Abstract
Stressful experiences are linked to anxiety disorders in humans. Similar effects are observed in rodent models, where anxiety is often measured in classic conflict tests such as the open-field test. Spontaneous rearing behavior, in which rodents stand on their hind legs to explore, can also be observed in this test yet is often ignored. We define two forms of rearing, supported rearing (in which the animal rears against the walls of the arena) and unsupported rearing (in which the animal rears without contacting the walls of the arena). Using an automated open-field test, we show that both rearing behaviors appear to be strongly context dependent and show clear sex differences, with females rearing less than males. We show that unsupported rearing is sensitive to acute stress, and is reduced under more averse testing conditions. Repeated testing and handling procedures lead to changes in several parameters over varying test sessions, yet unsupported rearing appears to be rather stable within a given animal. Rearing behaviors could therefore provide an additional measure of anxiety in rodents relevant for behavioral studies, as they appear to be highly sensitive to context and may be used in repeated testing designs.
Keywords:
Introduction
Stressful experiences have a powerful impact on brain function and can lead to both short and long-term behavioral alterations (Lupien, McEwen, Gunnar, & Heim, Citation2009). However, the cellular and behavioral response to stressors is always context-dependent, and various environmental factors can shape an individual’s response to stress (Boyce & Ellis, Citation2005; Joëls & Baram, Citation2009). Even in well-controlled experiments in rodents, accounting for contextual variables can be challenging. Observing stress-related anxiety in rodents typically relies on species-specific behaviors such as increased risk assessment, the reduction of exploration, seeking shelter, escape, burying or defecation (Bailey & Crawley, Citation2009). One of the most frequently used behavioral tests in rodents is the open-field test (OFT), a conflict test based on the opposing drives to explore new environments and fearful avoidance of bright/exposed areas. The OFT was originally developed by Calvin Hall in the 1930s to measure defecation as an index of timidity in mice (Walsh & Cummins, Citation1976). Nowadays, automated tracking allows the use of time spent in the center of the arena as a readout for anxiety, which has made the OFT the most widely used test for anxiety (Gould, Dao, & Kovacsics, Citation2009; Prut & Belzung, Citation2003). Unfortunately, automated open-field setups typically cannot measure exploratory behaviors, which are known to be highly sensitive to environmental factors and are suppressed under aversive, anxiety-provoking circumstances (Blanchard, Griebel, & Blanchard, Citation2003; McNaughton & Corr, Citation2004). The suppression of exploratory behavior in response to anxiety-provoking conditions is thought to be mediated by the hippocampal formation (Gray & McNaughton, Citation2003; Lever, Burton, & O’Keefe, Citation2006), a structure that is also a key target of the stress response (Ulrich-Lai & Herman, Citation2009). A classic exploratory behavior recorded in the open field is rearing behavior, in which the animal temporarily stands on its hind legs to sample the environment (Lever et al., Citation2006). Although initially thought to be highly correlated with activity (Walsh & Cummins, Citation1976), careful factor analyses of OFT data revealed that this relationship is more complicated and that it is vital to distinguish between supported rears (also called wall-leaning, where the animal stands on the hind limbs but touches the wall of the arena) and unsupported rears (briefly standing on hind legs without support) (Crusio, Citation2001; Crusio, Schwegler, Brust, & Van Abeelen, Citation1989). Both types of rearing load onto two different factors; While wall leaning only loads onto a factor related to locomotion/activity, rearing also loads onto a factor related to emotional behavior and is inversely correlated with defecation, a marker related to stress and anxiety (Whimbey & Denenberg, Citation1967). Crusio and colleagues also used elegant diallelic crosses of inbred mouse strains to show that unsupported rearing is linked to the size of the hippocampal intra- and infrapyramidal mossy fibers (Crusio et al. Citation1989). Based on this work, we ask whether recording supported and unsupported rears in an automated OFT setup renders the test more sensitive to changing contextual variables, and whether rearing behavior is influenced by acute stress. We show that both types of rearing are highly sensitive to the experimental context, including lighting conditions, noise, sex and stress exposure. Additionally, we find that unsupported and supported rears do indeed load onto different components that can be associated with emotionality or activity respectively, and that experimental groups account for much more of the variability in rearing (particularly unsupported rearing) than other OFT measures. Hence, future experiments should carefully record both types of rearing in the OFT, as this will increase both the amount and specificity of information gained about the animal’s behavioral state.
Materials and methods
Animals
C57BL/6 J (C57BL/6JRj) male and female mice (2.5 months) were obtained from Janvier (France), except for one group of males (n = 12) that was obtained from Charles River (Germany) henceforth referred to as V2 mice. Mice were maintained in a temperature- and humidity-controlled facility on a 12 hour reversed light–dark cycle (lights on at 08:15am) in individually ventilated cages (SealSafe PLUS, Tecniplast, Germany) with food (M/R Haltung Extrudat, Provimi Kliba SA, Switzerland, Cat.# 3436) and water ad libitum. Cages contained wood chip bedding (LIGNOCEL SELECT, J. Rettenmaier & Söhne, Germany) nesting material (tissue paper) and a cardboard shelter. Mice were housed in groups of 4 per-cage and used for experiments when 2.5–4 months old. All mice were given a minimum of 2 weeks to acclimatize to the light cycle and environmental conditions before testing. For each experiment, mice of the same age were used in all experimental groups to rule out confounding effects of age. All tests were conducted during the animals’ active (dark) phase from 12 to 5 pm. Mice were single housed 24 hours before behavioral testing in order to standardize their environment (Bohacek, Manuella, Roszkowski, & Mansuy, Citation2015). Independent animal groups were used for all experiments; however data from multiple experiments was pooled in and the associated analyses. All procedures were carried out in accordance to Swiss cantonal regulations for animal experimentation and were approved under license 155/2015.
