Abstract
Circulating concentrations of interleukin (IL)-6, an inflammatory biomarker widely assessed in humans to study the inflammatory response to acute psychological stress, have for decades been quantified using enzyme-linked immunosorbent assay (ELISA). However, biobehavioral researchers are increasingly using cytokine multiplex assays instead of ELISA to measure IL-6 and other cytokines. Despite this trend, multiplex assays have not been directly compared to ELISA for their ability to detect subtle stress-induced changes of IL-6. Here, we tested the prediction that a high-sensitivity multiplex assay (human Magnetic Luminex Performance Assay, R&D Systems, Minneapolis, MN) would detect changes in IL-6 as a result of acute stress challenge in a manner comparable to high-sensitivity ELISA. Blood was collected from 12 healthy adults immediately before and then 90 and 210 min after the start of the Trier Social Stress Test (TSST), an acute laboratory psychosocial stress challenge. In addition to quantifying IL-6 concentrations in plasma with both multiplex and ELISA, we also assessed concentrations of tumor necrosis factor-alpha, IL-8, IL-10, IL-5, and IL-2 with multiplex. The multiplex detected IL-6 in all samples. Concentrations strongly correlated with values determined by ELISA across all samples (r = 0.941, p < .001) as well as among samples collected at individual TSST time points. IL-6 responses to the TSST (i.e. area under the curve) captured by multiplex and ELISA were also strongly correlated (rs = 0.937, p < .001). While other cytokines were detected by multiplex, none changed as a result of TSST challenge at time points examined. These results suggest high-sensitivity magnetic multiplex assay is able to detect changes in plasma concentrations of IL-6 as a result of acute stress in humans.
Introduction
The physiological response to challenge with psychological stress includes changes in immune function and inflammation. The inflammatory response to stress can be measured using enumerative as well functional tests including assessment of circulating concentrations of inflammatory cytokines (Marsland, Walsh, Lockwood, & John-Henderson, Citation2017; Segerstrom & Miller, Citation2004). Inflammatory cytokines in the circulation originate from a variety of tissues including leukocytes, hepatocytes, and bone marrow cells (Turner, Nedjai, Hurst, & Pennington, Citation2014). Production of inflammatory cytokines from these cells in the context of stress is regulated in part by noradrenergic stimulation of various leukocyte subsets (Kolmus, Tavernier, & Gerlo, Citation2015; Padro & Sanders, Citation2014). In humans, responsiveness of circulating concentrations of inflammatory cytokines to stress has been found to associate with a number of variables including depression, prior stress exposure, cancer history, and tobacco smoking (Bennett et al., Citation2013; Marsland et al., Citation2017; Pace et al., Citation2006). Several inflammatory cytokines are responsive to acute stress challenge, including interleukin (IL)-6, IL-10, IL-1 beta, and tumor necrosis factor (TNF)-alpha (Marsland et al., Citation2017). A recent meta-analysis by Marsland et al. (Citation2017) suggests that among these biomarkers, IL-6 is measured most often.
Enzyme-linked immunosorbent assay (ELISA) has been the predominant method used by biobehavioral researchers to assess IL-6 in the context of stress. However, in recent years the use of multiplex assays to assess inflammatory cytokines has become more widespread (Marsland et al., Citation2017; Mills & Peterson, Citation2016). Multiplex assays are an attractive alternative to ELISA because they allow simultaneous assessment of multiple cytokines in one portion of plasma or serum. However, there are concerns about the reliability of these assays, as well as the absolute concentrations of cytokines detected by them (Breen et al., Citation2011). Accordingly, the purpose of this study was to compare a multiplex assay to high-sensitivity ELISA to detect stress-induced changes in circulating concentrations of IL-6 in healthy human adults.
