Abstract
Maternal deprivation (MD) disinhibits the adrenal glands, rendering them responsive to various stressors, including saline injection, and this increased corticosterone (CORT) response can last for as long as 2 h. In the present study, we tested the hypothesis that association of MD on day 11 with a saline injection would alter emotional behavior, CORT response, and brain monoamine levels, in male and female adult rats. Rats were submitted to the novelty suppressed feeding (NSF), the sucrose negative contrast test (SNCT), social investigation test (SIT), and the elevated plus maze (EPM). One quarter of each group was not tested (providing basal values of CORT and brain monoamines) and the remainder was decapitated 15, 45, or 75 min after the EPM, to assess CORT reactivity. Monoamine levels were determined in the hypothalamus (HPT), frontal cortex (FC), amygdala (AMY), ventral, and dorsal hippocampus (vHPC, dHPC, respectively). MD reduced food intake, in the home-cage, and latency to eat in the NSF in both sexes; females explored less the target animal in the SIT and explored more the open arms of the EPM than males; the CORT response to the EPM was greater in maternally-deprived males and females than in their control counterparts, and this response was further elevated in maternally-deprived females injected with saline. Regarding monoamine levels, females were less affected, showing isolated effects of the stressors, while in males, MD increased 5-HT levels in the HPT and decreased this monoamine in the FC, MD associated with saline reduced dopamine levels in all brain regions, except the HPT. MD at 11 days did not alter emotional behaviors in adult rats, but had an impact in neurobiological parameters associated with this class of behaviors. The impact of MD associated with saline on dopamine levels suggests that males may be vulnerable to motivation-related disorders.
1. Introduction
The proper development of altricial animals, such as rodents, non-human, and human primates, depends largely on attachment to the caregiver and the quality of parental care (Sullivan & Holman, Citation2010). In rats, the mother provides warmth, nourishment, and anogenital licking, which are important factors for adequate physical and emotional development of the pups. These behaviors have been coined “hidden regulators”, because they modulate physiological systems of the infant, besides their obvious functions (Hofer, Citation1994). One such system is the hypothalamus–pituitary–adrenal (HPA) axis, which undergoes a period of quiescence, ranging from postnatal day (PND) 4 to PND 14, characterized by an adrenal refractoriness to its trophic hormone, ACTH, and to stressors which in adults, result in increased corticosterone (CORT) levels (Witek-Janusek, Citation1988). This period is denominated Stress Hyporesponsive Period (SHRP) and is thought to be highly adaptive, due to maintenance of low and stable levels of CORT (Schoenfeld, Leathem, & Rabii, Citation1980), necessary for appropriate brain maturation (Meyer, Citation1983).
The seminal work by Seymour Levine demonstrated that separation of the infant from its mother for 24 h (herein designated Maternal Deprivation – MD) at different ages during the SHRP disinhibits the adrenal glands, which then become responsive to several stimuli, such as novelty, saline injection, and ACTH administration (Levine, Huchton, Wiener, & Rosenfeld, Citation1991; Rosenfeld et al., Citation1991). In this study, maternal deprivation did not alter the stress response of infants deprived on PND 3 (DEP3), but produced a twofold increase in saline injection-induced CORT response in DEP7 pups and a fivefold greater response to saline injection in DEP11 pups than control (CTL), non-deprived counterparts (Levine et al., Citation1991). Subsequent studies showed that the increase in CORT secretion to such a mild stressor, lasted for, at least, 2 h (Faturi et al., Citation2010; Suchecki, Nelson, Van Oers, & Levine, Citation1995), a far longer response than that observed in adult animals. Based on the evidence that high levels of CORT during development retard neuronal and glial maturation, impair myelination, and neurogenesis (Bohn, Citation1980; Matthews, Citation2000), leading to changes in the hippocampus (Huang, Harper, Evans, Newnham, & Dunlop, Citation2001), we hypothesized that prolonged elevation of CORT levels in response to saline injection in DEP11 pups could alter their brain development resulting in behavioral and neurobiological alterations. In previous studies, we showed that DEP 11 rats avoid the bright compartment of the light–dark box, but show no behavioral changes in the free exploration paradigm when compared to CTL rats (Faturi et al., Citation2010). These animals also display more risk assessment in the elevated plus maze (EPM), longer CORT response to this test and reduced levels of inhibitory aminoacids in the hippocampus, GABA in females and taurine in males (Barbosa Neto et al., Citation2012). In addition to changes in these neurotransmitters, MD increases dopamine levels in the striatum, prefrontal cortex (PFC), and amygdala (AMY) of DEP9 male rats (Rentesi et al., Citation2013), whereas it enhances the sensitivity to apomorphine, a D2 dopaminergic agonist in DEP3 adult rats (Rots et al., Citation1996). To the best of our knowledge, apart from Barbosa Neto and coworkers (2012) who measured monoamine and aminoacid neurotransmitters in the hippocampus, there has been no report on the effects of maternal deprivation on PND 11 on monoamine levels in specific brain areas associated with emotional behavior. Therefore, in the present study, we sought to further characterize the behavioral phenotype, CORT reactivity, and monoamine content in specific brain areas of DEP11 rats, stressed neonatally, by a saline injection, in order to test the hypothesis that association of these neonatal manipulations would lead to enduring outcomes in the neurobiology of emotional-related behaviors of adult male and female rats.
