, as well as the body weight, were recorded three times a week. Food intake was determined by subtracting the cumulative food consumed after 48 h from the known amount available two days early in each collective cage. Then, an average of the individual consumption was calculated by dividing the total consumption of the cage by the number of rats kept in it.
Chronic variable stress
The CVS was used as a heterotypic stress regimen and was based on protocols previously described (Duarte, Cruz, Leao, Planeta, & Crestani, Citation2015; Grippo, Citation2009). The CVS protocol consisted of daily exposure to stressors, varying the stressor during the days of the week. shows the weekly protocol, which was repeated for four weeks. During this period, rats of the control groups were left undisturbed, except for cleaning the cages, in the animal facility.
Table 1. Weekly protocol of chronic variable stress (CVS).
Surgical preparation
The rats were anesthetized with ketamine (80 mg/mL/kg, i.p.; Cristália, Itapira, SP, Brazil) and xylazine (7 mg/mL/kg, i.p.; Agener Union, Embu, SP, Brazil), and a polyethylene catheter (a 4-cm segment of PE-10 heat-bound to a 15-cm segment of PE-50; Clay Adams, Parsippany, NJ, USA) filled with a solution of saline (0.9%) containing heparin (50 UI/mL, Cristália, São Paulo, Brazil) was inserted into the abdominal aorta through the femoral artery for cardiovascular recording. A second catheter was implanted into the femoral vein for the infusion of drugs. Both catheters were tunneled under the skin and exteriorized on the rats’s dorsum. After the surgery, rats were treated with a polyantibiotic formulation of streptomycin and penicillin (560 mg/mL/kg, i.m.) to prevent infection, and received the non-steroidal anti-inflammatory drug flunixin meglumine (0.5 mg/mL/kg, s.c.) for postoperative analgesia. The rats were housed in individual cages throughout the period after the surgery. Similar surgical procedures have been used systematically (Costa-Ferreira, Vieira, Almeida, Gomes-de-Souza, & Crestani, Citation2016; Crestani, Alves, Busnardo, Resstel, & Correa, Citation2010; Crestani et al., Citation2011; Duarte et al., Citation2015) and our standardization experiments indicated that rats are completely recovered 24 h after the surgical procedure, without significant changes in body weight, mean arterial pressure (MAP) or heart rate (HR).
Measurement of cardiovascular parameters
The arterial cannula was connected to a pressure transducer (MLT0380; ADInstruments, Bella Vista, NSW, Australia). Pulsatile arterial pressure was recorded using an amplifier (AVS Projetos, São Carlos, Brazil) and an acquisition board (PowerLab, ADInstruments, Bella Vista, NSW, Australia) connected to a personal computer. The MAP and HR values were derived from pulsatile arterial pressure recordings.
Infusion of vasoactive agents
Intravenous infusion of the α1-adrenoceptor agonist phenylephrine hydrochloride (70 μg/mL at 0.4 mL/min/kg; Sigma-Aldrich, St. Louis, MO, USA) and the nitric oxide donor SNP (100 μg/mL at 0.8 mL/min/kg; Sigma-Aldrich, St. Louis, MO, USA) was performed using an infusion pump (K.D. Scientific, Holliston, MA, USA) (Costa-Ferreira et al., Citation2016; Duarte et al., Citation2015). The infusions of the vasoactive drugs were randomized, and the second treatment was only given when the cardiovascular parameters returned to values obtained before the administration of vasoactive drugs (the interval between the infusions was approximately 5 min). Phenylephrine caused an incremental pressor effect, whereas SNP evoked incremental depressor responses. The infusions lasted for 20–30 s, thus resulting in the administration of a total dose of 9–14 μg/kg of phenylephrine and 26–40 μg/kg of SNP.
Assessment of cardiac autonomic activity and intrinsic HR
Assessment of the cardiac autonomic tonus and intrinsic HR (iHR; i.e. the HR under complete pharmacological blockade of cardiac autonomic activity, thus representing the cardiac pacemaker activity) was performed by an intravenous bolus injection of the muscarinic receptor antagonist methylatropine (3 mg/ml/kg, i.v; Sigma-Aldrich, St Louis, MO, USA) and the β-adrenoceptor antagonist propranolol hydrochloride (4 mg/ml/kg, i.v.; Sigma-Aldrich, St. Louis, MO, USA). In order to facilitate comparisons, we selected these drug doses based on previous studies that evaluated autonomic changes evoked by chronic stressors and HFD (Carvalho de Lima et al., Citation2014; Duarte et al., Citation2015). Also, these doses are widely used in the literature (Franchini & Krieger, Citation1993; Negrao, Moreira, Santos, Farah, & Krieger, Citation1992; Sanches, Sartori, Jorge, Irigoyen, & De Angelis, Citation2009). The protocol was performed on two days (Costa-Ferreira et al., Citation2016; Duarte et al., Citation2015). On the first day, the rats from all experimental groups received intravenous administration of methylatropine and propranolol in a random order. The interval of treatment between drugs was 10 minutes. On the subsequent day, rats were treated with methylatropine and propranolol in the opposite sequence to that performed on the first day.
The parasympathetic activity was determined by the change in basal HR caused by methylatropine, whereas the sympathetic activity was determined by the HR change after propranolol treatment. The iHR was determined after combined treatment with propranolol and methylatropine (Costa-Ferreira et al., Citation2016; Duarte et al., Citation2015).