Open-field testing
All open-field testing took place inside sound insulated, ventilated multiconditioning chambers (TSE Systems Ltd, Germany). The open-field arena (45 cm (l) × 45 cm (w) × 40 cm (h)) consisted of four transparent Plexiglas walls and a light gray PVC floor. Control animals for all experiments were tested under four equally spaced yellow lights (4 Lux across the floor of the open field) with 75–77 dB of white noise playing through the speakers of each box. An infrared light also illuminated the boxes so that an infrared camera could be used to record the tests. Prior to testing each animal, the entire open-field arena was cleaned using 10 ml/l detergent (For, Dr .Schnell AG). Males and females were tested at least 2 days apart and boxes were cleaned at least twice between testing males and females. The room housing the multiconditioning chambers was illuminated with red LED lights (637 nm). Animals were removed from their home cage by the tail and placed directly into the center of the open field. The doors of the conditioning chamber were then swiftly closed. Tracking/recording was initiated upon first locomotion grid beam break. All open-field tests were 10 minutes in duration. For one experiment (summarized in ), the conditions of the OFT were altered. Animals tested in the “light” group were tested under 100 lux, whereas animals in the “dark” group were tested at 0 lux (no white light, only infrared camera recordings). Mice in the “silent” group were tested under the control conditions described above but without background noise.
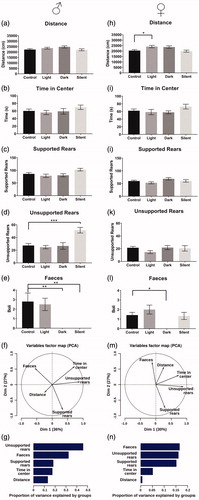
Figure 1. Impact of changing context on behavior of male and female mice in the open field test. In males (a–e), the number of unsupported rears is significantly increased in silent conditions whereas the number of fecal boli is significantly reduced in both the dark and silent conditions. Females (h–l) travel greater distance in the light condition, and fecal boli are reduced in the dark. Principle component analyses showing the loadings of the measured parameters onto principle components in males (f) and females (m). Supported and unsupported rears load differently onto the principle components. Variance analysis shows that unsupported rearing and feces explains a high percentage of variance between groups in males (g), whereas in females (n) feces, unsupported rears and supported rears explain the majority of variance. Male: control (n = 10), light (n = 12), dark (n = 10), silent (n = 9). Female: control (n = 26), light (n = 15), dark (n = 9), silent (n = 12). Data expressed as mean ± SEM, *p < .05, **p < .01, ***p < .001.
Recorded behaviors
Distance, time in center, supported rears, unsupported rears and the number of fecal boli were recorded. All parameters were scored automatically except fecal boli, which were counted manually during cleaning. Locomotion was tracked using an infrared beam grid; an additional beam grid was raised 6.5 cm above the locomotion grid to measure rearing. The central 50% (1012.5 cm2) was defined as the center of the arena. To automatically distinguish supported from unsupported rears, we empirically determined the area in which mice could not perform a supported rear. Thus, all rears within 12.5 cm of the walls were considered supported rears, while rears in the rest of the field were considered unsupported rears. Rearing was defined as an interruption of a beam in the z-axis for a minimum of 150 ms. If another rear was reported within 150 ms of the initial rear, it was counted as part of the initial rear. For a comparison of automated and manual scoring, see Supplementary Figure (S4).
Handling and repeated testing
Unless otherwise specified, mice were not handled before testing. When the effects of handling/taming mice were specifically assessed, mice were handled for three consecutive days prior to experiments. On the first day of handling the investigator’s hand was placed into the home cage for 10 minutes, allowing the mice to become accustomed to it, they were then each picked up and placed onto the investigator’s arm 5 times and cupped in the hands three times. This was repeated on the second day, but the mice were placed onto the investigators arm 10 times. On the third day, the procedure was repeated, but after the investigator’s hand was placed into the group house for 10 minutes, the animals were single housed before they were placed onto the investigators arm 10 times and cupped three times. The investigator who handled the animals and who performed the experiment was the only person to be in contact with the mice during this period. The same laboratory coat was worn for all interactions with these mice. On the first day after handling (day 1), mice were tested in the open field under control conditions, they were then tested again on day 2 and day 9.
Acute restraint
Animals were moved from the colony room to a holding room in which they were placed into a 50 ml falcon tube with an air hole for breathing. After placement in the falcon tube, each animal was carefully put back into its home cage ensuring the opening of the tube was not obstructed. Animals were restrained in this manner for 30 minutes. Upon release from restraint, animals were given 15 minutes in order to groom themselves before testing (45 minutes after the onset of the stressor).
Forced swim
Animals were moved from the colony room to a holding room before immediate forced swim testing in 17.9–18.1 °C water for 6 minutes. The forced swim took place in a plastic beaker (20 cm diameter, 25 cm deep, filled to 17 cm so no mouse could touch the bottom of the container with its tail or escape). Tracking/recording was remotely initiated as the mouse contacted the water. The water was changed and the beaker was cleaned shortly before each swim. Overhead red LED lights (637 nm, invisible to the mice) dimly illuminated both the holding and testing rooms. Animals were placed into the swimming beaker and into the open field 45 minutes after the onset of swim stress by the same investigator.