2. Materials and methods
2.1. Animals
Animals were Wistar rats, from Centro de Desenvolvimento de Modelos Experimentais para Medicina e Biologia (CEDEME), maintained under controlled temperature (22 ± 2 °C) and 12 h light–dark cycle (lights on at 7:00 h). Food and water were provided ad libitum and the experiments were previously approved by the Ethical Committee on the Use of Experimental Animals, in compliance with the Brazilian Law (protocol # 8465121114).
Litters were obtained after housing two virgin females with one experienced male, for 10 days, after which, males were taken away. Females remained together in the cages for another week and, then, housed individually in maternity cages. From this day on, nest building material – wood shavings and two leaves of paper towel – was provided. Nineteen days after the beginning of breeding, females were monitored, twice a day (at 9:00 h and 17:30 h), for the presence of pups. The day of birth was designated postnatal day (PND) 0 and on PND 1, litters were culled to four males and four females; when there were not enough pups, additional ones from other litters, born in the same day and period of the day, were adopted. Every third day, half of the material was replaced with clean bedding, and nest building material was provided again.
2.2. Maternal deprivation
Of a total of 33 litters, 16 remained with their mothers all the time (control, non-deprived group – CTL) and 17 were submitted to 24 h maternal deprivation (DEP) on postnatal day (PND) 11, at 10:00 h. Maternal deprivation ended at 10:00 h of PND 12.
Litters were removed from the home-cage and placed in different cages, containing bedding material from the home-cage, in a room far away from the maternity room. These cages were placed onto heating pads, set at 33 °C, for 24 h.
2.3. Saline injection and experimental groups
Saline injection was the inter-litter factor, so that all pups in a given litter were either injected or not, i.p., with sterile saline 0.9% (SAL), in a volume of 0.1 ml/10 g of body weight. Saline was administered to seven DEP litters, on PND 12, 2 h before the end of the maternal deprivation period, and to eight CTL litters on PND 12, at the time corresponding to the end of the deprivation period. The remainder of the litters were not submitted to this stressor, making up to four experimental groups: (1) control, no saline (CTL + NSAL, eight litters); (2) control + saline (CTL + SAL, eight litters); (3) deprived, no saline (DEP11 + NSAL, 10 litters); (4) deprived + saline (DEP11 + SAL, seven litters).
At the end of the deprivation period, pups were placed back with their mothers until PND 21, when they were weaned, separated by sex, and housed with their siblings, until the onset of behavioral testing.
2.4. Experimental protocol
On PND 55, three males and three females of each litter were randomly assigned to be exposed to a behavioral test battery. The remaining male and female from each litter were not tested and maintained in their home-cages until the day their siblings were tested. They were decapitated immediately before the onset of their siblings’ testing, in order to provide blood and brain sampling for determination of basal levels of CORT and brain monoamines. Testing was carried out between 8:00 h and 10:00 h for males and 10:10 h and 12:10 h for females, with an interval of, approximately, one week between tests (see ).
Figure 1. Experimental protocol of the study. Whole litters were designated to control, non-deprived (CTL), or to maternal deprivation on post-natal (PND) 11 (DEP11). On PND 12, one half of each group did not receive a saline injection (NSAL), whereas the other half received this stressor (SAL). From PND 60 onwards, males and females were exposed to different behavioral tests: novelty suppressed feeding (NSF); sucrose negative contrast test (SNCT); social investigation test (SIT), and elevated plus maze (EPM), approximately 7 days apart.
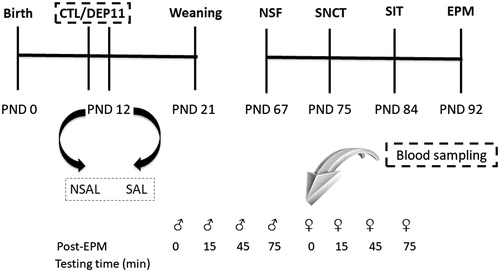
Females were tested, as much as possible, during estrous. In our previous studies, females were evaluated during diestrous, a phase of the estrous cycle when they are more responsive to stressful and axiogenic stimuli, contrary to the estrous phase (Lovick, Citation2012). Assessment of estrous cycle lasted for 10 days, commencing two weeks before the onset of behavioral testing or before blood sampling and brain harvesting for those females providing baseline values, in order to verify cycling regularity. For NSF and SNCT, the estrous phase was not considered because the tests lasted more than one day. After each behavioral test or decapitation, a vaginal smear was taken to confirm the phase of the cycle and those not in estrous were excluded from the analysis.
