Abstract
Adolescence is a distinct developmental period characterized by behavioral and physiological maturation. Rapid ongoing changes during neurodevelopment in particular present potential opportunities for stress to have lasting effects on longitudinal outcomes of behavioral and neuroendocrine function. While adult stress effects on outcomes during adulthood have been characterized, little is known about the lasting effects of adolescent repeated stressor exposure on outcomes during adolescence. We have previously reported different stress responses in adolescent rats relative to adult rats, including a blunted fear response outcome in adulthood in rats stressed during adolescence. The present study characterized the ontogeny of behavioral and neuroendocrine responses to eight underwater trauma (UWT) exposures in rats over a two week poststress time period during adolescence (P34) or adulthood (P83) relative to age-matched control groups that underwent eight swimming episodes without UWT. Repeated UWT exposures starting in adolescence, but not adulthood, resulted in adverse behavioral responses on the elevated plus maze 1 day post-stress. Corticosterone responses did not differ between UWT-exposed and controls for either age group at 1 day or at 7 days poststress, although there was an effect of age on corticosterone levels. We conclude that repeated UWT stress events have a lasting, negative behavioral effect on adolescent rats that is not observed in adult rats after the two-week exposure window. These results suggest that neurophysiological mechanisms underlying recovery from a repeated stressor are immature in adolescence relative to adulthood in rats.
Introduction
Exposure to a traumatic event may lead to long-lasting psychological health changes in some individuals. Post-traumatic stress disorder (PTSD) is one potential consequence following a traumatic exposure, although other mental health disorders may also result. Traumatic events may take place at any point across the developmental timeline, with potentially differing effects both acutely and in long-term outcomes depending on developmental events ongoing during the traumatic exposure. While traumatic stress is sometimes represented by a single punctuated event, multiple repeated exposures to the same traumatic event are also possible. Cumulative exposure to repeated traumatic stressors in humans has been associated with increased prevalence of PTSD and other poor psychological health outcomes in both civilian and military populations (Cabrera, Hoge, Bliese, Castro, & Messer, Citation2007). Additionally, developmental status at the time of traumatic stress exposure may be a contributing factor to the severity of the outcome.
Rat models have assisted in building an understanding of the unique characteristics of adolescence (for review, see Spear, Citation2000) and of the homeostatic responses to stress exposure using preclinical studies (Romeo, Patel, Pham, & So, Citation2016). Preclinical models of a traumatic stressor, or a stress event that represents a threat to life, are integral to understanding physiological responses to severe stressors. One such model representing a threat to life is underwater trauma (UWT) (Richter-Levin, Citation1998). UWT delivers a robust stressor event without causing hypoxic damage to lung or brain tissue (Moore, Gauchan, & Genovese, Citation2012). This brief severe stress exposure disrupts memory (Cohen, Liberzon, & Richter-Levin, Citation2009; Wang, Akirav, & Richter-Levin, Citation2000). Changes in signaling in brain areas relevant to emotional processing are evident acutely and chronically after a single UWT exposure (Ritov, Ardi, & Richter-Levin, Citation2014; Sood et al., Citation2014; Sood, Ritov, Richter-Levin, & Barki-Harrington, Citation2013). Long-term behavioral outcomes are also affected when adolescent rats undergo the UWT procedure (Moore, Altman, Gauchan, & Genovese, Citation2016; Moore, Gauchan, & Genovese, Citation2014). Baseline circulating corticosterone is decreased in adolescent rats at 7 days after UWT (Moore et al., Citation2012). These studies have built a basis for understanding a severe, acute stressor in adolescent and adult rats.
Still, many questions remain unanswered regarding lasting responses to a repeated traumatic stress event, particularly during a rapid phase of behavioral and neurophysiological change such as adolescence. It has been reported, for instance, that different phases of adolescent development impact behavioral and neuroendocrine response profiles to a chronic variable stress paradigm (Jankord et al., Citation2011), but little is known about the effect of developmental status on behavioral and neuroendocrine outcomes following repeated exposure to a traumatic stressor. We have previously reported that a single adolescent traumatic exposure blunts conditioned suppression of responding for food on an operant schedule of reinforcement and extinction of cued startle potentiation during adulthood (Moore et al., Citation2014), suggesting that adolescent trauamtic stress exposure dysregulates fundamental emotional learning capabilities. Additionally, further evidence from our lab demonstrates that adulthood outcomes of adolescent traumatic stress exposure vary depending on different stressors used (Moore et al., Citation2016). However, we did not directly compare outcomes between adolescents and adults in these studies. The present study extends these investigations of the ontogeny of stress response by characterizing the behavioral and neuroendocrine responses after repeated exposures to underwater trauma (UWT).