Statistical analysis
Statistical analyses were performed using SPSS 23 (IBM), Graphpad Prism 7 and R 3.4.1. Outliers were removed from all behavioral data using a ROUT test (Q = 5%). Any animal found to be an outlier in either of the following variables was excluded from all analyses: distance, time in center, supported rears, unsupported rears or feces (outliers in feces alone were not removed in all tests including a stressor, as mice defecate during stress exposure). A total of 275 animals were used overall, 37 of which were identified as outliers by the ROUT test and excluded. These exclusions do not alter the main conclusions presented in this manuscript. Animal numbers reported in the figure legends and statistics refer to the number of animals ultimately used (excluding outliers) Principal component analysis was performed in R 3.4.1 with the FactoMineR 1.36 package, after removal of outliers and scaling of the variables to unit variance (Lê, Josse, & Husson, Citation2008). The R script used is included in the supplementary information (see Supplementary Information S5)
Results
Context and anxiety measures
We first wanted to determine which of the recorded behaviors in the open-field test were most sensitive to environmental factors in females and males. Thus, we manipulated light intensity and background noise levels, two contextual parameters believed to impact the averseness of the test (Walsh & Cummins, Citation1976). Importantly, these two factors vary between laboratory setups but are often not clearly reported (Martin-Arenas & Pintado, Citation2014). Two-way ANOVAs (Group × Sex) were carried out for all dependent variables and are summarized in Supplementary Table 1. Because of significant interactions between “Group × Sex”, we plotted both males and females separately () and performed follow-up one-way ANOVAs. In males, changing brightness, background noise or vendor does not significantly impact total distance traveled (), time in center (), nor supported rears (). However, unsupported rears in males significantly change in response to context (ANOVA, F(3,37) = 9.702, p < .0001) and so does the number of fecal boli (Kruskal–Wallis, h = 17.86 df = 4, p < .01). The number of unsupported rears in males is significantly increased when mice are tested without background noise (Silent). Post hoc tests reveal that the number of fecal boli produced decreases when light intensity is reduced from 4 lux (Control) to 0 lux (Dark), and when the background noise is completely removed (). Therefore, testing mice without background noise appears to reduce anxiety, as the number of fecal boli decreases, and the number of unsupported rears increase. We hypothesized that if unsupported rears were related to anxiety, the number of unsupported rears should increase later in the test, as the test apparatus becomes more familiar (and thus less aversive). Indeed, when comparing the number of unsupported rears in the first five minutes and the last five minutes of the test session, we observed that males rear significantly more during the last 5 minutes (paired t-test; t = 5.971, df = 9, p < .001) (see Supplementary Figure S1).
Female mice were less responsive to the same contextual changes, yet there were group effects for distance traveled (ANOVA, F(3,58) = 3.894, p < .05), and for fecal boli (Kruskal–Wallis, h = 10.1, df = 3, p < .05). Increasing the light intensity from 4 lux (Control) to 100 lux (Light) increased the total distance traveled (), while a decrease in light intensity from 4 lux to 0 lux significantly reduced defecation (). In contrast to males, altering the levels of background noise had no significant effect on any of the recorded parameters in females ().
Additionally, we tested whether the same strain of mice from a different vendor (Charles River, Germany) would show a different phenotype at baseline. Interestingly, we found that (C57BL/6 J) mice from Charles River (Germany) perform significantly more unsupported rears (t = 3.49, df = 18, p < .05) than their Janvier counterparts (Figure S2).
To investigate the patterns of covariance between the different behaviors in the context of environmental variations in males, we performed a principal component analysis of all measurements scaled to unit variance (). The first two components together account for over 60% of the variability and reveal that supported and unsupported rears load differently and associate with distinct covariates. We used regression analyses to estimate the proportion of variance in each measured behavior that can be explained by differences across experimental groups (). While experimental groups explain nearly half of the variability in total unsupported rearing (46%), they account for much less of the variability in supported rears (18%), time in center (17%), or distance traveled (13%). This indicates that unsupported rears vary less within groups, and more across groups, than the other variables, thus representing a better variable for experimental studies. Indeed, multiple regression analysis revealed a significant association between unsupported rears and experimental groups (F(4,45) = 9.519, p < .0001), an association that remains unchanged when introducing supported rears in the model (F(4,44) = 8.032, p < .0001). This provides further evidence that supported and unsupported rearing represent two distinct behavioral variables, despite the similarities in the behaviors (standing on hind legs).
Although females respond very differently to context changes in comparison to males (), a principal component analysis shows – similar to males – that the different types of rearing load differently on the first two components (). The experimental groups explained a much smaller proportion of the variance in the different behaviors () than in males, most likely owing to the fact that females responded less strongly to alterations in context in these experiments. Nevertheless, feces, unsupported rears and supported rears were the behaviors most strongly associated with the experimental groups, explaining far more variance than the most frequently used OFT measures “time in center” and “distance traveled”.
