Abstract
Stress is a major risk factor in the development of various psychiatric disorders such as depression, anxiety and post-traumatic stress disorder. The use of stress paradigms in preclinical contexts is essential to advance our understanding of the pathophysiology of these disorders. However, they are not without their limitations and in this commentary, we have examined some of the practical issues associated with their use. We also highlight some of the latest techniques to identify their neuromolecular correlates as well as the potentially important and integrative role of computational neuroscience. Finally, we share our perspective on future directions in the field of preclinical stress research.
Introduction
Stress across the life-span is a potent risk factor for the onset of many psychiatric disorders such as depression, anxiety and post-traumatic stress disorder (Lupien, McEwen, Gunnar, & Heim, Citation2009; Prenderville, Kennedy, Dinan, & Cryan, Citation2015; Timmermans, Xiong, Hoogenraad, & Krugers, Citation2013). As such, a popular strategy to model aspects of these disorders in preclinical settings is to use various stressors, at various time points (e.g. early-life, adolescence or adulthood), acutely or chronically to gain insights into the underlying neuropathology. For example, recent discoveries include the delineation of distinct neural circuits that mediate behavioral despair and social avoidance following chronic psychosocial defeat stress (Knowland et al., Citation2017) as well as the identification of transcriptional networks that underscore stress resilience (Bagot et al., Citation2016) and stress-susceptibility in a sex-specific manner (Labonte et al., Citation2017).
However, important methodological issues still remain about the use of stress-based paradigms for the study of associated psychopathologies. For example, the utility of baseline phenotyping assays, effects of acute vs. chronic stressors and the importance of individual variability as well as sex differences in susceptibility to stress. In this commentary, we explore and discuss these and other issues relating to the strategies used to model stress-induced psychopathologies in rodents. We also discuss recent techniques to identify the neuronal and neuromolecular correlates of behavior and the role of computational neuroscience in integrating data from multiple methodologies. Lastly, we share our perspectives, as early-career investigators, on the future direction of this field.
Baseline phenotyping
Firstly, we discuss the concept of baseline behavioral phenotyping as a means to quantify the individual variability in rodents that are otherwise inbred and reared in the same environment. Each rodent will have its own “personality” that reflects the impact of a range of factors present at birth and which the rodent is exposed to over the course of its life outside the experimental context. At birth, these could include differences in mothering behavior, access to nutrition as well as stochastic genetic events (Lathe, Citation2004). Environmental factors which influence rodent “personality” may comprise access to enrichment, experimenter handling and position in a social hierarchy (Larrieu et al., Citation2017; Shemesh et al., Citation2013). Thus, the “personality” of a rodent manifests as baseline behaviors that could influence or even predict response to stress.
Baseline behavioral phenotyping could be done using strategies such as monitoring circadian rhythm in the homecage or exploratory activity. The circadian rhythm is intimately linked to the stress response pathway and as such baseline recordings may serve as a an important predictor of response to future stressors (Koch, Leinweber, Drengberg, Blaum, & Oster, Citation2017). Exploratory activity behavior in the elevated plus maze at baseline has, for example, been previously correlated with subsequent response to a sub-chronic unpredictable stress paradigm (Ducottet & Belzung, Citation2004).
However, caution must be exercised in regards to what impact such screening assays, especially those performed outside the homecage, may have on rodent behavior that could confound stress response (Voikar, Vasar, & Rauvala, Citation2004). It is also important to consider the types of statistical methods used when performing repeated behavioral assessments on the same rodent and the need to control for multiple comparisons (Festing & Altman, Citation2002). Lastly, the addition of another step to experimental protocols will increase the time it takes to complete experiments but we believe that this is outweighed by the value of the information to be gained, particularly in terms of understanding the links between the factors which define the “personality” of a rodent and its response to stress.
Acute or chronic?
It has been recently argued in an opinion piece by Musazzi, Tornese, Sala, and Popoli, (Citation2017) that the use of acute stress paradigms may be just as informative as chronic stress paradigms, particularly in terms of understanding how the response to an acute stressor can trigger a maladaptive pathway that leads to the onset of psychopathology. They described their work in which they observed dysregulation in glutamate release and apical dendritic atrophy in the prefrontal cortex of rats 24 h and 14 days after exposure to a single foot-shock stress, respectively. However, given that most of the other parameters analysed at this time point had returned to baseline, it is, in our view, difficult to argue that the effects of an acute stressor are as informative as a chronic stressor. Indeed, the long-term neurobiological or behavioral effects are not always observable even after chronic stressors (Cryan & Slattery, Citation2007).