2.5. Behavioral assessment
2.5.1. Novelty supressed feeding (NSF)
NSF is a test used to evaluate anxiety-like behavior, being also useful to assess the animal’s motivational state (Powell, Fernandes, & Schalkwyk, Citation2012). Its central paradigm is the conflict between hunger and aversiveness of open and unknown places (Bodnoff, Suranyi-Cadotte, Aitken, Quirion, & Meaney, Citation1988).
Four days before the onset of testing, the animals were individually housed, for the assessment of their diurnal and nocturnal food intake, which consisted of offering a known amount of chow at 9:00 h and weighing the remainder at 17:30 h (estimate of diurnal food intake). The remainder was weighed at 9:00 h on the next morning, providing an estimate of the nocturnal intake. This process was repeated for two days and the average diurnal and nocturnal food intake was calculated. Subsequently, food was removed and the animals were fasted for 48 h, in order to increase their motivation to seek food. The test commenced after the period of food deprivation, in a room under the same conditions as the animal facility. A chow pellet was placed in the center of a polypropylene cage (110 × 60 × 40 cm) and the animal, in one of the corners of the cage, with the nostril directed to the pellet. During the 10 min test period, latency for the first bite and the amount of food ingested were recorded. At the end of the testing period, animals were returned to their home-cages and food intake was assessed for 30 min, after which the animals were placed back in their original group in the home-cages. This test was carried out between PNDs 67 and 74.
2.5.2. Sucrose negative contrast test (SNCT)
The protocol was carried out according to Verma, Hellemans, Choi, Yu, and Weinberg (Citation2010). The goal of this test is to assess the rats’ hedonic profile, by offering a 15% sucrose solution (high concentration) for two consecutive days, and a 2.1% sucrose solution (low concentration) on the third day. Rats habituated to the high sucrose concentration do not consume as much of the low concentration solution (intake suppression), whereas those which are indifferent to the sudden change in the hedonic value of the solution consume the low concentrated sucrose as much as the high concentration, indicating impairment of the hedonic/motivational profile (Matthews, Wilkinson, & Robins, Citation1996).
Animals were housed individually and two bottles containing 200 ml of water or 200 ml of sucrose 15%, were weighed and provided to the animals at 11:30 h. Twenty-four hours later, the bottles were weighed again, to estimate the amount of liquid intake, and refilled with their respective liquid, weighed again, and replaced in the cage, with their positions switched, to avoid position preference bias. On the third day, the same procedure was repeated, except that, this time, the concentration of the sucrose solution was 2.1%. On the next day, the bottles were weighed for calculation of liquid intake. During the entire procedure, rat chow was offered ad libitum to the animals. For each day of the SNCT, sucrose preference index (SPI, %) was determined, using the equation: SPI = [sucrose intake/total liquid intake] × 100. This test was carried out between PNDs 75 and 84.
2.5.3. Social investigation test (SIT)
This test is used to assess changes in the animal interest to explore other individuals of the same species and of the same or opposite sex (Landauer & Balster, Citation1982) and may serve to evaluate behavioral aspects of social anxiety-like profile (File & Seth, Citation2003). The test was carried out in a circular arena 80 cm in diameter, closed by 50 cm high walls, which floor was divided by concentric lines. Two metal-grid containers (23.5 cm in diameter and 27 cm high), placed opposite to each other and rotated 90° at each test, were used. The sessions were carried out in a room under the same temperature and lighting conditions as the animal facility, and were video recorded for posterior behavioral analysis.
On the day before the SIT, each animal was habituated to the arena and containers for 10 min. On the following day, each animal was placed in the center of the arena, never facing the containers. One of the containers was empty and the other contained an unfamiliar rat, matched for sex and age (target rat). The analysis included the time of exploration of each container (in seconds), so the ratio between the time of exploration of the target rat/time of exploration of both containers was calculated and taken as the index of social investigation. This test was conducted between PNDs 84 and 95.
2.5.4. Elevated plus maze (EPM)
The EPM is used to assess anxiety-like behavior, based on the rat’s innate fear of open, elevated places, and was validated by Pellow, Chopin, File, and Briley (Citation1985). The maze was made of wood with four arms forming a cross 50 cm above the ground. All arms were 50 cm in length and 10 cm wide and two opposite arms, denominated closed, were enclosed by 40 cm high walls, while the open arms were surrounded by a 0.5 cm ledge, serving as tactile guide for the animals (Cohen, Zohar, & Matar, Citation2003). The test was 5 min long, and each rat was brought to the EPM cubicle and placed in the central square of the apparatus with its nostril facing one of the open arms (Pellow et al., Citation1985). All arms were divided in three segments of approximately 15 cm, which allowed the experimenter to evaluate the animal locomotor activity. After each test, the EPM was wiped out with a 20% alcohol solution. Each session was video-recorded for posterior behavioral analysis, with the aid of X-plo-Rat software (Laboratório de Comportamento Exploratório of Faculdade de Filosofia, Ciências e Letras at University of São Paulo - Campus Ribeirão Preto, São Paulo, Brazil). This test was carried out between PNDs 92 and 105.