Handling and repeated testing
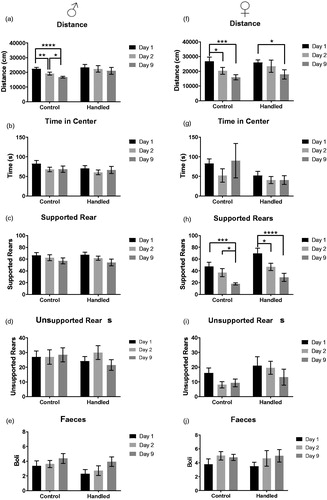
We were surprised to see the large contribution of unsupported rearing to overall behavioral variation in the open-field test. To get a better understanding of other contextual factors that influence open-field behavior in general, and rearing in particular, we next investigated the effects of repeated testing and handling in both male and female mice. Three-way ANOVA (Group, Sex, Day) for all dependent variables yielded complex, significant interactions between “Group × Sex” and “Sex × Day” (see Supplementary Table 2); therefore, we analyzed males and females differently (). In males, two-way ANOVAs (handling × day) showed a significant main effect of day for “distance” (ANOVA F(2,56) = 21.41, p < .0001). Males are significantly less active upon each subsequent exposure to the open field (). In females, ANOVAs revealed a significant main effect of day for “distance” and “supported rears” (ANOVA, F(2,30) = 17.53,p < .0001, ANOVA, F(2,30) = 25.86, p < .0001). Both the distance traveled and the number of supported rears decreases upon each subsequent exposure to the open field in females (). Overall, both repeated testing and handling seem to have only minor effects on behavioral performance in the open-field test in males and females. We also performed a correlation analysis and found that rearing was very consistent between days (days 1–2) in males (unsupported rearing: df = 14 r = 0.77 p < .001; supported rearing df = 14 r = 0.60, p < .05). Surprisingly, this was not the case in females, where there was only a significant correlation between supported rearing over subsequent days (days 1–2) of testing (df = 14, r = 0.94, p < .0001).
Figure 2. Effects of handling and repeated testing on behavior in the open-field test in males and females. In males, no significance was observed between the handled or control animals in any reported parameter or across days; however, there was a significant decrease in distance traveled in control males across all days (a–e). In females, both distance traveled and the number of supported rears decreased significantly across testing days. Male: control (n = 16), handled (n = 14). Female: control (n = 9), Handled (n = 8). Data expressed as mean ± SEM, *p < .05,**p < .01, ***p < .001.
Acute restraint/swim stress
Acute stress exposure is known to induce anxiety in rodents and humans (Grillon, Duncko, Covington, Kopperman, & Kling, Citation2007; Korte & De Boer, Citation2003). Given that unsupported rearing is a hippocampus-related exploratory behavior and showed the strongest response to contextual changes (), we wanted to test the hypothesis that rearing would be sensitive to the anxiety-inducing effects of acute stress. We first exposed mice to 30 min restraint stress and tested them on the open field 45 min after initiation of stress (). A two-way ANOVAs (Group × Sex) revealed significant main effects of Group for “time in center” and “unsupported rears”, and significant main effects of Sex for “distance” and “unsupported rearing”, but no significant interaction between “Group × Sex” for any dependent variable (see Supplementary Table 3). This indicates that restraint stress reduces center time and unsupported rears in both males and females, and that females travel greater distance and perform fewer unsupported rears. Follow-up t-tests reveal that the stress-induced changes reach statistical significance only in males (unsupported rears: t = 2.165, df = 24, p < .05; supported rears: t = 2.065, df = 24, p < .05; ).
Figure 3. The effect of acute stress on behaviors in the open-field test in males and females. In males (a–d), restraint stress significantly reduced supported and unsupported rears but was without effect in females (e-h). Swim stress significantly reduced distance, time in center and both types of rearing in males (i–l) and females (m-p). Male: restraint control (n = 12), stressed (n = 14). Swim stress: control (n = 10), stress (n = 11). Female: restraint control (n = 13), stressed (n = 10). Swim stress control (n = 11), stressed (n = 11). Data expressed as mean ± SEM, *p < .05, ***p < .001.
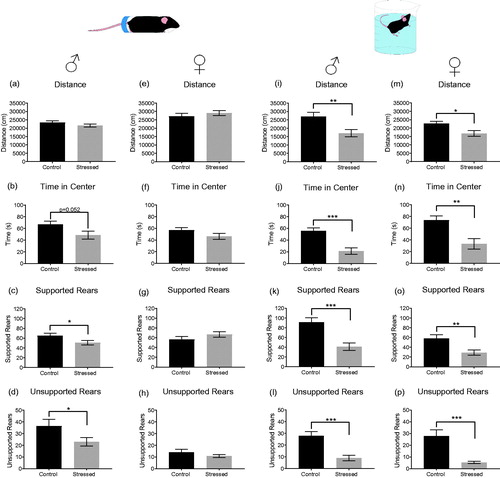
We next tested the impact of another acute stressor on open-field behavior. Mice were exposed to brief (6 minute) cold swim stress and tested on the open field 45 min after initiation of the stressor. Two-way ANOVA revealed significant main effects of Group for all dependent variables, and significant main effect of Sex for “time in center” and “supported rears”, but no interaction between “Group x Sex” (see Supplementary Table 4). This demonstrates that swim stress strongly decreases distance moved, time in center, unsupported rears and supported rears in both males and females, and that females spend more time in the center and show fewer supported rears than males (). Principal component and variance analyses of the effects of acute stress show that supported and unsupported rears continue to load onto the principal components differently and that experimental groups continue to explain large amounts of variance in unsupported rears (see Supplementary Figure S3).