Nevertheless, Musazzi et al. have correctly stated that more research is needed to examine the long-term effects of acute stressors. Future studies should be designed to monitor behavioral, physiological and neuromolecular trajectories from the point of exposure. Such an approach will help understand divergent pathways leading to states of stress-resilience and stress-susceptibility. In this context, one interesting variable that could be investigated further is the interaction between the impact of the acute stressor and the age of exposure.
Assessing individual variability in response to stressors
Extending the parameters which can be extracted from assays used to determine the impact of stress on rodent behavior enables analysis of individual variability in response to stress exposure (see above) and therapeutic strategies to counter its effects. This usually involves setting an a priori threshold score used to classify animals to specific groups, i.e. stress-susceptible vs. stress-resilient or treatment-responsive vs. treatment-resistant.
For example, with the chronic social defeat paradigm (CSDS), variability in the effects of stress on social behaviour is based on performance in the two-chamber social avoidance test. The setup is an open field arena with a cage adjacent to one of the walls. For phase 1, the test mouse is allowed to explore the arena for 2.5 min with the cage empty and this is followed by an inter-trial interval of 1 min during which the test mouse is returned to its home cage. Then, a novel aggressor mouse is placed inside the cage and then the test mouse is placed back in the arena for a further 2.5 min. The time spent in a defined zone outside the cage known as the interaction zone (IZ) is recorded. A ratio known as the social interaction ratio (SIT) is obtained (time spent in interaction zone in presence of aggressor/time spent interaction zone in absence of aggressor). A SIT score of greater than 1 is indicative of a resilient phenotype whereas a score of less than 1 suggests a susceptible phenotype. However, this binary approach fails to take into account that a stressed mouse that chooses to avoid the IZ could be doing so as an adaptive response to ensure it is not attacked any further, even though the aggressor is inside a cage. Furthermore, mice with SIT ratios that are close to the threshold of 1 are neither here nor there, making data interpretation a challenge. For this reason, others have chosen to include a third group known as stress-indifferent with ratios that are greater than 0.9 but less than 1.1 (Peña et al., Citation2017). A recent review of the CSDS paradigm has recommended further modifications to include other parameters to enable more accurate stratification of mice into stress-susceptible and stress-resilient groupings (Henriques-Alves & Queiroz, Citation2015). These include lengthening the duration of the test and including other measurable behaviors that relate to risk assessment (e.g. approach index and flight index).
Other assays which have been used to classify rodents as resilient or susceptible include the learned helplessness test (Landgraf, Long, Der-Avakian, Streets, & Welsh, Citation2015), the sucrose preference test (Zurawek et al., Citation2016) and the magnitude of the corticosterone response to an acute stressor (Rincón-Cortés et al., Citation2015). However, it is our opinion that rather than relying a single assay, assessing mice using several assays to come up with a composite score is likely to be more informative Citation.
Sex as biological variable
Females are at a higher risk of suffering from psychiatric disorders than males and this disparity in susceptibility starts in adolescence and maintained throughout adult life. However, very little is known about the sex-specific processes which underlie differences in pathology and susceptibility. As such, there is now a major push to incorporate female rodents in preclinical stress research studies; indeed this is now mandatory in several jurisdictions (Bale & Epperson, Citation2017; Shansky & Woolley, Citation2016).
Several chronic social stress protocols have been developed specifically for use with female rodents but which are equivalent to those used in males in terms of basic conditions such as housing and timing or duration of stress exposure (Jacobson-Pick, Audet, McQuaid, Kalvapalle, & Anisman, Citation2013; Schmidt et al., Citation2010; Trainor et al., Citation2011). More recently, two studies have modified the (CSDS) protocol for use in females. The first involves chemogenetic activation of the ventromedial hypothalamus of male mice so as to induce aggression against female conspecifics (Takahashi et al., Citation2017) and the second involves applying male odorants to females so as to mimic the male scent and rendering them susceptible to attack (Harris et al., Citation2017). Post-partum depression has been modeled using several approaches including gestational restraint stress in combination with overcrowding (Hillerer, Reber, Neumann, & Slattery, Citation2011) and administration of estradiol and progesterone via subcutaneous pellet for 3 weeks followed by acute withdrawal (Suda, Segi-Nishida, Newton, & Duman, Citation2008).