The following parameters were considered: (1) percentage of visits into the open arms (% VOA): this variable was calculated by dividing the entries in the OA by the number of entries in both arms; (2) percentage of time in the open arms (% TOA): represented by the ratio between the time spent in the OA and the time spent in both arms; (3) percentage of time in the central segment (% TC); (4) the number of segments crossed in closed arms; (5) time spent in the extremity of the OA. Entries were considered when the rats placed all four paws inside the arms and the same was true for motor activity.
Anxiety index was calculated according to Mazor and colleagues (Citation2009), considering the frequency and time spent in the OA in relation to the total exploration of the apparatus.
Anxiety index stands between 0 and 1, in which higher indices indicate higher anxiety level.
2.6. Blood sampling and determination of CORT plasma levels
The non-tested male and female from each litter were decapitated immediately after being taken from their home-cages (Basal or T0). Subsequently, one male and one female from the same litter were decapitated 15, 45, or 75 min after the EPM (T15, T45, and T75, respectively). Trunk blood was collected in EDTA containing tubes (Greiner bio-one, Brazil), centrifuged for 20 min (1209g at 4 °C) to obtain plasma, which was then frozen at −20 °C until determination of CORT plasma levels by adding 50 µl of standard solution (1 mg/ml of CORT methanol solution) and 100 µl of zinc sulfate 1 M (Sigma Aldrich, São Paulo, Brazil) to 100 µl of plasma. Afterwards, 1 ml of ether was added and the solution was centrifuged for 5 min at 1086.5g at room temperature. The supernatant was immediately frozen to −20 °C for 20 min; upon being defrost, the supernatant was transfer to another tube and submitted to a fast drying process with compressed nitrogen for 30 min. Afterwards, the sample was re-suspended in 100 µl water:methanol 1:1 v/v (J.T. Baker, São Paulo, Brazil) with 0.1% of phormic acid (J.T. Baker), and 20 µl were injected in the mass spectometer (Waters – model Quatromicro; based on Li et al. (Citation2014)).
2.7. Monoamine concentrations in discrete brain areas
The brains from non-tested animals were obtained frozen at −80 °C. Brains were divided and one hemisphere was used (in counterbalanced order among the animals in each group) and dissected to obtain frontal cortex (FC), dorsal and ventral hippocampus (dHPC and vHPC), amygdala (AMY), and hypothalamus (HPT). Noradrenaline (NA), serotonin (5-HT), dopamine (DA) and their metabolites homovanilic acid (HVA), 5-hydroxyindolacetic acid (5-HIAA), and 3,4-Dihydroxyphenylacetic acid (DOPAC) were detected by High Performance Liquid Chromatography (HPLC), with electrochemical detection and quantification (Machado, Tufik, & Suchecki, Citation2008).
The tissue samples were weighed individually and homogenized by sonication in 500 ml of extraction solution (0.1 M perchloric acid containing 0.4 mM sodium metabissulfite and 0.2 mM ethylenediaminetetraacetic acid). The homogenates were centrifuged at 20,000g for 10 min, then filtered through 0.22 mm membrane and stored at −80 °C for further analysis. Precipitates were dissolved in 0.1 N NaOH and assayed for protein levels (Bicinchoninic acid method, Pierce Chemical, Rockford, IL). Supernatants were submitted to fast isocratic separation through a C18 HPLC reversed-phase column system (Spheri-5, C18, ODS, 5 mm, 25 cm, 4.6 mm column; linked to a New-Guard Cartridge Column, RP-18, 7 mm pre-column; Perkin Elmer Brownlee Columns, Shelton, CT) and electrochemically detected using an amperometric detector (L-ECD-6A, Shimadzu, Japan), by oxidation on glass carbon electrode at +850 mV in relation to an Ag–AgCl reference electrode. The mobile phase consisted of 0.163 M citric acid, 0.06 M sodium phosphate dibasic anhydrous, 0.69 mM octyl sodium sulfate, 12 mM EDTA, acetonitrile 4%, tetrahydrofuran 1.7%, and orthophosphoric acid sufficient to bring the pH to 2.85, diluted in double distilled water. The mobile phase was filtered through a 0.2 mm filter membrane, degassed under vacuum, and delivered at a flow rate of 1.2 ml/min (HITACHI Pump System L-7100). Each sample was analyzed in duplicate and recovery of the analytes was determined by adding a fixed concentration of internal standard – dihydroxybenzylamine (DHBA) – before tissue homogenization. An automatic injector (HITACHI L-7250, cut injection method) was utilized to improve the reproducibility of injections. All standards and salts were purchased from Sigma (São Paulo, Brazil) and the solvents (HPLC grade) were purchased from J.T. Baker.
2.8. Statistical analysis
Behavioral parameters from food intake, NSF, SIT, and EPM were analyzed by three-way factorial ANOVA, with Group (CTL × DEP11), Stress (NSAL × SAL), and Sex (male × female) as main factors. Parameters obtained in the SNCT were subjected to repeated measures ANOVA, including Group, Stress, and Day (repeated measure: Day 1, Day 2, and Day 3) as main factors, separately by sex.