Methods
Subjects
A total of 48 adolescent (postnatal day, P22) and adult (P70-77) Sprague-Dawley rats were received from Charles River Laboratories and housed under a 12:12 light-dark cycle (lights on at 0600) with ad libitum access to standard laboratory rodent chow and water. Rats were pair-housed on arrival, and acclimated to the facility for one week. All subjects (n of 12 per group) were then acclimated to handling and daily weighing procedures for five days before beginning the experimental timeline.
Timeline of study
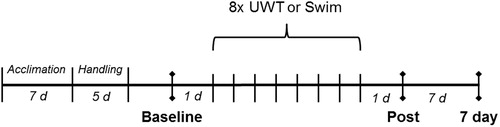
As shown in , baseline data were collected one day before the start of repeated UWT exposures. Baseline measures include behaviors on the elevated plus maze (EPM) and acoustic startle test (ASR) and neuroendocrine corticosterone values. UWT exposure began on P34-37 for adolescent rats and on P83-84 for adult rats. Behavioral data were subsequently collected one day after the final UWT exposure (i.e. “Post”), while corticosterone levels were measured at this timepoint as well as seven days (i.e. “7 day”) post-UWT exposure.
Figure 1. Timeline of study. Behavioral testing (EPM and ASR) occurred at “Baseline” and “Post” time points. Blood sample collection occurred at “Baseline”, “Post”, and “7 day” time points. Adolescent and adult rats were either exposed to eight underwater trauma (UWT) or swim-only sessions (n = 12/group) between Baseline and Post time points.
Underwater trauma (UWT)
Rats were placed into a Plexiglas tank containing approximately 12 L of normal saline at room temperature. UWT rats swam for 40 sec in the saline and were then gently submerged using an inverted colander. Rats were fully submerged for 20 sec, then removed from the tank and briefly dried. This procedure was repeated for a total exposure eight times over 10 days (Tuesday–Friday, Monday–Thursday). Control groups of rats were allowed to swim for the full 60-sec timeframe, then were removed from the tank and briefly dried. All rats resumed typical home cage behaviors such as grooming rapidly after the procedure, regardless of treatment.
Behavioral assays
Elevated plus maze (EPM)
Exploratory behavior was observed using a Kinder Scientific plus maze (Poway, CA). The maze consisted of four arms in a “plus” configuration and was made of tinted plastic. Each arm was 50 cm long and 10 cm wide. Closed arms (east and west) had walls 40 cm in height. Open arms (north and south) had no walls. The maze was elevated 80 cm above the floor. Testing on the EPM apparatus was conducted over five-minute sessions in dim lighting conditions (Open arms 7 lx, Closed arms 1-2 lx, Intersection 2 lx). Photobeam tracking was used to quantify behavioral performance across the maze. We previously reported change in basic movements as a measure to describe changes in exploratory behavior after stress exposure in rats (Genovese & Dobre, Citation2017; Genovese, Johnson, Tobin, & Gauchan, Citation2014; Moore et al., Citation2012; Moore et al., Citation2016).
Acoustic startle reflex
Startle amplitude was measured in Kinder Scientific chambers (Poway, CA). A five-minute acclimatization period preceded startle trials. Each session consisted of 30 trials, with 70 dB white noise background and a 115 dB white noise startle pulse. Intertrial interval (ITI) was randomized and varied from 10 to 30 sec with an average ITI of 20 sec. The maximum startle force (Newtons, N) was recorded for each trial.
Blood draws
Rats were briefly restrained in a Plexiglas restraint tube, and 0.2 mL of blood was collected by tail vein nick between the hours of 0930 and 1030. Sample collection was completed in under five minutes per rat, on average 2–3 minutes. Blood was stored on ice for 30 minutes and then centrifuged at 3000×g for 30 minutes at 4 °C. Serum was removed and stored at −80 °C until use. Corticosterone (ng/mL) was quantified using LC-MS-MS.
LC-MS-MS corticosterone quantification
Chemicals
Corticosterone, dimethyl sulfoxide (DMSO) and formic acid were purchased from Sigma-Aldrich (St Louis, MO). Norit-A neutral decolorizing carbon and HPLC grade acetonitrile were purchased from Fisher Scientific (Hanover Park, IL). Deionized water was obtained from a Solution 2000™ water purification system (Montreal, Canada).
Calibration standard curve and quality controls (QCs) preparation
Serum standard curves and quality control samples were prepared in blank rat serum. Serum was stripped of endogenous corticosterone prior to addition of the corticosterone analytical standard using Norit-A neutral decolorizing carbon as described in Samtani et al. (Samtani & Jusko, Citation2007). Charcoal was added to blank rat serum at a concentration of 1 g/25 ml. The suspension was stirred at room temperature for seven hours and centrifuged overnight at 16,000 × g using a bench-top PrismR centrifuge (Labnet International, Inc., Edison, NJ) at 4 °C. The supernatant was filtered using 0.45-µm sterile Nalgene filters (Nalgene Nunc International, Penfield, NY) and stored at −80 °C until use.