We obtained plasma before and after challenge with the Trier Social Stress Test (TSST), a widely-used acute psychosocial stress task that induces social evaluative threat using a public speaking task and mental arithmetic (Kirschbaum, Pirke, & Hellhammer, Citation1993). Plasma samples collected from participants were first analyzed for concentrations of IL-6 using high-sensitivity ELISA, and then with a high-sensitivity magnetic multiplex assay. In an exploratory manner, we also assessed the responsiveness of concentrations of additional inflammatory cytokines to the TSST with the multiplex assay (IL-2, IL-5, IL-8, IL-10, and TNF-α). While focusing primarily on the IL-6 response to the TSST, other cytokines were included because they have been previously explored in the context of the TSST (de Brouwer et al., Citation2014). We hypothesized that the multiplex assay would detect the change in IL-6 as a result of acute stress challenge in a manner comparable to the ELISA.
Methods
Participants
Twelve medically and psychiatrically healthy adults (seven women) (mean age 35.08 [SD = 8.88] years, mean body mass index 24.45 [SD = 3.93]) from metropolitan Atlanta took part in the study. Health status was determined by physical exam. Participant plasma sets were selected for multiplex analysis from a larger parent project on the effects of meditation on behavior and stress biology that included challenge with the TSST. Participants were included in the current investigation based on the magnitude of their IL-6 response to the TSST measured using high-sensitivity ELISA (see assay details below). We randomly selected individuals from the parent project with either below (attenuated) or above (robust) median IL-6 responses to the TSST, defined as an area under the curve from the initial baseline value (AUCi) (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, Citation2003) in order to select a subset of participants with IL-6 responses to the TSST across the response range. All participants of the parent study provided written informed consent and all study procedures were approved by the Emory University Institutional Review Board.
Trier Social Stress Test and blood collection
The TSST is a standardized laboratory psychosocial stress test that reliably activates inflammatory responsiveness to stress measurable at the level of several biomarkers, including inflammatory cytokines (Marsland et al., Citation2017), and consists of an instruction/anticipation phase followed by a 5 min public speaking task and a 5 min mental arithmetic task (Kirschbaum et al., Citation1993). Participants arrived at the Atlanta Clinical and Translational Science Institute at Emory University Hospital 6 h before the TSST stressor to allow for acclimatization and parent study assessments (physical exam and questionnaires). Participants were provided a standard low-fat lunch 2.5 h prior to the TSST, and an IV catheter was placed in the antecubital vein 1 h before the start of the TSST. Based on findings from a previous study by our group and the findings of others demonstrating a time-lag in IL-6 responses (Marsland et al., Citation2017; Pace et al., Citation2006), IL-6 concentrations were assessed in plasma collected immediately before the TSST and then again at 90 and 210 min after the start of the stressor. Whole blood was collected from the indwelling catheter into EDTA-coated monovettes and immediately centrifuged at 4 °C. Plasma was aliquoted and stored at −80 °C until batch assay. Thirty-six total plasma samples were analyzed.
Interleukin-6 determination by enzyme-linked immunosorbent assay
Unthawed plasma aliquots were assayed for IL-6 with a high-sensitivity ELISA (R&D Systems, Minneapolis, MN). The IL-6 ELISA was completed according to manufacturer instructions except for the use of a TomTec Quadra Tower (TomTec, Hamden, CT) to simultaneously load all samples and standards onto assay plates. All samples from a given individual were assayed within the same assay plate. The intra- and inter-assay variability for the IL-6 hs-ELISA were 7 and 13%, respectively. The lower limit of detection was 0.16 pg/ml and sensitivity of the assay (stated by the manufacturer) was 0.04 pg/ml. High- and low-concentration cytokine quality controls that included IL-6 were run on each plate (Randox Laboratories, Crumlin, UK).