CORT reactivity to the EPM was obtained by calculating the variation between each individual post-test value and the mean value of the respective non-tested subgroup, by the equation:
% of variation from basal = ([post-EPM level × 100]/mean value of the respective non-tested group) − 100
After this calculation, CORT reactivity was analyzed, separately by sex, by three-way factorial ANOVA with Group, Stress, and Time-point (15, 45, and 75 min) as main factors.
Monoamine levels and turnovers from each brain structure were analyzed separately by sex, using a two-way ANOVA, with Group and Stress as main factors. When appropriate, Duncan post hoc analysis was used for pairwise comparisons, using Statistica Software v.12 (Statsoft, Brazil) and results were considered significant when p ≤ .05.
3. Results
3.1. Food intake and novelty supressed feeding
Females ate less than males during the day [F(1,176) = 12.44; p < .001, ] and during the night [F(1,176) = 333.50; p < .00001, , whereas DEP11 animals ate less than CTL ones during the night (main effect of Group [F(1,176) = 3.82; p = .05, ). Cumulative food intake followed the same trend as the nocturnal intake (), with main effects of Sex [F(1,176) = 315.05; p < .00001] and Group [F(7,176) = 5.91; p < .02]; females and DEP11 animals ate less than males and CTL rats, respectively.
Figure 2. Assessment of food intake and parameters in the novelty suppressed feeding (NSF). (A) Diurnal food intake in grams (n = 19–29 animals/group). (B) Nocturnal food intake in grams (n = 19–29 animals/group). (C) Cumulative chow intake in grams (n = 19–29 animals/group). (D) Latency to eat in the NSF test, in seconds (n = 14–29 animals/group). (E) Food intake during the NSF test (n = 19–28 animals/group). (F) Food intake in the familiar cage, after the NSF test (n = 19–29 animals/group). *Different from respective CTL group. #Different from male counterparts. §Different from respective NSAL. CTL: control, non-deprived group; DEP11: maternally-deprived on postnatal day 11; NSAL: group not saline injected; SAL: group injected with saline on postnatal day 12. Data are presented as mean ± S.E.M.
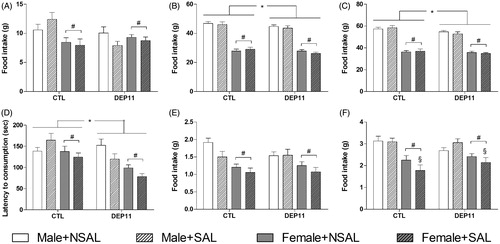
Females [F(1,163) = 14.58; p < .0002], as well as DEP11 rats [F(1,163) = 10.62; p < .002] took shorter than, respectively, males and CTL rats to eat. During the test, females ate less than males (main effect of Sex [F(1,161) = 16.61; p < .0001]) and SAL-injected rats ate less than NSAL ones (main effect of Stress [F(1,161) = 3.75; p = .05, ]). Upon returning to their home-cages, females ate less than males (main effect of Sex [F(1,160) = 39.05; p = .00001, ); an interaction between Stress and Sex [F(1,160) = 3.94; p < .05] was also revealed and the post hoc analysis indicated that SAL-treated rats ate less than NSAL ones (p < .04).
3.2. Sucrose negative contrast test
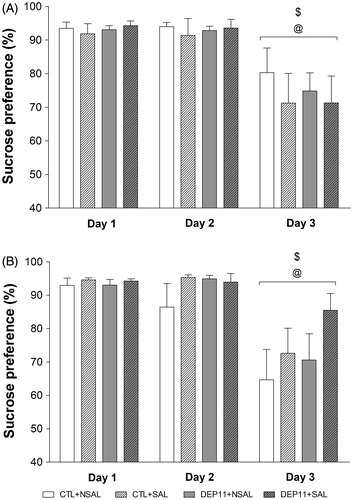
Both males and females showed suppression of intake of the lower sucrose concentration, main effect of Day, males () [F(2,116) = 28.29; p < .00001] and females [F(2,100) = 25.61; p < .00001] ().
Figure 3. Sucrose preference index in the sucrose negative contrast test of males (A) and females (B). @Different from Day 1. $Different from Day 2. CTL: control, non-deprived group; DEP11: maternally-deprived on postnatal day 11; NSAL: group not saline injected; SAL: group injected with saline on postnatal day 12. Data are presented as mean ± S.E.M. of 12–20 animals/group.
3.3. Social investigation test
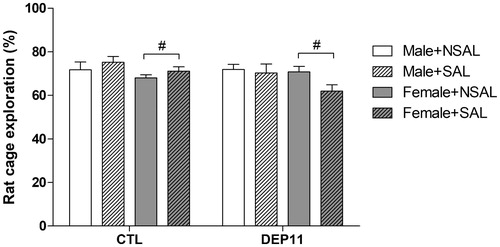
Females investigated the target conspecific less than males [F(1,143) = 3.90; p = .05]. There was a trend for interaction between Group and Stress [F(1,143) = 3.71; p = .056] ().