A standard corticosterone stock solution of 1.00 mg/ml was prepared in DMSO. This stock solution was then used to make up a 10 µg/ml stock in acetonitrile. The calibration standard curve and QCs were prepared by spiking the stripped rat serum with the 10 µg/ml corticosterone stock. The calibration standard curve ranged from 1.0 to 1000 ng/ml. QCs samples were prepared to cover the low (1.0 ng/ml, 2.5 ng/ml and 5.0 ng/ml), medium (300 ng/ml) and high (750 ng/ml) concentration ranges of the standard curve. Standard curve and QC samples were then extracted with two volumes of acetonitrile containing internal standard (mefloquine in acetonitrile) for each volume of sample. A blank (no corticosterone) sample was prepared in the same manner. Each sample was vortexed and centrifuged at 16,000 × g for 10 minutes at 4 °C. The supernatant was transferred to a 96-well plate for LC-MS/MS analysis. The corticosterone standard curve was constructed from peak area values obtained from LC-MS/MS analyzes. Corticosterone serum samples were extracted in the same manner with two volumes of internal standard. Corticosterone concentrations in samples were extrapolated from each corresponding standard curve.
Liquid chromatography -tandem mass spectrometry analysis
A Waters ACQUITY separation module UPLC coupled to an AB Sciex QTrap 4000 triple quadruple mass spectrometer equipped with a Turbo-V source was used for mass analyses. The analysis of corticosterone was performed using an Xterra® (2.1 × 50 mm, 3.5 µm) column, maintained at room temperature. The auto-sampler was maintained at 4 °C to minimize evaporation. 10 µl of sample was injected onto the column. Samples were eluted using a linear gradient of 5% to 98% (acetonitrile/0.1% formic acid in water) over 2.0 minutes at the flow rate of 0.400 ml/min. The analysis was performed in multiple reaction monitoring (MRM) in positive electrospray ionization mode by monitoring the ion transitions: m/z 347.10&cenveo_unknown_entity_wingdings_F0E0;121.20 (corticosterone) and m/z 379.10 &cenveo_unknown_entity_wingdings_F0E0; 361.10 (mefloquine). Compound parameters and source parameters were optimized to obtain the highest ion intensity of the analyte. The collision energy (CE), collision cell exit potential (CXP), declustering potential (DP) and entrance potential (EP) for corticosterone were 39.00 V, 10.00 V, 116.00 V, and 10.00 V, respectively. The collision energy (CE), collision cell exit potential (CXP), declustering potential (DP) and entrance potential (EP) for mefloquine were 35.00 V, 10.00 V, 71.00 V, and 10.00 V, respectively. The source parameters were optimized as follows: curtain gas (CUR), 20.00; collision activated dissociation (CAD), high; ionspray voltage (IS), 5500.00 V; nebulizer gas (GS1), 30.00; heater gas (GS2), 30.00; and source heater (TEM), 300.00. The instrument was controlled and data were collected using Analyst® software.
Data analysis
Data are presented as mean ± SEM. All data were analyzed using SPSS 24 (IBM Corporation, Armonk, NY) and graphed using Prism 7 (GraphPad Software Inc., San Diego, CA). Behavioral data (i.e. change from baseline scores) were analyzed using one-way ANOVA with Tukey post hoc tests to assess specific between-group differences of significant ANOVA terms. For corticosterone data, a two-way repeated measures ANOVA was used. Factors of group and time were tested, with a repeated measure of time and Tukey post hoc tests to assess specific between-group differences of significant ANOVA terms. An alpha level of 0.05 was considered significant in all tests.
Results
Exploratory behavior
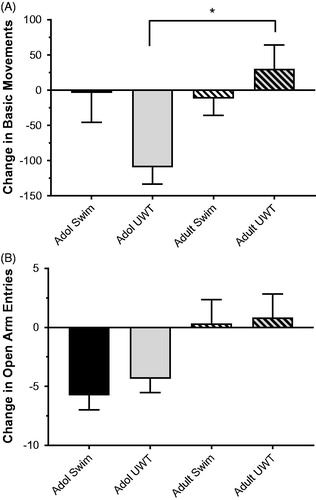
Exploratory behavior was tested on the EPM at baseline and one day after the last UWT (or swim) exposure. A measure of general locomotor activity on this task, Basic Movements, was used in lieu of tests specific to “anxiety-like behavior” as described in previously published studies (Genovese & Dobre, Citation2017; Genovese et al., Citation2014; Moore et al., Citation2016). Change in basic movements from baseline was measured as the principal dependent variable. For basic movements, adolescent and adult rats performed differently on the EPM (main effect of Age, F[1,48] = 4.240, p = .045. Also, adolescent and adults responded differently after traumatic stress exposure (Age × Treatment interaction, F[1, 44] = 5.32, p = .026; ). In both age groups, no change in exploratory behavior was observed across time points in the procedural control group (swim only). The sole change was observed after UWT exposure, which was different between adolescent UWT and adult UWT groups (Tukey post hoc test, p = .018; ). For open arm entries, there was a main effect of age (F[1, 44] = 11.51, p = .001), where the number of open arm entries was decreased in adolescent groups relative to baseline, whereas there was little change over time in adult groups. Repeated UWT exposures did not affect open arm entries in either age group.