We anticipate that in the coming years, this sub-field of stress-research will continue to expand and disentangle the complexity of gender bias in psychiatric disorders.
The brain at single-cell resolution
Single cells are the fundamental units of life. However, the function of most cell types in the brain remains unknown. For over a century, cell type classification and function in the nervous system has been traditionally defined by morphological studies, electrophysiological characteristics, and molecular composition. However, our understanding of these properties is now being challenged and advanced by emerging cutting-edge approaches, such as high-throughput single-cell RNA sequencing (Ofengeim, Giagtzoglou, Huh, Zou, & Yuan, Citation2017). Single-cell transcriptomics provides a powerful insight into the complexity of the brain by enabling the identification of molecular signatures at an extraordinary resolution, which will eventually reveal new dimensions of cell identity and its relationship with disease. For example, the vulnerability of specific cell types in stress-induced disorders might be explained, in part, by their transcriptomic profiles that could be the target of novel pharmacological interventions. Furthermore, identification of these populations can reveal more accurate and perhaps reliable signatures to monitor disease progression and efficacy of therapeutic strategies. However, the complexity of “Big Data” represents new scientific and analytical challenges. Nevertheless, exciting opportunities will emerge in the fields of molecular, cellular, systems, and computational neuroscience (see below) that will lead to the development of robust statistical and machine learning methods for the integration of such complex data sets. All things considered, we believe that with these techniques, we are on the verge of a paradigm shift in terms of how we interpret and describe the pathology of stress-induced disorders.
Activity-based genetic labeling of brain networks
Neuronal activity within specific brain nuclei is driven by a multitude of different cell populations. The collective activity of these neural networks is what drives behavior. Mapping these networks within the brain has been one of the fundamental challenges within neuroscience. There are several techniques such as pharmacological and physical lesions, metabolic imaging or electrophysiological recordings used to dissect some of the networks underlying behavior. While these techniques have provided valuable information on the fundamental basis of neural networks, they lack cell specificity and/or spatial resolution.
Recent developments in what is generally referred to as activity driven labeling of neuronal populations have opened up new avenues to dissect neural ensembles and mapping them in greater detail. More importantly, it has allowed researchers to access and manipulate these previously activated cell populations. A number of distinct labeling tools have been developed with varying properties in terms of specificity, signal to noise ratio and temporal activation. Central to most of the current available tools is the following observation that neural activity ultimately results in the expression of a number of immediate early genes (IEGs) (Guzowski, Setlow, Wagner, & McGaugh, Citation2001). By extracting promoter or enhancer sequences that drive the expression of IEGs and allowing them to control the expression of fluorescent reporters, opsins or other effector genes enables for permanent labeling of a previously transiently activated neuron. Some of the most widely used activity-dependent IEG promoter sequences are those of the Fos and Arc genes. An example of one of the possible applications of this technique was presented in a series of studies in which channelrhodopsin-2 expression was driven by c-fos activation (Liu et al., Citation2012; Ramirez et al., Citation2013; Redondo et al., Citation2014). Using this setup Ramirez et al. showed that optogenetic reactivation of a neural network associated with a positive experience within the hippocampus–amygdala–nucleus accumbens results in the suppression of depression-like behaviors induced by an acute stressor (Ramirez et al., Citation2015).
Combining activity-dependent labeling with brain clearing techniques such as 3DISCO and CLARITY (Vigouroux, Belle, & Chédotal, Citation2017) may make it possible to generate and visualize brain-wide activity maps of neuronal ensembles activated during a specific behavioral task. To further understand the molecular machineries that drive stress-induced pathologies, future studies could combine single-cell technologies with activity-dependent labeling techniques. This would enable researchers to ask questions such as what makes a particular stress activated neuronal ensemble transcriptionally or epigenetically distinct. It would also allow for the molecular identification of novel single-cell activity maps regulated during exposure to stress at an unprecedented level of specificity and resolution.