Assessment of baroreflex activity
Paired values of MAP and HR changes evoked by phenylephrine and SNP infusion were plotted to generate sigmoid logistic functions (Crestani et al., Citation2010; Head & McCarty, Citation1987; Korner, Shaw, West, & Oliver, Citation1972). Baroreflex analysis using sigmoid curves was characterized by five parameters: (a) lower HR plateau (P1; in beats/min; i.e. maximum reflex bradycardia); (b) upper HR plateau (P2; in beats/min; i.e. maximum reflex tachycardia); (c) HR range (in beats/min; i.e. difference between upper and lower plateau levels); (d) median blood pressure (BP50; in mm Hg), which is the MAP at 50% of the HR range; and (e) average gain (G; in beats/min per mm Hg), which is the average slope of the curves between +1 and −1 standard derivations from BP50 (Crestani et al., Citation2010; Head & McCarty, Citation1987).
Insulin tolerance test
The insulin tolerance test (ITT) was performed in conscious rats 24 h after the last stress stimulus to avoid the influence of acute effects of stress. Additionally, all rats received the ITT at the same time of day (16:00 h). After 8 h of fasting the rats were gently restrained and the tail tip (∼1–2 mm) was cut using a pair of surgical scissors for blood collection. The first drop was discarded, and the second drop was collected for the determination of fasting blood glucose concentration (time 0) using standard test strips (Accu-Chek Performa; Roche Diagnostics GmbH, Mannheim, Germany). Immediately, human recombinant insulin (Novolin R; Novo Nordisk, Montes Claros, MG, Brazil) was administered at a dose of 1.0 U/mL/kg (i.p.), and blood samples were collected at 10, 15, 20, 30 and 60 min from the tail tip for blood glucose measurements. The rate constant for plasma glucose disappearance (Kitt) was calculated from the slope of the regression line obtained with log-transformed glucose values between 0 and 60 min after insulin administration (Bonora et al., Citation1989; Speretta et al., Citation2016).
Analysis of serum biochemical parameters
Blood samples collected by cardiac puncture was centrifuged (400 g for 10 min at 4 °C) for the quantification of serum TGL and total cholesterol. All analyzes were performed using enzymatic colorimetric assays according to the manufacturer's instructions (Biotécnica® – Varginha, MG, Brazil) using a plate reader (SpectraMax® Paradigm®, Molecular Devices, USA). For TGL, the repeatability and reproducibility assay variation was 3.5% and 6.4%, respectively; and assay sensitivity was 4.0 mg/dL. For total cholesterol, the repeatability and reproducibility assay variation was 3.21% and 1.76%, respectively; and assay sensitivity was 1.9 mg/dL.
Data analysis
Data were expressed as mean ± standard error of the mean (SEM). Three-way ANOVA (stress and diet as independent factors and time as a repeated measurement) followed by Student–Newman–Keuls post hoc test was used to analyze time-course curves for body weight, food intake, and energy intake. The other data were analyzed using two-way ANOVA (stress and diet as independent factors) followed by Student–Newman–Keuls post hoc test. Differences were taken as significant at p < 0.05.
Results
Body weight and food intake
Analysis of body weight indicated a main effect of CVS [F (1, 140) = 35.51; p < 0.0001], HFD [F (1, 140) = 50.83; p < 0.0001] and time [F (4, 140) = 36.36; p < 0.0001]; as well as HFD × time interaction [F (4, 140) = 3.02; p < 0.05]. The post hoc analysis revealed that the control-HFD and CVS–HFD groups presented increase of body weight at week 4, when compared to control-SD and CVS–SD groups (p < 0.0001 and p < 0.05, respectively; ) and to week 0 (p < 0.0001 for both). However, control-HFD rats presented higher body weight at week 4 when compared to CVS–HFD (p < 0.01). The control-SD group also showed increased body weight at week 4 when compared to week 0 (p < 0.001), whereas CVS–SD rats presented decreased body weight gain (p < 0.05).
Figure 2. (A) Body weight, (B) food intake, and (C) energy intake in rats fed with either standard chow diet (SD) or high-fat diet (HFD) and exposed to chronic variable stress (CVS) or control. The results are presented as mean ± SEM. Three-way ANOVA followed by the Student–Newman–Keuls test. *Different from respective SD group within the same condition; #Different from respective control group; & Different from control-SD; @Different from week 0 for panel A, and different from week 1 for panels B and C; p < 0.05; n = 8 for all groups. Note: y axes do not start at zero.
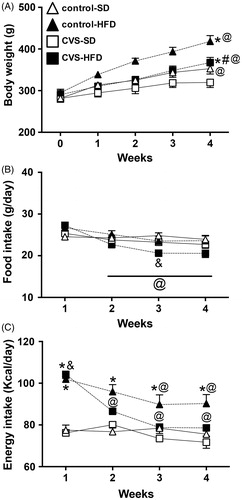
Analysis of food intake indicated a main effect of CVS [F (1, 112) = 11.03; p < 0.01] and time [F (3, 112) = 14.24; p < 0.0001]; as well as HFD × time [F (3, 112) = 5.07; p < 0.001] and CVS × time [F (3, 112) = 3.12; p < 0.05] interactions. The post hoc analysis revealed that the CVS–HFD rats presented reduced food intake from week 2 until the end of the experimental period, when compared to week 1 (p < 0.01; ). Furthermore, the CVS–HFD group presented reduced food intake at week 3 when compared to the control-SD group (p < 0.01).