Sex differences
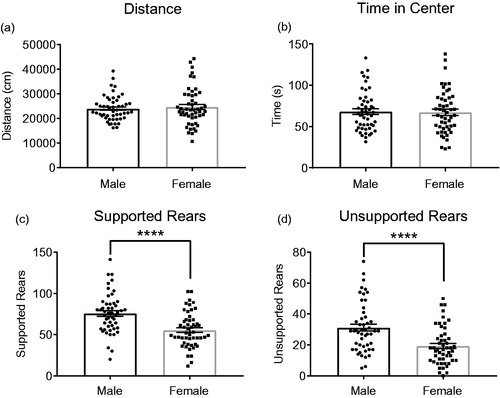
To test whether there are sex differences between males and females at baseline, we pooled the open-field data of control groups from all experiments reported here as well as several other experiments conducted in our laboratory (data not shown). Importantly, all pooled groups were tested in the open-field test under the exact same conditions (4 lux, 75–77 dB background noise, no handling). Here, we show no significant sex differences in the overall distance traveled or time in center (). However, we observe striking differences in both the number of supported rears (t = 4.494 df = 99, p < .0001) and unsupported rears (t = 4.227, df = 99, p < .0001), with females rearing much less than males ().
Figure 4. Sex differences in behavior the open-field test. Control groups of males and females were pooled across several independent experiments. There are no significant differences in either total distance traveled (a) or time in center (b), yet males show significantly more supported rears (c) and unsupported rears (d) than females. Males (n = 50), Females (n = 51). Data expressed as mean ± SEM, *p < .05, ***p < .001, ****p < .0001.
Discussion
Several previous studies have used factor analyses similar to those performed here, yet without including rearing behavior (Jähkel, Rilke, Koch, & Oehler, Citation2000; Whimbey & Denenberg, Citation1967). Meanwhile, others have included rearing, yet without distinguishing between supported and unsupported rears (Moser, Moser, Wultz, & Sagvolden, Citation1988; Tanaka, Young, Halberstadt, Masten, & Geyer, Citation2012). The principal component and variance analyses () indicate that unsupported and supported rearing give valuable information about an animal’s behavioral state. In support of the previous work of Crusio and colleagues (Crusio et al., Citation1989; Crusio, Citation2001), we show that unsupported rearing loads onto the principal components similarly to “time in center”, a parameter typically associated with emotionality, whereas supported rearing is more highly associated with a different principal component associated with distance/activity. Therefore, grouping the two rearing behaviors (or not including them in the overall behavioral analysis of the OFT) is likely to drastically reduce the deductions one can make about the behavioral state of the animals. Analysis of variance shows that selective manipulation of the environment explains more of the variability in unsupported rearing than in the classic measures “distance” or “time in center” (). This implies that by separating and measuring this particular aspect of rearing behavior independently one can improve the assessment of the animal’s emotional state.
Previous work has shown a strong relationship between the hippocampus and rearing behavior (Lever et al., Citation2006). More specific studies of hippocampal morphology and rearing have indicated that hippocampal intra- and infrapyramidal mossy fibers (IIPMF) are directly linked to rearing behavior, with mice selectively bred for high-frequency rearing (SHR mice) showing increases in IIPMF terminal field size (Crusio et al., Citation1989; van Abeelen, Citation1970). Factor analysis revealed that IIPMF density loaded very strongly onto the same factor as unsupported (but not supported) rearing, and defecation was inversely related to both measures (Crusio, Citation2001). This indicates that unsupported rearing is a specific trait with a morphological basis and that it is negatively correlated with the classic measure of anxiety in the OFT (defecation). Interestingly, the latency of rearing behavior in response to novelty was proposed as an early readout for neurodegenerative and cognitive disorders in a mouse model of Alzheimer’s disease (Torres-Lista et al., Citation2014). Although the authors do not define what type of rearing this relates to, it shows that rearing can also be an indication of degeneration of or increased metabolic/oxidative stress in the hippocampus. In their seminal work, Gray and McNaughton (Citation2003) have suggested that the hippocampus serves as a conflict resolution/inhibitory control center in the brain, that inhibits ongoing behavior in conflict situations and engages exploratory behavior if more information is required to resolve the conflict (Gray & McNaughton, Citation2003). In this context, it has been suggested that rearing would increase in situations of uncertainty (e.g. when the desire to explore the environment is roughly equal to the perceived danger in that environment), but decrease in situations where the balance is clearly tipped towards one of the two choices (e.g. very safe or very dangerous environment). Hence, it is intriguing that IIPMFs are closely related to unsupported rearing behavior and may serve to transfer information via the perforant pathway to the CA3 region.