A role for computational neuroscience
As previously alluded to, numerous assays exist to assess the impact of stress on behavioral processes such as coping behavior, anhedonia, decision-making and social behavior. However, in a recent critique, Krakauer et al. (Citation2017) have argued that an inadequate deconstruction of these behaviors has led to flawed causal explanations for experiments which seek to ascribe behavioral or psychological phenomena to specific brain structures, circuits or neurons. Rather, a more pluralistic, systems biology approach would be to perhaps use computational neuroscience to develop a layered and network-based understanding of what drives a specific behavior and then how experimental manipulations (e.g. stress) affects its many aspects.
A recent example of a study which used this approach is by the Frankland lab in the context of fear memory (Vetere et al., Citation2017). They quantified the expression of the immediate early gene, c-fos, in 84 brain regions following recall of fear memory and used this data to generate an in silico functional neural network of fear memory composed of nodes which represent brain regions and edges as the functional connections between them. To probe the importance of specific nodes, the authors deleted them as well as their edges and then remaining adjacent edges were readjusted according to weights of their neighboring edges and the weight of the deleted edge. This is done iteratively until edge weights are stabilized to produce the disrupted network. This disruption propagation model correlated well with the behavioral outcome of silencing nodes chemogenetically in vivo. In particular, the disruption/deletion of higher degree nodes had a greater impact on consolidation of fear memory compared to low-degree nodes.
Computational neuroscience clearly conveys a significant benefit in being able to weigh the importance of specific relationships between structures, sub-structures, circuits, microcircuits as well as the cellular, molecular and genetic systems that underpin behavior. This is consistent with the Research Domain Criteria (RDoC) framework for the study of psychiatric disorders which integrates data from multiple levels of research (i.e. from genetic analyses to patient self-reports) to understand the basis of normal and abnormal human behavior (Insel et al., Citation2010). In the context of stress research, such an understanding may reveal novel insights into the pathophysiology of various psychopathologies as well as strategies for their treatment which seek to rescue or normalize network-level disruptions, perhaps akin to the effects of deep-brain stimulation.
Concluding remarks
In this commentary, we have briefly discussed several practical issues as well as new experimental strategies in the field of stress research (). We believe that it is an exciting time to be a stress-researcher not least due to the breath-taking pace of technological developments which are improving our ability to probe the inner workings of the brain. Going forwards, one of the steps we need to take is to embrace the multidisciplinary nature of psychiatric neuroscience. This will involve stepping out of the lab to network and form extensive collaborations with colleagues from varied backgrounds. Combining a multitude of skill-sets allows for the design of more comprehensive studies which have a significant impact on the field. In the future, these are the types of studies which we anticipate will have the translational value to make a difference improving the lives of those suffering from stress-induced psychiatric disorders.
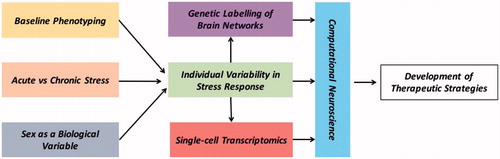
Figure 1. The individual variability in responses to stress paradigms can be linked to several factors such as the baseline phenotype or the “personality” of the rodent, sex, as well as the duration. These responses can be further informed and combined with data using high-resolution technologies to delineate stress-sensitive brain networks and relevant cell-specific transcriptomic signatures. Using computational neuroscience tools these multidimensional datasets can be further integrated to generate models of stress-induced psychiatric disorders. The goal of this approach would be to gain a deeper understanding of the neurobiology of stress-induced psychopathologies and the development of novel, more effective therapeutic strategies.
Acknowledgements
We would like to thank Professor Alon Chen and Professor John F. Cryan for their mentorship and unconditional support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bagot, R.C., Cates, H.M., Purushothaman, I., Lorsch, Z.S., Walker, D.M., Wang, J., … Nestler, E.J. (2016). Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron, 90, 969–983. doi:10.1016/j.neuron.2016.04.015
- Bale, T.L., & Epperson, C.N. (2017). Sex as a biological variable: Who, what, when, why, and how. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42, 386–396. doi:10.1038/npp.2016.215
- Cryan, J.F., & Slattery, D.A. (2007). Animal models of mood disorders: Recent developments. Current Opinion in Psychiatry, 20, 1–7. doi:10.1097/YCO.0b013e3280117733
- Ducottet, C., & Belzung, C. (2004). Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiology and Behavior, 81, 417–426. doi:10.1016/j.physbeh.2004.01.013
- Festing, M.F., & Altman, D.G. (2002). Guidelines for the design and statistical analysis of experiments using laboratory animals. Institute for Laboratory Animal Research, 43, 244–258. doi:10.1093/ilar.43.4.244
- Guzowski, J.F., Setlow, B., Wagner, E.K., & McGaugh, J.L. (2001). Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. Journal of Neuroscience, 21, 5089–5098.