Cytokine determination by magnetic multiplex assay
Unthawed plasma aliquots were analyzed for IL-6 and other cytokines (IL-2, IL-5, IL-8, IL-10, and TNF-α) using the human Magnetic Luminex Performance Assay (R&D Systems). A MAGPIX (Luminex, Austin, TX) was used to read the multiplex assay. As with the ELISA, all sample time points for a given individual were assayed within the same sample plate; only one assay plate was run. The magnetic multiplex assay and ELISA were run in the same laboratory on the University of Arizona campus. A 5-parameter logistic curve was used to interpolate cytokine concentrations using GraphPad Prism (La Jolla, CA) (Breen, Tan, & Khan, Citation2016). For IL-6, intra-assay variability was 5%, the lower limit of detection was 0.22 pg/ml and sensitivity of the assay (stated by the manufacturer) was 0.31 pg/ml.
Statistical analyses
We considered ELISA and multiplex assay performance in terms of the number of samples detected, the average concentration of IL-6 across all plasma samples, and concentrations detected for the low- and high-concentration quality controls. Concentrations of IL-6 and the other cytokines examined with the multiplex were first explored descriptively by computing means and standard error of the mean by TSST time point. Cytokine data that were not normally distributed (Shapiro–Wilk test) were natural log transformed before inferential testing. The association between plasma concentrations of IL-6 obtained with the ELISA and the multiplex was examined using a Pearson product-moment correlation coefficient. Plasma IL-6 concentration data returned by the ELISA and multiplex were also compared to one another using a 2-way ANOVA for repeated measures (assay X TSST time point), with follow-up paired sample t-tests corrected for multiple comparisons (to test for differences between assay methods at each TSST time point). Differences in concentrations of other cytokines determined by multiplex across the three TSST time points were analyzed using a 1-way ANOVA. Finally, in order to determine the association of IL-6 responses to the TSST determined by the ELISA and multiplex, TSST responses were aggregated as area under the curve for the initial value (AUCi) (Pruessner et al., Citation2003) before a Spearman’s rank correlation coefficient was computed. Non-log transformed values of IL-6 were used for this correlation because log transformed IL-6 concentrations were not amenable to an AUC approach.
Results
Both the ELISA and multiplex assays detected IL-6 in 100% of the samples tested, and absolute concentrations of IL-6 in low and high concentration quality controls were comparable (). Although the ELISA and the multiplex both detected comparable concentrations for the low concentration control, the ELISA appeared to under-detect the concentration of IL-6 in the high concentration control (). IL-6 concentrations were natural log transformed before further analyses, as data generated by both assays were not normally distributed. Across all plasma samples studied (N = 36), and regardless of TSST time point, concentrations of IL-6 determined by the ELISA were strongly correlated with concentrations of IL-6 determined by the multiplex, r = 0.941, p < .001 (). Concentrations of IL-6 between assays were also strongly correlated with one another within specific TSST time points (r = 0.808, p = .001, r = 0.958, p < .001, and r = 0.945, p < .001 in plasma collected before and 90 and 210 min after start of the TSST, respectively). Although challenge with the TSST significantly increased plasma concentrations of IL-6 from baseline to 90 and 210 min (F [1.37, 14.97] = 17.77, p < .001), IL-6 concentrations across the TSST did not differ between the high-sensitivity ELISA, and the multiplex (F [1, 22] = 0.20, p = .66). Concentrations of IL-6 from the two assay methods were also not different from one another at each of the three TSST time points (). Trajectories of IL-6 for each participant across the TSST and by assay method are shown in Supplemental Figure 1. IL-6 responses (aggregated as AUCi) determined by the high-sensitivity ELISA were strongly correlated with IL-6 responses determined by the magnetic multiple assays, rs = 0.937, p < .001 (). Of note, concentration of IL-6 in one of the samples fell below the assay sensitivity stated by the manufacturer for the magnetic multiplex assay. Removal of this data point from the analyses did not have a significant impact on the outcomes noted above. Finally, although IL-2, IL-5, IL-8, IL-10, and TNF-α were detected in 100% of the samples, there were no changes in plasma concentrations of these inflammatory biomarkers across the TSST at the time points assessed ().