Figure 4. Exploration of the container with the target rat during the social investigation test. #Different from males. Different from the respective CTL group. CTL: control, non-deprived group; DEP11: maternally-deprived on postnatal day 11; NSAL: group not saline injected; SAL: group injected with saline on postnatal day 12. Data are presented as mean ± S.E.M. of 12–27 animals/group.
3.4. Elevated plus maze
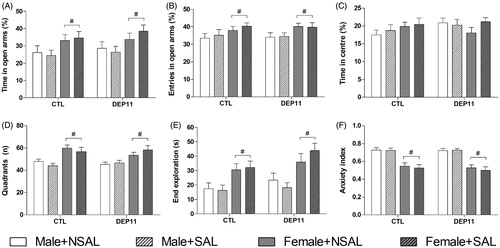
Females visited more the open arms (% VOA) [F(1,139) = 7.94; p < .006, ], spent more time in the open arms (% TOA) [F(1,139) = 10.37; p < .002, ] and crossed more segments in the closed arms [F(1,139) = 32.90; p < .0001, ] than males. Females also spent more time than males in the far end of the open arms [F(1,139) = 26.07; p < .0001] and there was also a trend for Group effect [F(1,139) = 3.63; p = .058] (). As a consequence, females exhibited lower anxiety index than males [F(1,139) = 79.70; p < .0001, ]. There were no effects on the % TC ().
Figure 5. Exploratory behavior of animals in the EPM. (A) % of visits to the open arms. (B) % of time spent in the open arms. (C) % of time spent in the central square. (D) Number of segments crossed in the closed arms. (E) Time in the far end of open arms. (F) Anxiety index. *Different from respective CTL group. #Different from respective males. CTL: control, non-deprived group; DEP11: maternally-deprived on postnatal day 11; NSAL: group not saline injected; SAL: group injected with saline on postnatal day 12. Data are presented as mean ± S.E.M. of 12–26 animals/group.
3.5. Percentage of CORT reactivity
3.5.1. Males
Main effects of Group [F(1,73) = 10.97; p < .002] and Time-point [F(2,73) = 9.93; p < .0002] were detected (). DEP11 animals secreted more CORT than CTL and for all rats, plasma levels were higher at 15 min than at 45 min (p < .04), which, in turn, were higher than at 75 min (p < .02).
Figure 6. Percentage of CORT reactivity (variation from basal levels, obtained in ng/ml) of males (A, n = 6–10 animals/group) and females (B, n = 3–7 animals/group) 15 min, 45 min, and 75 min after exposure to the EPM. *Different from respective CTL group. &Different from 15 min time-point. πDifferent from 45 min time-point. §Different from the respective NSAL groups. CTL: control, non-deprived group; DEP11: maternally-deprived on postnatal day 11; NSAL: group not saline injected; SAL: group injected with saline on postnatal day 12. Data are presented as mean ± S.E.M.
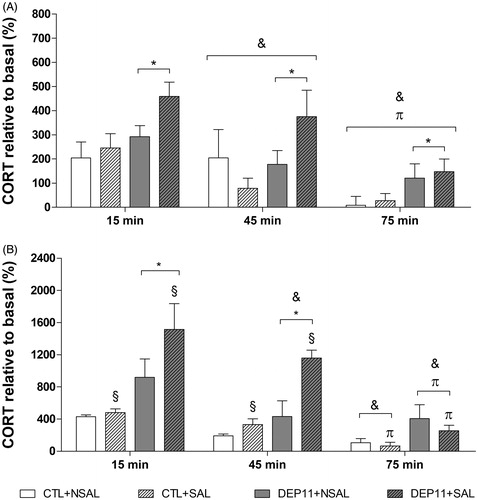
3.5.2. Females
There was an interaction between Group and Stress [F(1,51) = 5.69; p < .02], both the DEP11 + NSAL and DEP11 + SAL groups displayed greater stress reactivity than the CTL + NSAL group () (p < .05 and p < .0001, respectively); moreover, the DEP11 + SAL showed greater CORT variation than the CTL + SAL (p < .0001) and the DEP11 + NSAL counterparts (p < .005). There was also an interaction between Group and Time-point [F(2,51) = 4.44; p < .02] and CTL rats had lower CORT reactivity at 75 min than 15 min (p < .01), while for DEP11 rats CORT response lowered gradually from 15 min to 75 min (p < .001). At all time-points the CORT response was higher in DEP11 than in CTL rats (15 min, p < .001; 45 min, p < .001; and 75 min, p < .05). Finally, there was an interaction between Stress and Time-point [F(2,51) = 5.51; p < .007] and NSAL females exhibited lower CORT variation at 45 and 75 min than at 15 min (p < .05 for both Time-points), while SAL-injected rats showed a gradual decline from 15 to 75 min (p < .05). Finally, SAL rats had a higher CORT reactivity than NSAL ones (p < .005).