Analysis of energy intake indicated a main effect of CVS [F (1, 112) = 10.89; p < 0.01], HFD [F (1, 112) = 109.4; p < 0.0001] and time [F (3, 112) = 12.97; p < 0.0001]; as well as HFD × time [F (3, 112) = 8.30; p < 0.0001] and CVS × HFD [F (1, 112) = 4.29; p < 0.05] interactions. The post hoc analysis revealed that the CVS–HFD and control-HFD rats showed higher energy intake at week 1 when compared to CVS–SD and control-SD groups (p < 0.0001 for all; ). However, only control-HFD rats maintained greater intake throughout the experimental period (p < 0.001). The CVS-HFD group presented reduced energy intake from week 2 until the end of the experimental period when compared to week 1 (p < 0.01), and likewise the control-HFD group showed reduced intake from week 3 until the end of the experimental period when compared to week 1 (p < 0.05).
Adrenal weight, adiposity and metabolism
Adrenal weight
Analysis of the relative adrenal weight (adrenals/body weight) indicated effects of CVS [F (1, 25) = 25.63; p < 0.001] and HFD [F (1, 25) = 15.95; p < 0.001], without CVS × HFD interaction [F (1, 25) = 0.0005; p = 0.98]. The post hoc analysis revealed that the CVS–SD rats presented increased relative adrenal weight when compared to control-SD rats (p < 0.001), whereas both groups fed with HFD presented reduced relative adrenal weight when compared to their respective groups fed with SD (p < 0.05 and p < 0.01) (). However, comparison of actual adrenal weight did not indicate any effect of either CVS [F (1, 25) = 1.25; p = 0.27] or HFD [F (1, 25) = 28; p = 0.59] ().
Table 2. Combined adrenal weight, adiposity, lipid profile, and fasting blood glucose in control and chronic variable stress (CVS)-exposed rats fed with either standard chow diet (SD) or high-fat diet (HFD) for four weeks.
Adipose stores
Analysis of relative weight of epidydimal adipose tissue showed effects of CVS [F (1, 25) = 4.72; p < 0.05] and HFD [F (1, 25) = 26.24; p < 0.0001], but without CVS × HFD interaction [F (1, 25) = 0.61; p = 0.43] (). Analysis of relative retroperitoneal adipose tissue weight revealed effects of CVS [F (1, 25) = 6.44; p < 0.05] and HFD [F (1, 25) = 42.00; p < 0.0001], but without CVS × HFD interaction [F (1, 25) = 0.33; p = 0.56] (). Analysis of relative mesenteric adipose tissue weight demonstrated effects of HFD [F (1, 25) = 19.07; p < 0.001], but not CVS [F (1, 25) = 2.67; p = 0.11] and no CVS × HFD interaction [F (1, 25) = 0.004; p = 0.94] ().
The post hoc analysis demonstrated that the CVS–HFD and control-HFD groups presented increased relative epididymal (p < 0.05 and p < 0.01, respectively) (p < 0.0001 and p < 0.001, respectively) and mesenteric (p < 0.05 and p < 0.05, respectively) adipose tissue weights when compared to their respective groups fed with SD. Additionally, CVS–SD rats presented reduced relative weight of retroperitoneal adipose tissue when compared to control-SD rats (p < 0.001) ().
Blood glucose
Analysis of fasting blood glucose concentration indicated effects of HFD [F (1, 25) = 27.25; p < 0.0001], but without effects of CVS [F (1, 25) = 0.75; p = 0.10] and CVS × HFD interaction [F (1, 25) = 0.74; p = 0.78] (). The post hoc analysis revealed that control-HFD and CVS–HFD groups presented increased blood glucose when compared to control-SD and CVS–SD, respectively (p < 0.01 for both).
Insulin tolerance test
Analysis of the insulin tolerance test indicated effects of HFD [F (1, 25) = 11.98; p < 0.01], but without effects of CVS [F (1, 25) = 0.81; p = 0.37] or CVS x HFD interaction [F (1, 25) = 0.32; p = 0.57]. The post hoc analysis revealed that CVS-HFD rats presented decreased insulin tolerance when compared to CVS-SD rats (p < 0.05; ).
Plasma triglyceride and cholesterol
Analysis of TGL concentrations indicated effects of CVS [F (1, 25) = 10.47; p < 0.01] and HFD [F (1, 25) = 17.25; p < 0.001], but without CVS × HFD interaction [F (1, 25) = 0.02; p = 0.87] (). The post hoc analysis demonstrated that TGL concentrations were higher in control-HFD and CVS-HFD rats when compared to control-SD and CVS-SD rats, respectively (p < 0.05 for both), and lower in the CVS-SD rats when compared to control-SD rats (p < 0.05). Comparison of total cholesterol concentration did not indicate effects of either CVS [F (1, 25) = 0.26; p = 0.61] or HFD [F (1, 25) = 3.15; p = 0.09] ().
Basal cardiovascular parameters
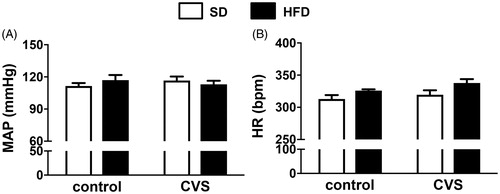
Comparisons of MAP did not indicate effects of either CVS [F (1, 25) = 0.02; p = 0.88] or HFD [F (1, 25) = 0.06; p = 0.79] (). Analysis of the HR data indicated effects of HFD [F (1, 25) = 7.55; p < 0.05], but without effects of CVS [F (1, 25) = 2.61; p = 0.11] or CVS × HFD interaction [F (1, 25) = 0.21; p = 0.64]. However, the post hoc analysis did not demonstrate differences among groups (p > 0.05; ).