The hippocampus is a key target of the stress response, and hippocampus-dependent behaviors are strongly influenced by stress. We show that in male mice, unsupported rearing, a hippocampus-dependent behavior, increases if the environment becomes safer/less aversive (shown by removing background noise during testing, ), but is suppressed after exposure to an acute swim or restraint stress challenge (). Interstingly, studies that failed to differentiate the different types of rearing, still found that a range of stressors/stress models and modulators in mice and rats can impact overall rearing behavior: restraint (Zimprich et al., Citation2014), novelty (Torres-Lista et al., Citation2014), aversive odors (McGregor, Citation2004), loss of olfactory capacity (Thakare, Aswar, Kulkarni, Patil, & Patel, Citation2017), exposure to toxins such as lead (Rodrigues, Rocha, Mello, & Souza, Citation1996), psychoactive pharmacological compounds including the endocannabinoid analog oleoylethanolamide (Jin, Yu, Tian-Lan, Zhang, & Quan, Citation2015), caffeine (Onaolapo, Onaolapo, Akanmu, & Olayiwola, Citation2016) and ketamine (McGowan et al., Citation2017). As supported and unsupported rearing behaviors were grouped in these studies, it is possible that significant alterations in the number of supported and unsupported rears could have been overlooked, giving less information about the behavioral state of the animal. Our observations seem to support the previous claim that rearing behavior appears to follow an inverse U curve with little rearing at the extremes of anxiety/fear (Gray & McNaughton, Citation2003; Lever et al., Citation2006; McNaughton & Corr, Citation2004). However, this can make the interpretation of rearing difficult, as rearing can be either increased or decreased by making the test more aversive, depending on the baseline level of activation along the inverted-U-curve (Ennaceur, Citation2014). Yet, if the context of the experiment is carefully considered and controlled, unsupported rearing could provide a useful readout of anxiety. It is tempting to speculate that swim stress led to a stronger, more anxiogenic stress response than restraint stress, as swim stress more strongly suppressed several anxiety-related behaviors (time in center, unsupported rears, supported rears). This could be due to the fact that swim stress (particularly in cold water) strongly increases the release of norepinephrine (NE) in the hippocampus (Stone, Citation1970), and selective release of NE has been shown to induce anxiety (McCall et al., Citation2015). The strength of NE release in the hippocampus, or activation of the hippocampus via the amygdala, could therefore explain the different reactions to the stressors. However, swim stress also suppressed locomotor activity, thus making it difficult to conclusively interpret whether the general increase in anxiety-related behavior was secondary to a decrease in activity due to exhaustion. However, previous experiments evaluating the immediate effects of stress on anxiety have frequently used even shorter delays, which might add confounding variables such as grooming or physical exhaustion (McCall et al., Citation2015). In our experiments, mice were tested 45 minutes after stress onset. We have shown previously that, at this 45 minute time point, mice have recovered physically from the stress exposure (Roszkowski & Bohacek, Citation2016), while still showing peak corticosterone levels (Bohacek et al., Citation2015) and dramatic changes in (noradrenaline-dependent) hippocampal gene expression (Roszkowski et al., Citation2016).
We observed striking sex differences, with females showing much less supported and unsupported rearing than males, despite comparable levels of locomotor activity (). While sex differences in stress-responsiveness have been well-documented in rodents (Bangasser & Wicks, Citation2017; McLaughlin, Baran, & Conrad, Citation2009), sex differences regarding supported and unsupported rears in relation to stress are reported much less frequently. Previous work has shown baseline differences in unsupported rearing between males and females from various mouse strains (Delprato et al., Citation2017). Although several experimental parameters vary between this report and our study (size of the open field, test duration, sexes tested on the same day), our results are in general agreement and extend these findings by also showing strong sex differences in supported rearing. Additionally, we find that there are also differences in rearing behavior between the sexes under specific conditions (e.g. light, noise). In contrast to males, rearing in females changes in response to repeated testing (). This highlights the importance of routinely including females in basic research, and the use of multiple different stressors before drawing conclusions about the generalizability of observed effects.
Importantly, the hormonal status of females was not recorded in the current experiments. It is thus not clear whether the observed sex differences are due to organizational effects of sex hormones, or influenced by fluctuating hormone levels. Ovarian hormones elicit clear effects on hippocampal morphology and function (Woolley, Citation2007), and impact hippocampus-dependent behaviors (Bohacek & Daniel, Citation2007; Daniel, Citation2006). However, the results showing pronounced sex differences in the total number of rearing at baseline () were generated by comparing large numbers of mice tested across different days. Therefore, if ovarian hormones had a strong impact on rearing, we would have expected to observe higher variance (between pooled control animals) in females, but variance tended to be slightly smaller in females (). This is consistent with previous reports showing low variance between females relative to males in many behavioral tests (Fritz, Amrein, & Wolfer, Citation2017; Prendergast, Onishi, & Zucker, Citation2014).
While other researchers have occasionally included many other ethologically relevant variables to their analyses (e.g. sniffing, grooming, gnawing, etc.), the advantage of studying rearing behavior is that it can be measured automatically by using an elevated beam grid. Others have discussed the advantages and disadvantages of automated semi-high throughput behavioral apparatus in detail (Hånell & Marklund, Citation2014), In short, automated behavioral scoring enables a higher throughput and faster data gathering. Although it adds a certain degree of (systematic) error, it guarantees consistency between mice, days and experimenters. The widespread use of behavioral chambers similar to the ones used in our experiments could help to increase the reproducibility of behavioral data between labs. This is of extreme relevance, given the effects of slight changes in experimental conditions and the wide variance between open-field setups (Martin-Arenas & Pintado, Citation2014).
Conclusions
We show that supported and unsupported rears in the open-field test load onto different principal components and that unsupported rearing often explains the highest amount of variance between groups under different experimental conditions. Unsupported rearing appears to be a sensitive measure of anxiety in both males and females. Therefore, future studies using the OFT should systematically record supported and unsupported rears. Given the ubiquitous use of the OFT in basic research, this would generate large amounts of data to help understand which variables shape animal behavior and which brain structures and molecules mediate these behaviors.
Oliver_et_al._Supplementary_Material.pdf
Download PDF (1.1 MB)Acknowledgements
We thank Shiree Sutton for illustrations and Yvonne Zipfel for animal caretaking. We thank Prof. Isabelle Mansuy for generously providing labspace and sharing resources.
Disclosure statement
The authors have read and understood the journal of stress policy on declaration of interests and declare that they have no competing interests.