3.6. Basal levels of monoamines
3.6.1. Males
The results of brain monoamines of males are presented in and the respective turnovers, in .
Figure 7. Basal monoamine levels in the hypothalamus (HPT), frontal cortex (FC), amygdala (AMY), dorsal hippocampus (dHPC), and ventral hippocampus (vHPC) of male rats. (A) Noradrenaline. (B) Dopamine. (C) Serotonin. *Different from the respective CTL group. + Different from CTL + NSAL. α Different from CTL + SAL. β Different from DEP11 + NSAL.CTL, control, non-deprived group; DEP11: maternally-deprived on postnatal day 11; NSAL: group not saline injected; SAL: group injected with saline on postnatal day 12. Data are presented as mean ± S.E.M. of 7–10 animals/group.
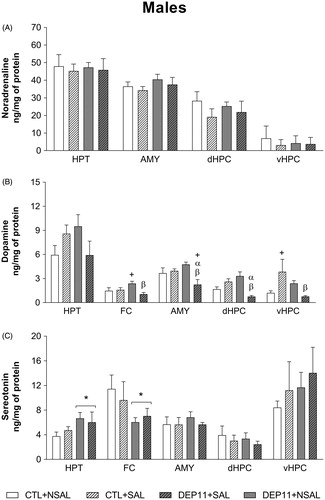
Table 1. Basal dopamine and serotonin turnover rates in male rats under basal condition.
NA (upper panel): no changes were observed and this monoamine was not detected in the FC; therefore this structure is not represented in the figure.
DA (middle panel): in the FC, DEP11 + NSAL animals exhibited higher levels than DEP11 + SAL (p < .005) and CTL + NSAL (p < .05), due to an interaction between Group and Stress [F(3,28) = 5.86; p < .05]. In the AMY, the lowest levels were observed in DEP11 + SAL animals (interaction between Group and Stress [F(3,29) = 8.86; p < .01]). Likewise, in the dHPC, DEP11 + SAL displayed lower concentrations than CTL + SAL (p < .005) and DEP11 + NSAL (p < .001), whereas DEP11 + NSAL animals exhibited higher DA levels than CTL + NSAL ones (p < .05) (Group and Stress interaction [F(3,29) = 18.99; p < .001]). Finally, in the vHPC the interaction between Group and Stress [F(3,27) = 6.26; p < .05] indicated that the DEP11 + SAL and CTL + NSAL groups showed lower levels than the CTL + SAL (p < .05).
5-HT (lower panel): in the HPT there was a main effect of Group [F(3,29) = 4.26; p < .05] and DEP11 males showed higher levels than CTL ones. There was also a main effect of Group in the FC [F(3,28) = 4.22; p < .05], but contrary to the HPT, DEP11 showed lower levels than CTL animals.
No further differences were found in monoamine levels or turnovers.
3.6.2. Females
The results of brain monoamines in females are presented in and their respective turnover rates, in .
Figure 8. Basal monoamine levels in the hypothalamus (HPT), frontal cortex (FC), amygdala (AMY), dorsal hippocampus (dHPC), and ventral hippocampus (vHPC) of female rats. (A) Noradrenaline. (B) Dopamine. (C) Serotonin. *Different from the respective CTL group. δDifferent from the respective NSAL group. CTL: control, non-deprived group; DEP11: maternally-deprived on postnatal day 11; NSAL: group not saline injected; SAL: group injected with saline on postnatal day 12. Data are presented as mean ± S.E.M. of 5–7 animals/group.
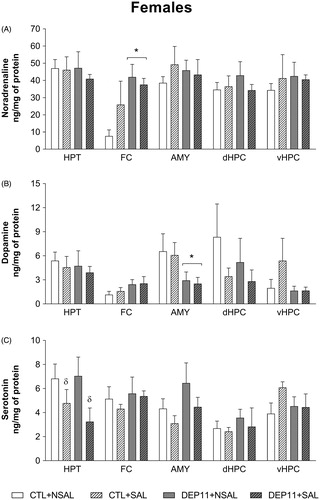
Table 2. Dopamine and serotonin turnover rates in female rats in estrous under basal condition.
NA (upper panel): there was a main effect of Group only in the FC [F(3,19) = 7.60; p < .05], with DEP11 animals showing higher levels than CTL ones.
DA (middle panel): DEP11 had lower levels in the AMY than CTL rats (Group effect [F(3,19) = 4.45; p < .05]).
5-HT (lower panel): in the HPT there was a main effect of Stress [F(3,19) = 5.18; p < .05], and higher levels were obtained in SAL than in NSAL counterparts.