Figure 3. (A) Mean arterial pressure (MAP) and (B) heart rate (HR) in rats fed with either standard chow diet (SD) or high-fat diet (HFD) and exposed to chronic variable stress (CVS) or control. The results are presented as mean ± SEM. Two-way ANOVA followed by the Student–Newman–Keuls test. n = 7 for all groups.
Intrinsic HR and cardiac autonomic modulation
Analysis of the HR change induced by intravenous administration of propranolol (i.e. to test for cardiac sympathetic activity) indicated effects of CVS [F (1, 25) = 4.75; p < 0.05], but without effects of HFD [F (1, 25) = 2.82; p = 0.10] or CVS × HFD interaction [F (1, 25) = 3.74; p = 0.06]. The post hoc analysis demonstrated that CVS-SD and CVS-HFD rats presented increased HR response to propranolol when compared to control-SD rats (all p < 0.05; ).
Figure 4. Autonomic activity, Δ heart rate (HR) evoked by administration of (A) propranolol and (B) methylatropine, and (C) intrinsic HR (iHR), HR values after combined treatment with methylatropine and propranolol in rats fed with either standard chow diet (SD) or high-fat diet (HFD) and exposed to chronic variable stress (CVS) or control. The results are presented as mean ± SEM. Two-way ANOVA followed by the Student–Newman–Keuls test. *Different from respective SD group within the same condition; #Different from respective control group; &Different from control-SD; p < 0.05; n = 7 for all groups.
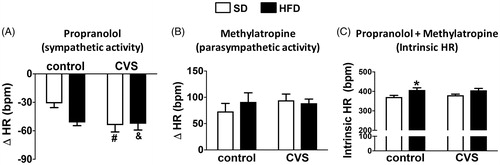
Analysis of the HR change induced by intravenous administration of methylatropine (i.e. to test cardiac parasympathetic activity) did not indicate effect of either CVS [F (1, 25) = 0.45; p = 0.50] or HFD [F (1, 25) = 0.20; p = 0.65] ().
Analysis of the HR values after combined treatment with propranolol and methylatropine (i.e. to assess iHR) indicated effects of HFD [F (1, 25) = 11.71; p < 0.01], but without effects of CVS [F (1, 25) = 0.16; p = 0.68] and CVS × HFD interaction [F (1, 25) = 0.33; p = 0.56]. The post hoc analysis revealed that control-HFD rats presented increased iHR when compared to control-SD rats (p < 0.05; ).
Baroreflex function
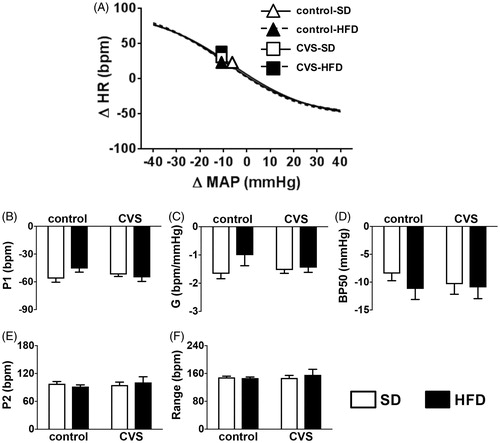
Analysis of P1, Gain (G), BP50, P2, and HR range parameters of baroreflex sigmoidal curves did not indicate effects of either CVS [P1: (1, 25) = 0.53; p = 0.46]; G: (1, 25) = 0.37; p = 0.54; BP50: F (1, 25) = 0.18; p = 0.67; P2: F (1, 25) = 0.20; p = 0.65; HR Range: F (1, 25) = 0.23; p = 0.62], or HFD [P1: (1, 25) = 1.21; p = 0.28]; G: (1, 25) = 2.24; p = 0.14; BP50: F (1, 25) = 0.77; p = 0.38; P2: F (1, 25) = 0.001; p = 0.96; HR Range: F (1, 25) = 0.18; p = 0.66] ().
Figure 5. (A) Nonlinear regression analysis of the baroreflex correlating Δ mean arterial pressure (MAP) and Δ heart rate (HR); symbols on curves indicate the BP50, (B) P1, bradycardic response, (C) baroreflex gain (G), (D) BP50, (E) P2, tachycardic response, and (F) baroreflex range in rats fed with either standard chow diet (SD) or high-fat diet (HFD) and exposed to chronic variable stress (CVS) or control. The results are presented as mean ± SEM. Two-way ANOVA followed by the Student–Newman–Keuls test. n = 7 for all groups.
Discussion
The present study provides evidence about cardiovascular function and autonomic activity in rats subjected to the association between chronic stress exposure and consumption of high-fat hypercaloric food. The main findings of the present study are that (i) either CVS or HFD increased cardiac sympathetic tone, however the association between these factors did not affect this response; (ii) HFD increased HR; (iii) HFD increased iHR in both control and CVS rats; (iv) HFD promoted metabolic alterations which were independent of CVS exposure; and (v) CVS decreased weight gain in both HFD and SD fed rats, whereas food and energy intake were reduced by CVS only in the HFD fed rats. The hypothesis that the association between HFD and CVS might exacerbate the cardiovascular and metabolic disturbances in rats when compared to these factors singly was not confirmed, since our results indicate absence of interaction on autonomic imbalance evoked by either CVS or HFD and on HR, which was influenced only by the HFD. Additionally, HFD evoked metabolic disruptions which seem to be independent of stress exposure.