Figure 1. Plasma concentrations of interleukin-6 detected by magnetic multiplex assay (R&D Systems) and high-sensitivity enzyme-linked immunosorbent assay (ELISA) are strongly and positively correlated with one another (r = 0.941, p < .001).
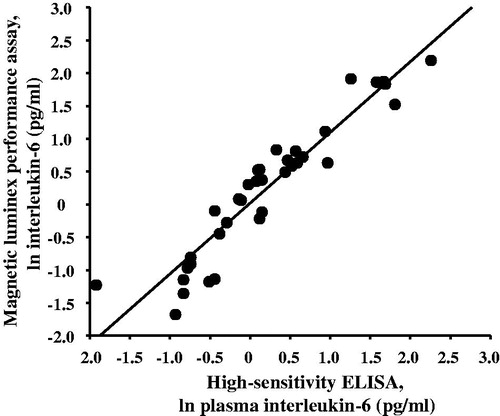
Figure 2. Concentrations of interleukin (IL)-6 were measured in plasma collected before, 90 and 210 min after the start of the Trier Social Stress Test (TSST) using high-sensitivity enzyme-linked immunosorbent assay (ELISA) or magnetic multiplex assay (R&D Systems). *vs. pre-TSST baseline IL-6 determined by ELISA; **vs. pre-TSST baseline IL-6 determined by multiplex.
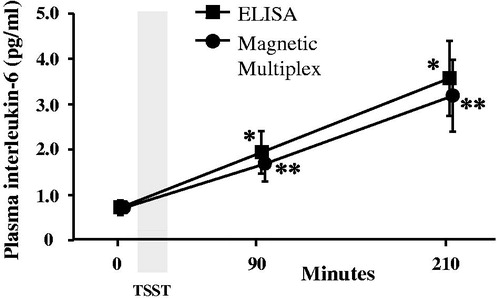
Figure 3. Plasma interleukin (IL)-6 responses to the TSST determined by either high-sensitivity enzyme-linked immunosorbent assay (ELISA) or magnetic multiplex assay (R&D Systems) and aggregated as area under the curve from the baseline (initial) value (AUCi) are strongly correlated with one another, rs = 0.937, p < .001.
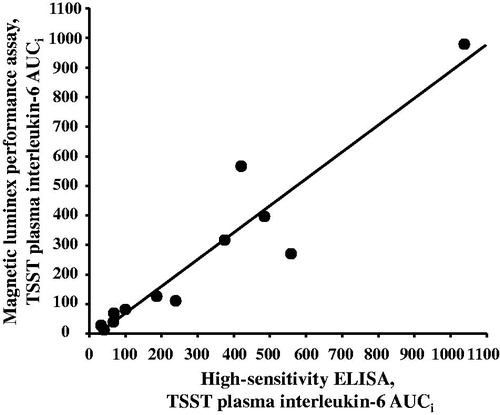
Figure 4. Concentrations of TNF-α (panel A), IL-10 (panel B), IL-8 (panel C), IL-5 (panel D), and IL-2 (Panel E) were determined by magnetic multiplex assay in plasma samples collected before, 90 and 210 min after the start of the Trier Social Stress Test (TSST).
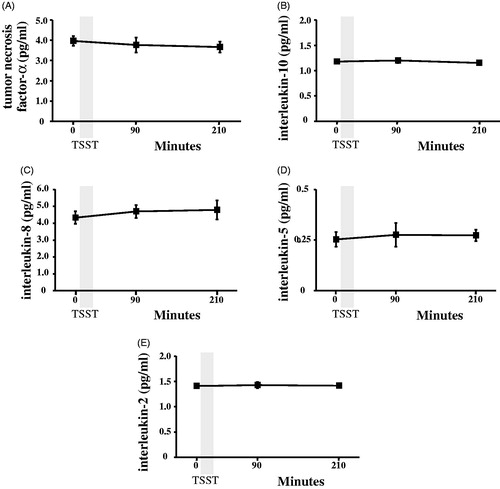
Table 1. High-sensitivity enzyme-linked immunosorbent assay (ELISA) and magnetic multiplex assay performance.