Additional information
Funding
References
- Bailey, K.R., & Crawley, J.N. (2009). Anxiety-related behaviors in mice. In J. Buccafusco (Ed.), Methods of behavior analysis in neuroscience. Boca Raton: CRC Press/Taylor & Francis.
- Bangasser, D.A., & Wicks, B. (2017). Sex-specific mechanisms for responding to stress. Journal of Neuroscience Research, 95, 75–82. doi:10.1002/jnr.23812
- Blanchard, D.C., Griebel, G., & Blanchard, R.J. (2003). The mouse defense test battery: Pharmacological and behavioral assays for anxiety and panic. 463, 97–116. European Journal of Pharmacology, 463, 97–116. doi:10.1016/S0014-2999(03)01276-7
- Bohacek, J., & Daniel, J.M. (2007). Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Hormones and Behavior, 52, 237–243. doi:10.1016/j.yhbeh.2007.04.010
- Bohacek, J., Manuella, F., Roszkowski, M., & Mansuy, I.M. (2015). Hippocampal gene expression induced by cold swim stress depends on sex and handling. Psychoneuroendocrinology, 52, 1–12. doi:10.1016/j.psyneuen.2014.10.026
- Boyce, W.T., & Ellis, B.J. (2005). Biological sensitivity to context: I. an evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17, 271–301. doi:10.1017/S0954579405050145
- Crusio, W.E. (2001). Genetic dissection of mouse exploratory behaviour. Behavioural Brain Research, 125, 127–132. doi:10.1016/S0166-4328(01)00280-7
- Crusio, W.E., Schwegler, H., Brust, I., & Van Abeelen, J.H. (1989). Genetic selection for novelty-induced rearing behavior in mice produces changes in hippocampal mossy fiber distributions. Journal of Neurogenetics, 5, 87–93. doi:10.3109/01677068909167267
- Crusio, W.E., Schwegler, H., & van Abeelen, J.H.F. (1989). Behavioral responses to novelty and structural variation of the hippocampus in mice. II. Multivariate genetic analysis. Behavioural Brain Research, 32, 81–88. doi:10.1016/S0166-4328(89)80075-0
- Daniel, J.M. (2006). Effects of oestrogen on cognition: What have we learned from basic research? Journal of Neuroendocrinology, 18, 787–795. doi:10.1111/j.1365-2826.2006.01471.x
- Delprato, A., Algéo, M.P., Bonheur, B., Bubier, J.A., Lu, L., Williams, R.W., … Crusio, W.E. (2017). QTL and systems genetics analysis of mouse grooming and behavioral responses to novelty in an open field. Genes, Brain and Behavior, 16, 790–799. doi:10.1111/gbb.12392
- Ennaceur, A. (2014). Tests of unconditioned anxiety – Pitfalls and disappointments. Physiology and Behavior, 135, 55–71. doi:10.1016/j.physbeh.2014.05.032
- Fritz, A.K., Amrein, I., & Wolfer, D.P. (2017). Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 175, 380–391. doi:10.1002/ajmg.c.31565
- Gould, T.D., Dao, D.T., & Kovacsics, C.E. (2009). Mood and anxiety related phenotypes in mice. Neuromethods, 42, 1–20. doi:10.1007/978-1-60761-303-9
- Gray, J. A., & McNaughton, N. (2003). The neuropsychology of anxiety: An enquiry into the function of the septo-hippocampal system. NY: Oxford University Press.
- Grillon, C., Duncko, R., Covington, M.F., Kopperman, L., & Kling, M.A. (2007). Acute stress potentiates anxiety in humans. Biological Psychiatry, 62, 1183–1186. doi:10.1016/j.biopsych.2007.06.007
- Hånell, A., & Marklund, N. (2014). Structured evaluation of rodent behavioral tests used in drug discovery research. Frontiers in Behavioral Neuroscience, 8, 252. doi:10.3389/fnbeh.2014.00252
- Jähkel, M., Rilke, O., Koch, R., & Oehler, J. (2000). Open field locomotion and neurotransmission in mice evaluated by principal component factor analysis-effects of housing condition, individual activity disposition and psychotropic drugs. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 24, 61–84. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10659984
- Jin, P., Yu, H.L., Tian-Lan, Zhang, F., & Quan, Z.S. (2015). Antidepressant-like effects of oleoylethanolamide in a mouse model of chronic unpredictable mild stress. Pharmacology Biochemistry and Behavior, 133, 146–154. doi:10.1016/j.pbb.2015.04.001
- Joëls, M., & Baram, T.Z. (2009). The neuro-symphony of stress. Nature Reviews. Neuroscience, 10, 459–466. doi:10.1038/nrn2632
- Korte, S.M., & De Boer, S.F. (2003). A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. European Journal of Pharmacology, 463, 163–175. doi:10.1016/S0014-2999(03)01279-2
- Lê, S., Josse, J., & Husson, F. (2008). FactoMineR: An R package for multivariate analysis. Journal of Statistical Software, 25, 253–258. doi:10.18637/jss.v025.i01
- Lever, C., Burton, S., & O’keefe, J. (2006). Rearing on hind legs, environmental novelty, and the hippocampal formation. Reviews in the Neurosciences, 17, 111–133. doi:10.1515/REVNEURO.2006.17.1-2.111
- Lupien, S.J., McEwen, B.S., Gunnar, M.R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. doi:10.1038/nrn2639
- Martin-Arenas, F.J., & Pintado, C.O. (2014). Results of the open field test at different light intensities in C57 mice. Proceedings of Measuring Behavior, 1–5. Retrieved from http://www.measuringbehavior.org
- McCall, J.G.G., Al-Hasani, R., Siuda, E.R.R., Hong, D.Y.Y., Norris, A.J.J., Ford, C.P.P., & Bruchas, M.R.R. (2015). CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron, 87, 605–620. doi:10.1016/j.neuron.2015.07.002
- McGowan, J.C., LaGamma, C.T., Lim, S.C., Tsitsiklis, M., Neria, Y., Brachman, R.A., & Denny, C.A. (2017). Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 42, 1577–1589. doi:10.1038/npp.2017.19
- McGregor, I.S. (2004). Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. Journal of Neuroscience, 24, 4134–4144. doi:10.1523/JNEUROSCI.0187-04.2004
- McLaughlin, K.J., Baran, S.E., & Conrad, C.D. (2009). Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Molecular Neurobiology, 40, 166–182. doi:10.1007/s12035-009-8079-7
- McNaughton, N., & Corr, P.J. (2004). A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews, 28, 285–305. doi:10.1016/j.neubiorev.2004.03.005
- Moser, M.B., Moser, E.I., Wultz, B., & Sagvolden, T. (1988). Component analyses differentiate between exploratory-behavior of spontaneously hypertensive rats and wistar kyoto rats in a 2-compartment free-exploration open-field. Scandinavian Journal of Psychology, 29, 200–206.