It is well established that stress changes food preferences and the amount of food intake (Ulrich-Lai et al., Citation2015). Several pre-clinical studies have explored the effect of chronic stress on palatable food intake, but the results are diverse (Torres & Nowson, Citation2007). Indeed, either increase (Ely et al., Citation1997; Rowland & Antelman, Citation1976) or decrease in palatable food intake (Gronli et al., Citation2005; Papp, Willner, & Muscat, Citation1991) in animals subjected to chronic stressors has been reported. Increases in palatable food intake may reduce the responses to stress and promote a sense of well-being (Ulrich-Lai et al., Citation2015), whereas decreases in food intake may reflect the development of depressive behavior and anhedonia (Papp et al., Citation1991; Willner, Citation2005). More severe stressors favor a decrease in food intake (Torres & Nowson, Citation2007). Our data showed decreased food and energy intake in rats exposed to CVS and fed with HFD for four weeks, resulting in lower body weight gain and retroperitoneal adipose tissue weight compared to control rats fed with the same diet. These results are in line with a previous study (Aslani et al., Citation2015) that also demonstrated decreased total caloric intake and body weight gain in rats fed with HFD and exposed to CVS for six weeks. Furthermore, the authors found depressive- and anxiety-like behaviors in these rats. Stress-induced inhibition of feeding behavior may also have an endocrine basis. For instance, noradrenaline and corticotropin-releasing factor (CRF) released in response to stress seems to suppress appetite during stress (Halford, Citation2001; Takeda et al., Citation2004), which can explain the reduced food intake in rats exposed to CVS and fed with HFD.
Interestingly, while the food and energy intake was similar among control and CVS rats fed with SD, the rats exposed to CVS and fed with SD decreased body weight gain. The increased sympathetic nervous system activity and hypertrophied adrenals found in these rats may be involved in this response. For instance, the sympathetic nervous system directly targets peripheral organs and stimulate the adrenal medulla to increase circulating catecholamines, so allowing an immediate “fight-or-flight” response to the threat. Besides, the increase in circulating concentrations of corticosterone modifies tissue metabolism to inhibit energy storage and increases glucose availability to muscle and brain (Harris, Citation2015). Moreover, brown adipose tissue thermogenesis seems to be activated by CRF and the sympathetic nervous system (Harris, Citation2015), which could also increase energy expenditure. Indeed, a recent study demonstrated increased interscapular brown adipose tissue accumulation, increased energy expenditure and lipid oxidation, and lower body weight in a mouse model of chronic psychosocial defeat, even with high food intake (Coccurello et al., Citation2017).
In the present study, HFD decreased insulin sensitivity, increased blood glucose and triglyceride concentrations, and CVS did not exacerbate these responses. These results are in line with previous studies that showed metabolic alterations in rats fed with HFD (Speretta et al., Citation2012, Citation2016) and in rats fed with HFD and exposed to CVS (Aslani et al., Citation2015). Furthermore, a recent study reported few metabolic consequences in rats fed with SD and exposed to CVS (Thompson et al., Citation2015). Additionally, their data suggest that stressor intensity and/or frequency may be one factor contributing to the metabolic consequences (Thompson et al., Citation2015).
Pre-clinical studies suggest that cardiovascular diseases are associated with dysfunctions in cardiac autonomic balance and baroreflex activity (Grippo, Moffitt, & Johnson, Citation2002, Citation2008), which are important measures to evaluate autonomic function (Crestani et al., Citation2011; Flues et al., Citation2010; Grippo, Moffitt, & Johnson, Citation2008; Negrao et al., Citation1992). In the present study, HFD and CVS independently or combined increased cardiac sympathetic tone. Furthermore, we found increased baseline values of HR in rats fed with HFD. It is well established that increased sympathetic tone to the heart and baseline HR contribute to the development of hypertension in animals and humans (do Carmo et al., Citation2016). In this sense, it is possible that a longer association between CVS and HFD than the four weeks used in the present study could promote an increase in blood pressure. However, more studies are necessary to support this hypothesis.
In contrast, we did not find alterations on MAP, HR and baroreflex function in rats subjected to HFD and/or CVS. Our results contrast with previous studies that reported resting tachycardia related to increased cardiac sympathetic activity in rats exposed to either CVS or HFD (Duarte et al., Citation2015; Grippo, Beltz, & Johnson, Citation2003; Grippo et al., Citation2002; Speretta et al., Citation2016). Moreover, it has been recently demonstrated that rats fed with HFD showed increases in blood pressure, which was accompanied by increased HR and reduced baroreflex bradycardic response (Speretta et al., Citation2016). However, our data are in line with other studies that did not find increases in blood pressure following CVS or HFD (Costa-Ferreira et al., Citation2016; Fardin, Oyama, & Campos, Citation2012). These discrepancies in the cardiovascular responses to CVS and HFD may be related to differences in age, rat strains, period and/or types of CVS, period and/or composition of HFD. Importantly, the alterations in the sympathetic activity found in HFD rats may contribute to the development of obesity-induced hypertension (Stocker, Meador, & Adams, Citation2007). Moreover, the present data indicate an interface between HFD and CVS in increasing HR.