Figure 2. Change in basic movements (A) and open arm entries (B) in the EPM after eight exposures to UWT. Adolescent rats in the UWT group exhibited an adverse behavioral response relative to adult UWT rats, with a negative change between Baseline and Post time points. Data represent mean ± SEM. *significantly different from adult UWT, p < .05. N = 12 per group.
Acoustic startle
Acoustic startle response was tested at baseline and one day after the last UWT exposure. Percent change between baseline and postexposure max startle values was determined. ANOVA revealed no significant effects of age (F[1, 44] = 1.36, p = .249), treatment (F[1, 44] = 0.018, p = .895) or interaction (F[1,48] = 1.620, p = .210) resulting from the procedural control or UWT (data not shown).
Corticosterone
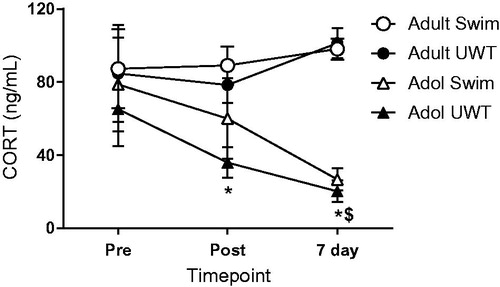
Blood was drawn via tail nick at baseline, one day after the last UWT exposure, and again at the same time of day seven days later (i.e. eight days after the last UWT exposure). Baseline values had the largest variance, which may have been related to the variance in baseline blood draw times. Adolescents and adults responded differently (effect of Age, F[1, 44] = 14.026, p = .001) and showed divergent trends across the time points collected (Age × Time interaction, Greenhouse–Geisser corrected, F [1.614, 71.013] = 4.783, p = .017; ), where circulating corticosterone levels decreased in adolescent groups over time (effect of Time, Greenhouse–Geisser corrected, F[1.476, 34.464] = 5.882, p = .012) but not in adult groups. Repeated UWT exposures, however, did not significantly affect corticosterone levels for either adolescent or adult groups. Post hoc analyses revealed a significant difference at post and 7-day time points between adolescent and adult UWT groups (post: p = .026; 7-day: p < .0001), as well as a significant difference at the 7-day time point between adolescent and adult swim-only control groups (p < .0001; ).
Figure 3. Corticosterone changes as a percent of baseline measurements as a function of age across timepoints. Generally, adolescents decreased and adults increased circulating CORT over the timepoints measured. *significantly different from Adult UWT, $significantly different from Adult Swim, p < .05. N = 12 per group.
Discussion
Exposure to acute or repeated traumatic stress in adolescence, a period in which fluctuation of hormones and developmental changes occur, may result in a host of potential negative consequences including a hindered ability for effective coping in adulthood, adverse behavioral changes, and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Horovitz, Tsoory, Hall, Jacobson-Pick, & Richter-Levin, Citation2012; for review, Eiland & Romeo, Citation2013; Romeo, Citation2010; Tsoory & Richter-Levin, Citation2006). Therefore, a susceptibility to stress in adolescence may translate to persistent adverse effects on various biologically-relevant processes that may hinder daily functioning. In rats, a single UWT exposure or its contextual reminder has been shown to have robust detrimental effects on behavioral outcomes, perhaps due to dysregulation of amygdalar neuronal activity (Ardi, Ritov, Lucas, & Richter-Levin, Citation2014; Ritov & Richter-Levin, Citation2014). Our laboratory has previously reported that a single UWT exposure during the adolescence period in Sprague-Dawley rats resulted in acute and lasting adverse behavioral outcomes, suggesting the susceptibility of maturing pathways to stressful events (Moore et al., Citation2012; Moore et al., Citation2014; Moore et al., Citation2016).
Here, we investigated the impact of repeated stress exposure via UWT on behavioral and neuroendocrine response adaptation. We observed that exposures to repeated stress in adulthood resulted in little change in behavioral and neuroendocrine outcome measures; however, adolescent exposure to repeated stress resulted in significant effects on behavioral measures after the repeated stressor exposure, suggesting that adolescents may have under-developed stress coping mechanisms. These and other findings highlight that the temporal window during which stress exposure takes place is an impactful factor on stress-related outcomes and thus should be taken in to account in evaluating preclinical models designed to understand stress effects. Moreover, these findings have clinical implications for patient populations with a trauma history during adolescence and complement reports of adverse behavioral and neuroendocrine changes especially among those who were exposed to repeated stress at a young age, who have enhanced susceptibility to anxiety disorders and risk-taking behaviors later in life.