- Onaolapo, J.O., Onaolapo, Y.A., Akanmu, A.M., & Olayiwola, G. (2016). Caffeine and sleep-deprivation mediated changes in open-field behaviours, stress response and antioxidant status in mice. Sleep Science, 9, 236–243. doi:10.1016/j.slsci.2016.10.008
- Prendergast, B.J., Onishi, K.G., & Zucker, I. (2014). Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews, 40, 1–5. doi:10.1016/j.neubiorev.2014.01.001
- Prut, L., & Belzung, C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European Journal of Pharmacology, 463, 3–33. doi:10.1016/S0014-2999(03)01272-X
- Rodrigues, A.L.S., Rocha, J.B.T., Mello, C.F., & Souza, D.O. (1996). Effect of perinatal lead exposure on rat behaviour in open-field and two-way avoidance tasks. Pharmacology and Toxicology, 79, 150–156. doi:10.1111/j.1600-0773.1996.tb00259.x
- Roszkowski, M., & Bohacek, J. (2016). Stress does not increase blood-brain barrier permeability in mice. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 36, 1304–1315. doi:10.1177/0271678X16647739
- Roszkowski, M., Manuella, F., Von Ziegler, L., Durán-Pacheco, G., Moreau, J.-L., Mansuy, I.M., & Bohacek, J. (2016). Rapid stress-induced transcriptomic changes in the brain depend on beta-adrenergic signaling. Neuropharmacology, 107, 329–338. doi:10.1016/j.neuropharm.2016.03.046
- Stone, E.A. (1970). Swim-stress-induced inactivity: relation to body temperature and brain norepinephrine, and effects of d-Amphetamine. Psychosomatic Medicine, 32, 51–59. doi:10.1097/00006842-197001000-00004
- Tanaka, S., Young, J.W., Halberstadt, A.L., Masten, V.L., & Geyer, M.A. (2012). Four factors underlying mouse behavior in an open field. Behavioural Brain Research, 233, 55–61. doi:10.1016/j.bbr.2012.04.045
- Thakare, V.N., Aswar, M.K., Kulkarni, Y.P., Patil, R.R., & Patel, B.M. (2017). Silymarin ameliorates experimentally induced depressive like behavior in rats: Involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response. Physiology and Behavior, 179, 401–410. doi:10.1016/j.physbeh.2017.07.010
- Torres-Lista, V., Parrado-Fernández, C., Alvarez-Montón, I., Frontiñán-Rubio, J., Durán-Prado, M., Peinado, J.R., … Giménez-Llort, L. (2014). Neophobia, NQO1 and SIRT1 as premorbid and prodromal indicators of AD in 3xTg-AD mice. Behavioural Brain Research, 271, 140–146. doi:10.1016/j.bbr.2014.04.055
- Ulrich-Lai, Y.M., & Herman, J.P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews. Neuroscience, 10, 397–409. doi:10.1038/nrn2647
- van Abeelen, J.H.F. (1970). Genetics of rearing behavior in mice. Behavior Genetics, 1, 71–76. doi:10.1007/BF01067372
- Walsh, R.N., & Cummins, R. A. (1976). The open-field test: A critical review. Psychological Bulletin, 83, 482–504. doi:10.1037/0033-2909.83.3.482
- Whimbey, A. E., & Denenberg, V.H. (1967). Two independent behavioral dimensions in open-field performance. Journal of Comparative and Physiological Psychology, 63, 500–504. doi:10.1037/h0024620
- Woolley, C.S. (2007). Acute effects of estrogen on neuronal physiology. Annual Review of Pharmacology and Toxicology, 47, 657–680. doi:10.1146/annurev.pharmtox.47.120505.105219
- Zimprich, A., Garrett, L., Deussing, J.M., Wotjak, C.T., Fuchs, H., Gailus-Durner, V., … Hölter, S.M. (2014). A robust and reliable non-invasive test for stress responsivity in mice. Frontiers in Behavioral Neuroscience, 8, 125. doi:10.3389/fnbeh.2014.00125