Despite the absence of changes in basal values of HR, we found increased values of iHR in the HFD groups. This response contributes to resting tachycardia identified in rats exposed to CVS and fed with HFD. The mechanisms involved in the increase of iHR in animals fed with HFD is not known, but it is possibly related to leptin. In this sense, leptin seems to increase the thyrotropin hormone in the pituitary (Pinkney et al., Citation1998; Reinehr & Andler, Citation2002), which in turn may increase iHR (Fellet, Arza, Arreche, Arranz, & Balaszczuk, Citation2004). Expression of leptin receptors was documented at the cell surface of the sinus node, but in the presence of sympathetic blockade (to investigate effects independent of a sympathetic modulation) leptin decreases the HR (Lin et al., Citation2015), thus reinforcing the idea that any involvement of leptin is possibly related to a stimulation of the thyroid gland. However, further studies are necessary to investigate the mechanisms involved in increased iHR evoked by HFD.
Importantly, our research group and others have demonstrated the participation of central and peripheral renin-angiotensin system (RAS) function in the development of obesity-induced hypertension (do Carmo et al., Citation2016; Speretta et al., Citation2016). In particular, the sodium concentration in the HFD used in the present study was considerably lower compared to SD and this could influence the cardiovascular parameters due to RAS activation. Preliminary results from our group showed that serum sodium levels and osmolality are not different between animals fed with SD or HFD (Sá, Citation2017). Although we have not directly evaluated the activation of the RAS, these data do not support the idea of RAS activation related to differences in sodium concentration in the HFD compared to SD rats.
In conclusion, four weeks of either CVS exposure or HFD promoted an increase in sympathetic tone to the heart, but the present data did not indicate an association between stress and obesity in this response. Additionally, rats fed with HFD showed increased basal values of HR and metabolic disorders, which were independent of CVS exposure. These results confirm the concept that the modern lifestyle, which includes increased intake of high-fat hypercaloric food associated with emotional stress situations, promotes metabolic alterations and increases the risk for development of cardiovascular diseases.
Acknowledgements
The authors thank Amanda Marreiro Barbosa, Thayz Rodrigues Chagas, Natália Engroff do Canto and Tamires Gregorio for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aslani, S., Vieira, N., Marques, F., Costa, P.S., Sousa, N., & Palha, J.A. (2015). The effect of high-fat diet on rat's mood, feeding behavior and response to stress. Translational Psychiatry, 5, e684. doi: 10.1038/tp.2015.178
- Bonora, E., Moghetti, P., Zancanaro, C., Cigolini, M., Querena, M., Cacciatori, V., … Muggeo, M. (1989). Estimates of in vivo insulin action in man: Comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. Journal of Clinical Endocrinology & Metabolism, 68, 374–378. doi: 10.1210/jcem-68-2-374
- Carvalho de Lima, D., Guimaraes, J.B., Rodovalho, G.V., Silveira, S.A., Haibara, A.S., & Coimbra, C.C. (2014). Exercise training starting at weaning age preserves cardiac pacemaker function in adulthood of diet-induced obese rats. Applied Physiology, Nutrition, and Metabolism, 39, 888–894. doi: 10.1139/apnm-2013-0529
- Coccurello, R., Romano, A., Giacovazzo, G., Tempesta, B., Fiore, M., Giudetti, A.M., … Gaetani, S. (2017). Increased intake of energy-dense diet and negative energy balance in a mouse model of chronic psychosocial defeat. European Journal of Nutrition, Advance Online publication. doi: 10.1007/s00394-00017-01434-y
- Costa-Ferreira, W., Vieira, J.O., Almeida, J., Gomes-de-Souza, L., & Crestani, C.C. (2016). Involvement of Type 1 Angiontensin II Receptor (AT1) in cardiovascular changes induced by chronic emotional stress: Comparison between homotypic and heterotypic stressors. Frontiers in Pharmacology, 7, 262. doi: 10.3389/fphar.2016.00262
- Crestani, C.C. (2016). Emotional stress and cardiovascular complications in animal models: A review of the influence of stress type. Frontiers in Physiology, 7, 251. doi: 10.3389/fphys.2016.00251
- Crestani, C.C., Alves, F.H., Busnardo, C., Resstel, L.B., & Correa, F.M. (2010). N-methyl-D-aspartate glutamate receptors in the hypothalamic paraventricular nucleus modulate cardiac component of the baroreflex in unanesthetized rats. Neuroscience Research, 67, 317–326. doi: 10.1016/j.neures.2010.05.001
- Crestani, C.C., Tavares, R.F., Guimaraes, F.S., Correa, F.M., Joca, S.R., & Resstel, L.B. (2011). Chronic fluoxetine treatment alters cardiovascular functions in unanesthetized rats. European Journal of Pharmacology, 670, 527–533. doi: 10.1016/j.ejphar.2011.09.030
- Despres, J.P., & Lemieux, I. (2006). Abdominal obesity and metabolic syndrome. Nature, 444, 881–887. doi: 10.1038/nature05488