Discussion
The goal of this study was to determine whether a magnetic multiplex assay could detect acute stress-induced changes in plasma concentrations of IL-6 in healthy adults that were first determined by a high-sensitivity ELISA. We also explored the responsiveness of other cytokines (IL-2, IL-5, IL-8, IL-10, and TNF-α) to the same acute stress challenge using the multiplex assay. Performance of the multiplex assay was good compared to the high-sensitivity ELISA in terms of absolute concentrations of IL-6 detected in both plasma samples and quality controls. More importantly, the multiplex assay and high-sensitivity ELISA detected comparable IL-6 responses to the TSST. There was also a strong association between responses aggregated as areas under the curve, and there were no significant differences in IL-6 concentrations detected by the two assay methods among samples collected at each TSST time point. While the multiplex assay detected concentrations of the other cytokines (IL-2, IL-5, IL-8, IL-10, and TNF-α) in all samples, none of these changed as a result of challenge with the TSST at the time points assessed.
Although these findings may at first seem to be of secondary interest, this is the first report that we are aware of empirically demonstrate a cytokine multiplex assay can detect the subtle yet biologically relevant psychosocial stress-induced changes in IL-6. Breen and colleagues (Citation2011) reported several years ago that IL-6 concentrations returned by some but not all multiplex assays were associated with concentrations revealed by high-sensitivity ELISA, although performance was poorer in samples with concentrations ≤10 pg/ml (determined by ELISA). As an inflammatory biomarker, IL-6 is widely assessed in the circulation of humans in the context of acute stress challenge. IL-6 will likely remain popular in human studies that examine the acute response to stress and how it is related to, or modulated by, various conditions including stress-related psychiatric disorders (Fagundes, Glaser, Hwang, Malarkey, & Kiecolt-Glaser, Citation2013; Pace et al., Citation2006), and interventions intended to promote resilience to stress (Pace et al., Citation2009; von Kanel et al., Citation2008). Historically, ELISAs have been the most commonly used laboratory method to quantify changes in IL-6 as a result of acute stress challenge (Marsland et al., Citation2017). Findings presented here on the ability of multiplex assays to detect stress-induced changes in IL-6 may encourage others to use multiplex assays to assess not only IL-6 before and after stress challenge in humans, but other cytokines as well. Exploration of other cytokines in the context of stress is desirable because their production in the context of stress may interact with IL-6. In this sense, future studies would do well to move away from only assessing a single immune biomarker in the context of stress, and instead measure multiple inflammatory biomarkers before and after acute stress challenge. Of note, current multiplex technology allows for multiple markers to be assessed at the same time (Luminex, Citation2017), although this number is limited by related biochemical factors, such as matrix effects in plasma and serum, the combination of different cytokines selected to be included in a panel (Rosenberg-Hasson, Hansmann, Liedtke, Herschmann, & Maecker, Citation2014), and the dilution factor of plasma or serum recommended by a manufacturer in order to run a certain panel of cytokines. de Brouwer et al. (Citation2014) measured multiple cytokines in the context of stress, but they did not explore their interactions. Their efforts may have been hampered by a limited blood collection time courses from before to after acute stress challenge (de Brouwer et al., Citation2014). Indeed, the recent review by Marsland et al. (Citation2017) indicates that different cytokines might have different response time courses to acute stress. This may be because some cytokines regulate the release of others that are “downstream” in the inflammatory signaling cascade and because different cytokines may be differentially regulated by inflammatory signaling pathways, such as nuclear factor-κB (Zhang, Lenardo, & Baltimore, Citation2017). Future studies will need to carefully select the time points at which samples are collected in order to understand how cytokines and other components of inflammation respond to acute stress. In this study, we also did not attempt to analyze the interactions of all of the cytokines examined because of the small sample size, and because the time course of plasma samples examined. Although optimal for IL-6, the time course was likely too limited for the other cytokines. The time points selected for blood collection in this study (before and then 90 and 210 min after the TSST) may be one reason why changes were not observed in other cytokines assessed by the magnetic multiplex assay. These cytokines may be responsive to the TSST, but at different points in time. Readers are directed to the meta-analysis by Marsland et al. (Citation2017) for more information about the optimal time course of these cytokines after acute stress challenge.