In the present study, we observed a significant reduction in exploratory behavior in the EPM only for adolescent rats that had undergone repeated UWT exposures, a finding that is in agreement with previous preclinical reports demonstrating adverse behavioral effects after adolescent stress exposure (Bazak et al., Citation2009; Moore et al., Citation2012; for review, McCormick & Green, Citation2013; Luo et al., Citation2014). Moreover, the present results are concordant with previous observations in our laboratory that adverse behavioral change on the EPM decreases in severity after a greater number of predator presentations in adult rats (Genovese et al., Citation2014). Evidence from the literature suggests that adverse behavioral outcomes following adolescent stress likely stem from the many effects of adolescent stress exposure on brain function. For example, adolescent rodents display a greater susceptibility to detrimental stress-related behavioral effects and subsequent prolonged activation of the locus coeruleus-norepinephrine system than adult rats (Stone & Quartermain, Citation1997; Zitnik, Curtis, Wood, Arner, & Valentino, Citation2016). Adolescents also display delayed adaptation to other experimental conditions, such as tolerance to delta-9-tetrahydrocannibinol and associated learning impairments in a Morris water maze task (Moore et al., Citation2010). Therefore, delayed or immature adaptive processes during adolescence may impact the behavioral responsiveness to repeated stress exposure during this critical period. This phenomenon may also play a role in the lasting effects of an insult experienced during development on adaptive processes later in life.
An underlying developmental difference in physiology between adolescents and adults may lead to greater detrimental effects on lifetime functionality among those who experienced stress during adolescence. Indeed, differences in physiological processes like hormonal reactivity, HPA responsiveness to stress, as well as stress-induced changes to relevant neurocircuitry and gene expression may make the adolescent rat more vulnerable to behavioral impairments later in life relative to adult counterparts (Coppens et al., Citation2011; de Araujo Costa Folha et al., Citation2017; McCormick, Mathews, Thomas, & Waters, Citation2010; Romeo et al., Citation2016). In the present study, we observed that adults have overall greater levels of circulating corticosterone at multiple timepoints, regardless of stress treatment. In the adolescent groups, corticosterone release decreased over time, similar to previous findings in our lab (Moore et al., Citation2012; Moore et al., Citation2014; Moore et al., Citation2016). The effects of repeated stress on corticosterone release in rats have varied in the literature, with some studies reporting increases (Doremus-Fitzwater, Varlinskaya, & Spear, Citation2009; McCormick, Merrick, Secen, & Helmreich, Citation2007) and others decreases in corticosterone after repeated stress exposure. These conflicting observations mimic clinical inconsistencies in cortisol after stress exposure (Wust, Federenko, van Rossum, Koper, & Hellhammer, Citation2005). Inconsistencies in directionality of post-stress HPA responses indeed pose challenges in interpretation, and it is therefore difficult to determine if long-term decreases in cortisol/corticosterone are eventually detrimental or beneficial to recovery efforts. Consistent with our present findings, differences between adolescent and adult corticosterone release following mild acute stress or habituation of corticosterone release after repeated stress have been reported (Doremus-Fitzwater et al., Citation2009; Hodges & McCormick, Citation2015; McCormick, Smith, & Mathews, Citation2008; Weintraub, Singaravelu, & Bhatnagar, Citation2010). It remains unclear why all adolescent corticosterone values decreased over the course of the study, this effect may have been due to lingering effects of the swim-only procedure among control subjects and/or blood draws. These may have produced an inhibitory tone on the HPA axis in adolescents despite the adverse behavioral response. Previously, we found that 7 days after a single UWT exposure, adolescent rats had decreased basal CORT, but not after swim (Moore et al., Citation2012). In the present study, this effect was present only for adolescent rats, though adults received the same experimental manipulations. While more studies are required to directly examine this phenomenon, the unusual corticosterone responses in the adolescent groups suggests further that this development period carries the risk of enhanced vulnerability to stress.
Importantly, inconsistencies across the preclinical stress research literature may certainly be due to age among other methodological differences, such as the type of stressor used. Inconsistent findings may also reflect the rapid neurodevelopmental changes that occur throughout adolescence, and associated impact of stress on neurodevelopment throughout this timeline. Notably, previous findings in our lab were conducted within the same time window in adolescence (P34-P41) as the present study and have consistently shown blunted corticosterone over time after adolescent stress exposure, regardless of stressor type (Moore et al., Citation2012; Moore et al., Citation2014; Moore et al., Citation2016). It is possible that the effect of age on corticosterone response in the present study was an underlying factor in the lower impact of the repeated stressors in the adult group.