- do Carmo, J.M., da Silva, A.A., Wang, Z., Fang, T., Aberdein, N., de Lara Rodriguez, C.E., & Hall, J.E. (2016). Obesity-induced hypertension: Brain signaling pathways. Current Hypertension Reviews, 18, 58. doi: 10.1007/s11906-016-0658-1
- Dorresteijn, J.A., Visseren, F.L., & Spiering, W. (2012). Mechanisms linking obesity to hypertension. Obesity Reviews, 13, 17–26. doi: 10.1111/j.1467-789X.2011.00914.x
- Duarte, J.O., Cruz, F.C., Leao, R.M., Planeta, C.S., & Crestani, C.C. (2015). Stress vulnerability during adolescence: Comparison of chronic stressors in adolescent and adult rats. Psychosomatic Medicine, 77, 186–199. doi: 10.1097/PSY.0000000000000141
- Ely, D.R., Dapper, V., Marasca, J., Correa, J.B., Gamaro, G.D., Xavier, M.H., … Dalmaz, C. (1997). Effect of restraint stress on feeding behavior of rats. Physiology & Behavior, 61, 395–398. doi: 10.1016/S0031-9384(96)00450-7
- Fardin, N.M., Oyama, L.M., & Campos, R.R. (2012). Changes in baroreflex control of renal sympathetic nerve activity in high-fat-fed rats as a predictor of hypertension. Obesity (Silver Spring), 20, 1591–1597. doi: 10.1038/oby.2012.4
- Fellet, A.L., Arza, P., Arreche, N., Arranz, C., & Balaszczuk, A.M. (2004). Nitric oxide and thyroid gland: Modulation of cardiovascular function in autonomic-blocked anaesthetized rats. Experimental Physiology, 89, 303–312. doi: 10.1113/expphysiol.2004.027201
- Flues, K., Paulini, J., Brito, S., Sanches, I.C., Consolim-Colombo, F., Irigoyen, M.C., & De Angelis, K. (2010). Exercise training associated with estrogen therapy induced cardiovascular benefits after ovarian hormones deprivation. Maturitas, 65, 267–271. doi: 10.1016/j.maturitas.2009
- Ford, D.E., Mead, L.A., Chang, P.P., Cooper-Patrick, L., Wang, N.Y., & Klag, M.J. (1998). Depression is a risk factor for coronary artery disease in men: The precursors study. Archives of Internal Medicine, 158, 1422–1426. doi: 10.1001/archinte.158.13.1422
- Franchini, K.G., & Krieger, E.M. (1993). Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. Journal of the Autonomic Nervous System, 42, 63–69. doi: 10.1016/0165-1838(93)90342-r
- Grippo, A.J. (2009). Mechanisms underlying altered mood and cardiovascular dysfunction: The value of neurobiological and behavioral research with animal models. Neuroscience & Biobehavioral Reviews, 33, 171–180. doi: 10.1016/j.neubiorev.2008.07.004
- Grippo, A.J., Beltz, T.G., & Johnson, A.K. (2003). Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiology & Behavior, 78, 703–710. doi: 10.1016/S0031-9384(03)00050-7
- Grippo, A.J., Moffitt, J.A., & Johnson, A.K. (2002). Cardiovascular alterations and autonomic imbalance in an experimental model of depression. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 282, R1333–R1341. doi: 10.1152/ajpregu.00614.2001
- Grippo, A.J., Moffitt, J.A., & Johnson, A.K. (2008). Evaluation of baroreceptor reflex function in the chronic mild stress rodent model of depression. Psychosomatic Medicine, 70, 435–443. doi: 10.1097/PSY.0b013e31816ff7dd
- Gronli, J., Murison, R., Fiske, E., Bjorvatn, B., Sorensen, E., Portas, C.M., & Ursin, R. (2005). Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiology & Behavior, 84, 571–577. doi: 10.1016/j.physbeh.2005.02.007
- Haines, A.P., Imeson, J.D., & Meade, T.W. (1987). Phobic anxiety and ischaemic heart disease. British Medical Journal (Clinical Research Ed.), 295, 297–299. doi: 10.1136/bmj.295.6593.297
- Halford, J.C. (2001). Pharmacology of appetite suppression: Implication for the treatment of obesity. Current Drug Targets, 2, 353–370. doi: 10.2174/1389450013348209
- Harris, R.B. (2015). Chronic and acute effects of stress on energy balance: Are there appropriate animal models? American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 308, R250–R265. doi: 10.1152/ajpregu.00361.2014
- Head, G.A., & McCarty, R. (1987). Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. Journal of the Autonomic Nervous System, 21, 203–213. doi: 10.1016/0165-1838(87)90023-3
- Inoue, N. (2014). Stress and atherosclerotic cardiovascular disease. Journal of Atherosclerosis and Thrombosis, 21, 391–401. doi: 10.5551/jat.21709
- Kawachi, I., Sparrow, D., Vokonas, P.S., & Weiss, S.T. (1994). Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation, 90, 2225–2229. doi: 10.1161/01.CIR.90.5.2225
- Kim, Y., Yang, H.Y., Kim, A.J., & Lim, Y. (2013). Academic stress levels were positively associated with sweet food consumption among Korean high-school students. Nutrition, 29, 213–218. doi: 10.1016/j.nut.2012.08.005
- Knox, S.S., & Uvnas-Moberg, K. (1998). Social isolation and cardiovascular disease: An atherosclerotic pathway? Psychoneuroendocrinology, 23, 877–890. doi: 10.1016/S0306-4530(98)00061-4
- Korner, P.I., Shaw, J., West, M.J., & Oliver, J.R. (1972). Central nervous system control of baroreceptor reflexes in the rabbit. Circulation Research, 31, 637–652. doi: 10.1161/01.RES.31.5.637