- Harris, A.Z., Atsak, P., Bretton, Z.H., Holt, E.S., Alam, R., Morton, M.P., … Gordon, J.A. (2017). A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology, in press. doi:10.1038/npp.2017.259
- Henriques-Alves, A.M., & Queiroz, C.M. (2015). Ethological evaluation of the effects of social defeat stress in mice: Beyond the social interaction ratio. Frontiers in Behavioral Neuroscience, 9, 364. doi:10.3389/fnbeh.2015.00364
- Hillerer, K.M., Reber, S.O., Neumann, I.D., & Slattery, D.A. (2011). Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: Implications for postpartum anxiety and mood disorders. Endocrinology, 152, 3930–3940. doi:10.1210/en.2011-1091
- Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D.S., Quinn, K., … Wang, P. (2010). Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167, 748–751. doi:10.1176/appi.ajp.2010.09091379
- Jacobson-Pick, S., Audet, M.-C., McQuaid, R.J., Kalvapalle, R., & Anisman, H. (2013). Social agonistic distress in male and female mice: Changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PLoS One, 8, e60133. doi:10.1371/journal.pone.0060133
- Knowland, D., Lilascharoen, V., Pacia, C.P., Shin, S., Wang, E.H.-J., & Lim, B.K. (2017). Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell, 170, 284–297.e218. doi:10.1016/j.cell.2017.06.015
- Koch, C.E., Leinweber, B., Drengberg, B.C., Blaum, C., & Oster, H. (2017). Interaction between circadian rhythms and stress. Neurobiology of Stress, 6, 57–67. doi: https://doi.org/10.1016/j.ynstr.2016.09.001
- Krakauer, J.W., Ghazanfar, A.A., Gomez-Marin, A., MacIver, M.A., & Poeppel, D. (2017). Neuroscience needs behavior: Correcting a reductionist bias. Neuron, 93, 480–490. doi:10.1016/j.neuron.2016.12.041
- Labonte, B., Engmann, O., Purushothaman, I., Menard, C., Wang, J., Tan, C., … Nestler, E.J. (2017). Sex-specific transcriptional signatures in human depression. Nature Medicine, 23, 1102–1111. doi:10.1038/nm.4386
- Landgraf, D., Long, J., Der-Avakian, A., Streets, M., & Welsh, D.K. (2015). Dissociation of learned helplessness and fear conditioning in mice: A mouse model of depression. PLoS One, 10, e0125892. doi:10.1371/journal.pone.0125892
- Larrieu, T., Cherix, A., Duque, A., Rodrigues, J., Lei, H., Gruetter, R., & Sandi, C. (2017). Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress. Current Biology, 27, 2202–2210.e2204. doi:10.1016/j.cub.2017.06.027
- Lathe, R. (2004). The individuality of mice. Genes, Brain and Behavior, 3, 317–327. doi:10.1111/j.1601-183X.2004.00083.x
- Liu, X., Ramirez, S., Pang, P.T., Puryear, C.B., Govindarajan, A., Deisseroth, K., & Tonegawa, S. (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature, 484, 381–385. doi:10.1038/nature11028
- Lupien, S.J., McEwen, B.S., Gunnar, M.R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. [Review Article]. Nature Reviews Neuroscience, 10, p 434. doi:10.1038/nrn2639
- Musazzi, L., Tornese, P., Sala, N., & Popoli, M. (2017). Acute or chronic? A stressful question. Trends in Neurosciences, 49, 525–535. doi:10.1016/j.tins.2017.07.002
- Ofengeim, D., Giagtzoglou, N., Huh, D., Zou, C., & Yuan, J. (2017). Single-cell RNA sequencing: Unraveling the brain one cell at a time. Trends in Molecular Medicine, 23, 563–576. doi:10.1016/j.molmed.2017.04.006
- Peña, C.J., Kronman, H.G., Walker, D.M., Cates, H.M., Bagot, R.C., Purushothaman, I., … Nestler, E.J. (2017). Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science, 356, 1185–1188. doi:10.1126/science.aan4491
- Prenderville, J.A., Kennedy, P.J., Dinan, T.G., & Cryan, J.F. (2015). Adding fuel to the fire: the impact of stress on the ageing brain. Trends in Neurosciences, 38, 13–25.