This study has other limitations that bear consideration besides the plasma collection time points examined. First, this study only profiled one multiplex kit against ELISA. The high-sensitivity multiplex kit by R&D systems was selected given the authors’ prior experience with other assays products from this company, as well as “word-of-mouth” recommendations from laboratory immunologists. It may be that other products from other manufacturers may be equally adequate compared to high-sensitivity ELISA to detect IL-6 in the context of acute stress challenge, although the experience of our group suggests some kits may not be. For example, initial testing in our laboratory revealed that another magnetic multiplex assay kit (Human Cytokine/Chemokine Magnetic Bead Panel, HCYTOMAG-60 K, Millipore, Billerica, MA) was not adequate for observing stress-induced changes in IL-6 (see Supplementary Figures 2–4). It is also important to note that data obtained from multiplex kits from different manufacturers may not be easily interchangeable (Elshal & McCoy, Citation2006). Second, this study only included plasma samples (N = 36) from 12 participants. Participants were included in the study based on IL-6 concentrations in their samples before and after the TSST as determined by high-sensitivity ELISA. While the study was designed to ensure that there was a range of IL-6 concentrations in their plasma samples, and the test of the association between the magnitude of IL-6 responses to the TSST had very good power post-hoc (with a two-tailed test and alpha = 0.05, power = 0.85), the sample size likely also limited the ability to detect TSST-induced changes in the other cytokines examined. These other cytokines were also not analyzed with ELISA, because there was not enough plasma. Third, we only examined plasma collected from healthy adults. Future studies should assess IL-6 and the other cytokines in samples collected from those with stress-related disorders, such as major depression and posttraumatic stress disorder, and across a more thorough sampling time course. Finally, in this study, we did not attempt to determine whether similar agreement between the magnetic multiplex and high-sensitivity ELISA would hold for serum. This is an important point, because evidence suggests that concentrations of some cytokines may be higher when assayed in serum than in plasma (Rosenberg-Hasson et al., Citation2014).
In conclusion, findings suggest that the multiplex assay by R&D Systems tested here is able to detect the same acute stressor-induced change in IL-6 as conventional high-sensitivity ELISA. These findings should give confidence to researchers who would like to implement multiplex kits to explore other inflammatory biomarkers that may be responsive to acute stress, while still capturing subtle yet important changes in circulating concentrations of IL-6.
Thaddeuset_Pace_et_al_supplemental_content.zip
Download Zip (285.6 KB)Disclosure statement
Although not in conflict with the study presented here, Dr. Raison serves in a consultant role for Novartis Pharmaceuticals, Usona Institute, and Emory Healthcare. Dr. Pace and the other authors report no conflicts of interest.