It is important to note that the present findings were observed at experimentally-defined time points and do not preclude the possibility of eventual recovery later in life. Others have reported intact adaptation or even a strengthened form of stress-coping or resilience after repeated stress exposure during adolescence (Sadler & Bailey, Citation2016). It is possible that these previous reports captured an eventual recovery period in rodents repeatedly-stressed during adolescence. Recovery of neuronal morphology changes in the hippocampus and amygdala vary after repeated stress in the adult rat and delayed hippocampal volume deficits occur in rats given a longer recovery time (Isgor, Kabbaj, Akil, & Watson, Citation2004; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, Citation2002; Vyas, Pillai, & Chattarji, Citation2004). Recovery from the effects of chronic stressors therefore depends on the stage of development of an organism (for reviews, see Eiland & Romeo, Citation2013; Hollis, Isgor, and Kabbaj, Citation2013). As adolescence is a developmental period marked by enriched plasticity of mechanisms underlying brain function relative to adulthood, it is also possible that neurophysiological recovery is more likely or more rapid among this population. Additionally, a limitation of the present study was the number and time of collections for circulating corticosterone (taken only once on the days of collection in the early part of the light phase). Therefore, diurnal and ultradian rhythmic patterns of corticosterone and the influence of repeated stress on disrupting these patterns must be considered (for review, see Young, Abelson, and Lightman, Citation2004; Atkinson, Wood, Kershaw, Bate, and Lightman, Citation2006). Taken together, these findings reaffirm the need to better understand the adolescent responses to traumatic stress and the timecourse for which recovery, if any, occurs.
Adaptive responsiveness to environmental stress is a necessary process to promote organismal and species survival. Exposure to early life stress (e.g. physical or sexual abuse, traumatic life changes, military combat, etc.) increases the risk to develop stress- and anxiety-related disorders, such as PTSD (Kilpatrick et al., Citation2003; van der Kolk, Roth, Pelcovitz, Sunday, & Spinazzola, Citation2005). Here, we show that stress response is immature in adolescent rats, with robust adverse behavioral responses following eight repeated underwater trauma exposures. Neuroendocrine shifts were also observed at parallel time points, suggesting that adolescence is a period of particular vulnerability to stress. Further studies should explore the timecourse of the persistence of these behavioral and neuroendocrine effects, and whether the immature response observed acutely in the present study produces similarly robust behavioral and neuroendocrine deficits into adulthood. Moreover, behavioral and neuroendocrine changes measured over the course of repeated stress exposure may further characterize differences between adolescent and adult response to stress.
Ethical approval
Studies were conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals and adhere to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition. All procedures were reviewed and approved by the Walter Reed Army Institute of Research/Naval Medical Research Center Institutional Animal Care and Use Committee, and performed in facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Acknowledgements
The authors thank Stefania Dobre and Christina Johnson for laboratory assistance, Dr. Emily G. Lowery-Gionta for helpful comments on the manuscript, and Dr. Raymond F. Genovese.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ardi, Z., Ritov, G., Lucas, M., & Richter-Levin, G. (2014). The effects of a reminder of underwater trauma on behaviour and memory-related mechanisms in the rat dentate gyrus. International Journal of Neuropsychopharmacology, 17, 571–580. doi:10.1017/S1461145713001272
- Atkinson, H., Wood, S., Kershaw, Y., Bate, E., & Lightman, S. (2006). Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. Journal of Neuroendocrinology, 18, 526–533. doi:10.1111/j.1365-2826.2006.01444.x
- Bazak, N., Kozlovsky, N., Kaplan, Z., Matar, M., Golan, H., Zohar, J., … Cohen, H. (2009). Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor. Psychoneuroendocrinology, 34, 844–858. doi:10.1016/j.psyneuen.2008.12.018
- Cabrera, O.A., Hoge, C.W., Bliese, P.D., Castro, C.A., & Messer, S.C. (2007). Childhood adversity and combat as predictors of depression and post-traumatic stress in deployed troops. American Journal of Preventive Medicine, 33, 77–82. doi:10.1016/j.amepre.2007.03.019