- Ramirez, S., Liu, X., Lin, P.A., Suh, J., Pignatelli, M., Redondo, R.L., … Tonegawa, S. (2013). Creating a false memory in the hippocampus. Science, 341, 387–391. doi:10.1126/science.1239073
- Ramirez, S., Liu, X., MacDonald, C.J., Moffa, A., Zhou, J., Redondo, R.L., & Tonegawa, S. (2015). Activating positive memory engrams suppresses depression-like behaviour. Nature, 522, 335–339. doi:10.1038/nature14514
- Redondo, R.L., Kim, J., Arons, A.L., Ramirez, S., Liu, X., & Tonegawa, S. (2014). Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature, 513, 426–430. doi:10.1038/nature13725
- Rincón-Cortés, M., Barr, G.A., Mouly, A.M., Shionoya, K., Nuñez, B.S., & Sullivan, R.M. (2015). Enduring good memories of infant trauma: Rescue of adult neurobehavioral deficits via amygdala serotonin and corticosterone interaction. Proceedings of the National Academy of Sciences of the United States of America, 112, 881–886. doi:10.1073/pnas.1416065112
- Schmidt, M.V., Scharf, S.H., Liebl, C., Harbich, D., Mayer, B., Holsboer, F., & Muller, M.B. (2010). A novel chronic social stress paradigm in female mice. Hormones & Behavior, 57, 415–420. doi:10.1016/j.yhbeh.2010.01.010
- Shansky, R.M., & Woolley, C.S. (2016). Considering sex as a biological variable will be valuable for neuroscience research. Journal of Neuroscience, 36, 11817–11822. doi:10.1523/JNEUROSCI.1390-16.2016
- Shemesh, Y., Sztainberg, Y., Forkosh, O., Shlapobersky, T., Chen, A., & Schneidman, E. (2013). High-order social interactions in groups of mice. Elife, 2, e00759. doi:10.7554/eLife.00759
- Suda, S., Segi-Nishida, E., Newton, S.S., & Duman, R.S. (2008). A postpartum model in rat: Behavioral and gene expression changes induced by ovarian steroid deprivation. Biological Psychiatry, 64, 311–319. doi:10.1016/j.biopsych.2008.03.029
- Takahashi, A., Chung, J.-R., Zhang, S., Zhang, H., Grossman, Y., Aleyasin, H., … Russo, S.J. (2017). Establishment of a repeated social defeat stress model in female mice. Scientific Reports, 7, 12838. doi:10.1038/s41598-017-12811-8
- Timmermans, W., Xiong, H., Hoogenraad, C.C., & Krugers, H.J. (2013). Stress and excitatory synapses: from health to disease. Neuroscience, 248, 626–636. doi:10.1016/j.neuroscience.2013.05.043
- Trainor, B.C., Pride, M.C., Villalon Landeros, R., Knoblauch, N.W., Takahashi, E.Y., Silva, A.L., & Crean, K.K. (2011). Sex differences in social interaction behavior following social defeat stress in the monogamous california mouse (Peromyscus californicus). PLoS One, 6, e17405. doi:10.1371/journal.pone.0017405
- Vetere, G., Kenney, J.W., Tran, L.M., Xia, F., Steadman, P.E., Parkinson, J., … Frankland, P.W. (2017). Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron, 94, 363–374.e364. doi:10.1016/j.neuron.2017.03.037
- Vigouroux, R.J., Belle, M., & Chédotal, A. (2017). Neuroscience in the third dimension: Shedding new light on the brain with tissue clearing. Molecular Brain, 10, 33. doi:10.1186/s13041-017-0314-y
- Voikar, V., Vasar, E., & Rauvala, H. (2004). Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes, Brain and Behavior, 3, 27–38.
- Zurawek, D., Kusmider, M., Faron-Gorecka, A., Gruca, P., Pabian, P., Kolasa, M., … Dziedzicka-Wasylewska, M. (2016). Time-dependent miR-16 serum fluctuations together with reciprocal changes in the expression level of miR-16 in mesocortical circuit contribute to stress resilient phenotype in chronic mild stress – An animal model of depression. European Neuropsychopharmacology, 26, 23–36. doi:doi.org/10.1016/j.euroneuro.2015.11.013