- Kyrou, I., & Tsigos, C. (2008). Chronic stress, visceral obesity and gonadal dysfunction. Hormones (Athens), 7, 287–293. doi: 10.14310/horm.2002.1209
- Laugero, K.D., Falcon, L.M., & Tucker, K.L. (2011). Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite, 56, 194–204. doi: 10.1016/j.appet.2010.11.001
- Lin, Y.C., Huang, J., Hileman, S., Martin, K.H., Hull, R., Davis, M., & Yu, H.G. (2015). Leptin decreases heart rate associated with increased ventricular repolarization via its receptor. American Journal of Physiology-Heart and Circulatory Physiology, 309, H1731–H1739. doi: 10.1152/ajpheart.00623.2015
- Negrao, C.E., Moreira, E.D., Santos, M.C., Farah, V.M., & Krieger, E.M. (1992). Vagal function impairment after exercise training. Journal of Applied Physiology (1985), 72, 1749–1753. doi: 10.1152/jappl.1992.72.5.1749
- Papp, M., Willner, P., & Muscat, R. (1991). An animal model of anhedonia: Attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berlin), 104, 255–259. doi: 10.1007/BF02244188
- Pinkney, J.H., Goodrick, S.J., Katz, J., Johnson, A.B., Lightman, S.L., Coppack, S.W., & Mohamed-Ali, V. (1998). Leptin and the pituitary-thyroid axis: A comparative study in lean, obese, hypothyroid and hyperthyroid subjects. Clinical Endocrinology (Oxford), 49, 583–588. doi: 10.1046/j.1365-2265.1998.00573.x
- Poirier, P., Giles, T.D., Bray, G.A., Hong, Y., Stern, J.S., Pi-Sunyer, F.X., & Eckel, R.H., … American Heart, A., Obesity Committee of the Council on Nutrition, P. A., & Metabolism. (2006). Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation, 113, 898–918. doi: 10.1161/CIRCULATIONAHA.106.171016
- Reinehr, T., & Andler, W. (2002). Thyroid hormones before and after weight loss in obesity. Archives of Disease in Childhood, 87, 320–323. doi: 10.1136/adc.87.4.320
- Rowland, N.E., & Antelman, S.M. (1976). Stress-induced hyperphagia and obesity in rats: A possible model for understanding human obesity. Science, 191, 310–312. doi: 10.1126/science.1246617
- Rozanski, A., Blumenthal, J.A., & Kaplan, J. (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation, 99, 2192–2217. doi: 10.1161/01.CIR.99.16.2192
- Sá, J.M. (2017). High-fat diet changes fluid–electrolyte balance in rats. In L.A. De Luca Júnior (Chair), I Physiology of Appetitive, Thirst and Salt Intake Behaviors Symposium. Satellite Symposium conducted before the XL SBNec Annual Meeting, Araraquara, SP, Brazil.
- Sanches, I.C., Sartori, M., Jorge, L., Irigoyen, M.C., & De Angelis, K. (2009). Tonic and reflex cardiovascular autonomic control in trained-female rats. Brazilian Journal of Medical and Biological Research, 42, 942–948. doi: 10.1590/S0100-879X2009001000011
- Sgoifo, A., Carnevali, L., & Grippo, A.J. (2014). The socially stressed heart. Insights from studies in rodents. Neuroscience & Biobehavioral Reviews, 39, 51–60. doi: 10.1016/j.neubiorev.2013.12.005
- Speretta, G.F., Rosante, M.C., Duarte, F.O., Leite, R.D., Lino, A.D., Andre, R.A., … Duarte, A.C. (2012). The effects of exercise modalities on adiposity in obese rats. Clinics (Sao Paulo), 67, 1469–1477. doi: 10.6061/clinics/2012(12)19
- Speretta, G.F., Silva, A.A., Vendramini, R.C., Zanesco, A., Delbin, M.A., Menani, J.V., … Colombari, D.S. (2016). Resistance training prevents the cardiovascular changes caused by high-fat diet. Life Science, 146, 154–162. doi: 10.1016/j.lfs.2016.01.011
- Steptoe, A., & Kivimaki, M. (2012). Stress and cardiovascular disease. Nature Reviews Cardiology, 9, 360–370. doi: 10.1146/annurev-publhealth-031912-114452
- Stocker, S.D., Meador, R., & Adams, J.M. (2007). Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension, 49, 640–646. doi: 10.1161/01.HYP.0000254828.71253.dc
- Takeda, E., Terao, J., Nakaya, Y., Miyamoto, K., Baba, Y., Chuman, H., … Rokutan, K. (2004). Stress control and human nutrition. Journal of Medical Investigation, 51, 139–145. doi: 10.2152/jmi.51.139
- Thompson, A.K., Fourman, S., Packard, A.E., Egan, A.E., Ryan, K.K., & Ulrich-Lai, Y.M. (2015). Metabolic consequences of chronic intermittent mild stress exposure. Physiology & Behaviour, 150, 24–30. doi: 10.1016/j.physbeh.2015.02.038
- Timpson, N.J., Harbord, R., Davey Smith, G., Zacho, J., Tybjaerg-Hansen, A., & Nordestgaard, B.G. (2009). Does greater adiposity increase blood pressure and hypertension risk?: Mendelian randomization using the FTO/MC4R genotype. Hypertension, 54, 84–90. doi: 10.1161/HYPERTENSIONAHA.109.130005
- Torres, S.J., & Nowson, C.A. (2007). Relationship between stress, eating behavior, and obesity. Nutrition (Burbank, Los Angeles County, Calif.), 23, 887–894. doi: 10.1016/j.nut.2007.08.008
- Ulrich-Lai, Y.M., Fulton, S., Wilson, M., Petrovich, G., & Rinaman, L. (2015). Stress exposure, food intake and emotional state. Stress (Amsterdam, Netherlands), 18, 381–399. doi: 10.3109/10253890.2015.1062981
- Willner, P. (2005). Chronic mild stress (CMS) revisited: Consistency and behavioural–neurobiological concordance in the effects of CMS. Neuropsychobiology, 52, 90–110. doi: 10.1159/000087097