- Cohen, H., Liberzon, I., & Richter-Levin, G. (2009). Exposure to extreme stress impairs contextual odour discrimination in an animal model of PTSD. International Journal of Neuropsychopharmacology, 12, 291–303. doi:10.1017/S146114570800919X
- Coppens, C.M., Siripornmongcolchai, T., Wibrand, K., Alme, M.N., Buwalda, B., de Boer, S.F., … Bramham, C.R. (2011). Social defeat during adolescence and adulthood differentially induce BDNF-regulated immediate early genes. Frontiers in Behavioral Neuroscience, 5, 72. doi:10.3389/fnbeh.2011.00072
- de Araujo Costa Folha, O.A., Bahia, C.P., de Aguiar, G.P.S., Herculano, A.M., Coelho, N.L.G., de Sousa, M.B.C., … Pereira, A. (2017). Effect of chronic stress during adolescence in prefrontal cortex structure and function. Behavioral and Brain Research, 326, 44–51. doi:10.1016/j.bbr.2017.02.033
- Doremus-Fitzwater, T.L., Varlinskaya, E.I., & Spear, L.P. (2009). Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology and Behavior, 97, 484–494. doi:10.1016/j.physbeh.2009.03.025
- Eiland, L., & Romeo, R.D. (2013). Stress and the developing adolescent brain. Neuroscience, 249, 162–171. doi:10.1016/j.neuroscience.2012.10.048
- Genovese, R.F., & Dobre, S. (2017). Mitigation of adverse behavioral impact from predator exposure by the nociceptin/orphanin FQ peptide antagonist J-113397 in rats. Behavioral Pharmacology, 28, 521–530. doi:10.1097/FBP.0000000000000329
- Genovese, R.F., Johnson, C.C., Tobin, C.A., & Gauchan, S. (2014). Multiple presentations reduce the behavioral impact of protected predator exposure in rats. Behavioral Processes, 108, 105–109. doi:10.1016/j.beproc.2014.09.032
- Hodges, T.E., & McCormick, C.M. (2015). Adolescent and adult male rats habituate to repeated isolation, but only adolescents sensitize to partner unfamiliarity. Hormones and Behavior, 69, 16–30. doi:10.1016/j.yhbeh.2014.12.003
- Hollis, F., Isgor, C., & Kabbaj, M. (2013). The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience, 249, 232–241. doi:10.1016/j.neuroscience.2012.09.018
- Horovitz, O., Tsoory, M.M., Hall, J., Jacobson-Pick, S., & Richter-Levin, G. (2012). Post-weaning to pre-pubertal ('juvenile') stress: a model of induced predisposition to stress-related disorders. Neuroendocrinology, 95, 56–64. doi:10.1159/000331393
- Isgor, C., Kabbaj, M., Akil, H., & Watson, S.J. (2004). Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus, 14, 636–648. doi:10.1002/hipo.10207
- Jankord, R., Solomon, M.B., Albertz, J., Flak, J.N., Zhang, R., & Herman, J.P. (2011). Stress vulnerability during adolescent development in rats. Endocrinology, 152, 629–638. doi:10.1210/en.2010-0658
- Kilpatrick, D.G., Ruggiero, K.J., Acierno, R., Saunders, B.E., Resnick, H.S., & Best, C.L. (2003). Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the national survey of adolescents. Journal of Consulting and Clinical Psychology, 71, 692–700. doi:10.1037/0022-006X.71.4.692
- Luo, X.M., Yuan, S.N., Guan, X.T., Xie, X., Shao, F., & Wang, W.W. (2014). Juvenile stress affects anxiety-like behavior and limbic monoamines in adult rats. Physiology and Behavior, 135, 7–16. doi:10.1016/j.physbeh.2014.05.035
- McCormick, C.M., & Green, M.R. (2013). From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience, 249, 242–257. doi:10.1016/j.neuroscience.2012.08.063
- McCormick, C.M., Mathews, I.Z., Thomas, C., & Waters, P. (2010). Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition, 72, 73–85. doi:10.1016/j.bandc.2009.06.003
- McCormick, C.M., Merrick, A., Secen, J., & Helmreich, D.L. (2007). Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. Journal of Neuroendocrinology, 19, 116–126. doi:10.1111/j.1365-2826.2006.01515.x
- McCormick, C.M., Smith, C., & Mathews, I.Z. (2008). Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behaviral Brain Research, 187, 228–238. doi:10.1016/j.bbr.2007.09.005
- Moore, N.L., Altman, D.E., Gauchan, S., & Genovese, R.F. (2016). Adulthood stress responses in rats are variably altered as a factor of adolescent stress exposure. Stress, 19, 295–302. doi:10.1080/10253890.2016.1191465
- Moore, N.L., Gauchan, S., & Genovese, R.F. (2012). Differential severity of anxiogenic effects resulting from a brief swim or underwater trauma in adolescent male rats. Pharmacology, Biochemistry, and Behavior, 102, 264–268. doi:10.1016/j.pbb.2012.05.002
- Moore, N.L., Gauchan, S., & Genovese, R.F. (2014). Adolescent traumatic stress experience results in less robust conditioned fear and post-extinction fear cue responses in adult rats. Pharmacology Biochemistry and Behavior, 120, 17–24. doi:10.1016/j.pbb.2014.01.011