Additional information
Funding
References
- Bennett, J.M., Glaser, R., Andridge, R.R., Peng, J., Malarkey, W.B., & Kiecolt-Glaser, J.K. (2013). Long lasting effects of smoking: Breast cancer survivors’ inflammatory responses to acute stress differ by smoking history. Psychoneuroendocrinology, 38, 179–187. doi: 10.1016/j.psyneuen.2012.05.012
- Breen, E.C., Reynolds, S.M., Cox, C., Jacobson, L.P., Magpantay, L., Mulder, C.B., … Norris, P.J. (2011). Multisite comparison of high-sensitivity multiplex cytokine assays. Clinical and Vaccine Immunology, 18, 1229–1242. doi: 10.1128/CVI.05032-11
- Breen, E.J., Tan, W., & Khan, A. (2016). The statistical value of raw fluorescence signal in Luminex xMAP based multiplex immunoassays. Scientific Reports, 6, 26996. doi: 10.1038/srep26996
- de Brouwer, S.J., van Middendorp, H., Stormink, C., Kraaimaat, F.W., Joosten, I., Radstake, T.R., … Evers, A.W. (2014). Immune responses to stress in rheumatoid arthritis and psoriasis. Rheumatology (Oxford), 53, 1844–1848. doi: 10.1093/rheumatology/keu221
- Elshal, M.F., & McCoy, J.P. (2006). Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods, 38, 317–323. doi: 10.1016/j.ymeth.2005.11.010
- Fagundes, C.P., Glaser, R., Hwang, B.S., Malarkey, W.B., & Kiecolt-Glaser, J.K. (2013). Depressive symptoms enhance stress-induced inflammatory responses. Brain Behavior and Immunity, 31, 172–176. doi: S0889-1591(12)00113-4
- Kirschbaum, C., Pirke, K.M., & Hellhammer, D.H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. doi: 10.1159/000119004
- Kolmus, K., Tavernier, J., & Gerlo, S. (2015). beta2-Adrenergic receptors in immunity and inflammation: Stressing NF-kappaB. Brain Behavior and Immunity, 45, 297–310. doi: 10.1016/j.bbi.2014.10.007
- Luminex. (2017). Flexmap 3D. Retrieved 2017 from https://www.luminexcorp.com/research/instruments/flexmap-3d/
- Marsland, A.L., Walsh, C., Lockwood, K., & John-Henderson, N.A. (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behavior and Immunity, 64, 208–219. doi: 10.1016/j.bbi.2017.01.011
- Mills, P.J., & Peterson, C.T. (2016). Multiplexing and beyond in biobehavioral research. Psychosomatic Medicine, 78, 642–645. doi: 10.1097/PSY.0000000000000329
- Pace, T.W.W., Mletzko, T.C., Alagbe, O., Musselman, D.L., Nemeroff, C.B., Miller, A.H., & Heim, C.M. (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry, 163, 1630–1633. doi: 10.1176/ajp.2006.163.9.1630
- Pace, T.W.W., Negi, L.T., Adame, D.D., Cole, S.P., Sivilli, T.I., Brown, T.D., … Raison, C.L. (2009). Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology, 34, 87–98. doi: 10.1016/j.psyneuen.2008.08.011
- Padro, C.J., & Sanders, V.M. (2014). Neuroendocrine regulation of inflammation. Seminars in Immunology, 26, 357–368. doi: 10.1016/j.smim.2014.01.003
- Pruessner, J.C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D.H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. doi: S0306453002001087
- Rosenberg-Hasson, Y., Hansmann, L., Liedtke, M., Herschmann, I., & Maecker, H.T. (2014). Effects of serum and plasma matrices on multiplex immunoassays. Immunologic Research, 58, 224–233. doi: 10.1007/s12026-014-8491-6
- Segerstrom, S.C., & Miller, G.E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130, 601–630. doi: 10.1037/0033-2909.130.4.6012004-15935-004
- Turner, M.D., Nedjai, B., Hurst, T., & Pennington, D.J. (2014). Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica Et Biophysica Acta, 1843, 2563–2582. doi: 10.1016/j.bbamcr.2014.05.014
- von Kanel, R., Kudielka, B.M., Metzenthin, P., Helfricht, S., Preckel, D., Haeberli, A., … Fischer, J.E. (2008). Aspirin, but not propranolol, attenuates the acute stress-induced increase in circulating levels of interleukin-6: A randomized, double-blind, placebo-controlled study. Brain Behavior and Immunity, 22, 150–157. doi: 10.1016/j.bbi.2007.07.005
- Zhang, Q., Lenardo, M.J., & Baltimore, D. (2017). 30 Years of NF-kappaB: A blossoming of relevance to human pathobiology. Cell, 168, 37–57. doi: 10.1016/j.cell.2016.12.012