- Moore, N.L., Greenleaf, A.L., Acheson, S.K., Wilson, W.A., Swartzwelder, H.S., & Kuhn, C.M. (2010). Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. Journal of Pharmacology and Experimental Therapeutics, 335, 294–301. doi:10.1124/jpet.110.169359
- Richter-Levin, G. (1998). Acute and long-term behavioral correlates of underwater trauma–potential relevance to stress and post-stress syndromes. Psychiatry Research, 79, 73–83. doi:10.1016/S0165-1781(98)00030-4
- Ritov, G., Ardi, Z., & Richter-Levin, G. (2014). Differential activation of amygdala, dorsal and ventral hippocampus following an exposure to a reminder of underwater trauma. Frontiers in Behavioral Neuroscience, 8, 18. doi:10.3389/fnbeh.2014.00018
- Ritov, G., & Richter-Levin, G. (2014). Water associated zero maze: a novel rat test for long term traumatic re-experiencing. Frontiers in Behavioral Neuroscience, 8, 1. doi:10.3389/fnbeh.2014.00001
- Romeo, R.D. (2010). Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Frontiers in Neuroendocrinology, 31, 232–240. doi:10.1016/j.yfrne.2010.02.004
- Romeo, R.D., Patel, R., Pham, L., & So, V.M. (2016). Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neuroscience and Biobehavioral Reviews, 70, 206–216. doi:10.1016/j.neubiorev.2016.05.020
- Sadler, A.M., & Bailey, S.J. (2016). Repeated daily restraint stress induces adaptive behavioural changes in both adult and juvenile mice. Physiology and Behavior, 167, 313–323. doi:10.1016/j.physbeh.2016.09.014
- Samtani, M.N., & Jusko, W.J. (2007). Quantification of dexamethasone and corticosterone in rat biofluids and fetal tissue using highly sensitive analytical methods: assay validation and application to a pharmacokinetic study. Biomedical Chromatography, 21, 585–597. doi:10.1002/bmc.788
- Sood, R., Ritov, G., Richter-Levin, G., & Barki-Harrington, L. (2013). Selective increase in the association of the β2 adrenergic receptor, β Arrestin-1 and p53 with Mdm2 in the ventral hippocampus one month after underwater trauma. Behavioural Brain Research, 240, 26–28. doi:10.1016/j.bbr.2012.11.009
- Sood, R., Ritov, G., Boltyansky, B., Spector-Chotiner, A., Richter-Levin, G., & Barki-Harrington, L. (2014). Underwater trauma causes a long-term specific increase in the expression of cyclooxygenase-2 in the ventral CA1 of the hippocampus. Pyschoneuroendocrinology, 49, 62–68. doi:10.1016/j.psyneuen.2014.06.015
- Spear, L.P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24, 417–463. doi:10.1016/S0149-7634(00)00014-2
- Stone, E.A., & Quartermain, D. (1997). Greater behavioral effects of stress in immature as compared to mature male mice. Physiology and Behavior, 63, 143–145. doi:10.1016/S0031-9384(97)00366-1
- Tsoory, M., & Richter-Levin, G. (2006). Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. The International Journal of Neuropsychopharmacology, 9, 713–728. doi:10.1017/S1461145705006255
- van der Kolk, B.A., Roth, S., Pelcovitz, D., Sunday, S., & Spinazzola, J. (2005). Disorders of extreme stress: The empirical foundation of a complex adaptation to trauma. Journal of Trauma Stress, 18, 389–399. doi:10.1002/jts.20047
- Vyas, A., Mitra, R., Shankaranarayana Rao, B.S., & Chattarji, S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience, 22, 6810–6818. doi:20026655
- Vyas, A., Pillai, A.G., & Chattarji, S. (2004). Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience, 128, 667–673. doi:10.1016/j.neuroscience.2004.07.013
- Wang, J., Akirav, I., & Richter-Levin, G. (2000). Short-term behavioral and electrophysiological consequences of underwater trauma. Physiology and Behavior, 70, 327–332. doi:10.1016/S0031-9384(00)00274-2
- Weintraub, A., Singaravelu, J., & Bhatnagar, S. (2010). Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Research, 1343, 83–92. doi:10.1016/j.brainres.2010.04.068
- Wust, S., Federenko, I.S., van Rossum, E.F., Koper, J.W., & Hellhammer, D.H. (2005). Habituation of cortisol responses to repeated psychosocial stress-further characterization and impact of genetic factors. Psychoneuroendocrinology, 30, 199–211. doi:10.1016/j.psyneuen.2004.07.002
- Young, E.A., Abelson, J., & Lightman, S.L. (2004). Cortisol pulsatility and its role in stress regulation and health. Frontiers in Neuroendocrinology, 25, 69–76. doi:10.1016/j.yfrne.2004.07.001
- Zitnik, G.A., Curtis, A.L., Wood, S.K., Arner, J., & Valentino, R.J. (2016). Adolescent social stress produces an enduring activation of the rat locus coeruleus and alters its coherence with the prefrontal cortex. Neuropsychopharmacology, 41, 1376–1385. doi:10.1038/npp.2015.289