4. Discussion
The present study was undertaken to test the hypothesis that a saline injection in animals previously submitted to MD on PND 11 would lead to enduring changes in emotional behaviors, CORT stress response, and constitutive monoamine levels in emotion-related brain areas. It is well established that 24 h MD during the SHRP disinhibits the adrenal glands, which then become responsive to various stressors, including saline injection (Levine et al., Citation1991). Previous studies have shown that the CORT response to a saline injection lasts for, at least, 2 h in neonates maternally-deprived on PND 9 (Girardi, Zanta, & Suchecki, Citation2014) and on PNDs 8 and 11 (Suchecki et al., Citation1995). The results of the present study partially supported our hypothesis, mainly regarding the biological parameters. Furthermore, a clear sex-dependent effect was observed at the behavioral and neurobiological levels.
In the NSF, both male and female DEP adult rats ate less in the home-cage than CTL counterparts, replicating our previous findings on adolescent rats, which is paralleled by a reduction in neuropeptide Y (NPY) immunoreactivity in the arcuate nucleus of the hypothalamus (Wertheimer, Girardi, de Oliveira, Monteiro Longo, & Suchecki, Citation2016). Some studies demonstrate that MD reduces leptin levels in adult males and females and insulin levels in males (Viveros, Díaz, Mateos, Rodríguez, & Chowen, Citation2010; Viveros, Llorente, et al., Citation2010), suggesting that these animals would be likely to overeat. Indeed, DEP5 adult rats consume more high fat, instead of regular and carbohydrate-rich, chow than CTL and DEP14 rats (Penke et al., Citation2001). These findings indicate that disruption of maternal care permanently alters the regulation of hunger and satiety in both sexes. In addition, the present results replicated the previously reported sexual dimorphism in the amount of food intake (Applegate, Upton, & Stern, Citation1982; Asarian & Geary, Citation2013; Wertheimer et al., Citation2016). Although we did not assess the animals’ body weight, food intake relative to body weight might not have been different between females and males, since the former weighs less than the latter. Interestingly, despite reduced spontaneous motivation to eat in the home-cage, DEP11 rats displayed shorter latency to initiate feeding in a novel cage after fasting for 48 h, a condition that increases CORT plasma levels (McGhee, Jefferson, & Kimball, Citation2009), serotoninergic activity in the frontal cortex (Beck & Luine, Citation1999), and reduces DA availability in the nucleus accumbens (Pothos, Hernandez, & Hoebel, Citation1995). There are two possible interpretations for the results obtained in the NSF test. First, DEP11 rats displayed better adaptability to stressful situations (48 h of fasting), resulting in lesser anxiety-like behavior and, consequently, faster approach to the pellet in a novel environment. Similar results were reported in adult rats raised by dams displaying better maternal care, compared to animals raised by less caring mothers (Caldji et al., Citation1998). We assessed maternal care upon reunion of mothers with their litters and, although frequency of maternal care was measured only during that day, mothers kept away from their offspring for 24 h displayed more arched back nursing and anogenital licking and grooming than mothers of control litters (data not shown). Alternatively, the shorter latency to eat in the NSF test could represent increased impulsivity. Impulsive behavior can be classified as a predisposition to act prematurely, with poor planning and high risk-taking actions, being the simplest form of motor disinhibition and impulsive decision-making (Jupp, Caprioli, & Dalley, Citation2013). Brain monoamine levels of non-tested animals were useful to indicate which of these interpretations was more appropriate (discussed below).
In the SNCT, all animals, regardless of the neonatal manipulation and sex, reduced the intake of the less concentrated sucrose solution, indicating that their infancy history did not interfere with their ability to discriminate salient stimulus. This result is in contrast to a previous study from our group showing that saline injection on PND 10 caused adolescent males to suppress less the low sucrose solution (Girardi et al., Citation2014). In addition, we found that MD on PNDs 3 or 11 impaired the discrimination of male, but not female, adolescent rats (unpublished data). Importantly, methodological differences between these studies could explain the divergent results. Perhaps, the most important difference is the fact that in the present study, animals were submitted to a battery of tests, whereas in the unpublished one, animals were tested only once. The second difference is the age of the animals at testing (late adolescence × adulthood).
Impairment of social behavior is commonly observed in emotional disorders (Kennedy & Adolphs, Citation2012; Allsop, Vander Weele, Wichmann, & Tye, Citation2014). In males, MD had no impact on social investigation. Again, this result is in contrast with our previous study, in which DEP9 males displayed less social investigation regardless of whether they had or not received a saline injection (Girardi et al., Citation2014). MD impact on social investigation appears to be age-dependent, since DEP3, but not DEP11, adolescent males, displayed impairment in this social behavior (unpublished data). Furthermore, CTL females already displayed less motivation to explore an unfamiliar conspecific (present results and unpublished data), suggesting a sex-dependent pattern of social behavior, with males being more affected than females, especially when MD is imposed at earlier ages.
Females were clearly less anxious than males in the EPM, regardless of the neonatal manipulations, a result that is in line with data suggesting that they explore the open arms more often than males (Johnston & File, Citation1991; Imhof, Coelho, Schmitt, Morato, & Carobrez, Citation1993). The lack of effect of maternal deprivation on PND 11 on classical spatial-temporal parameters in the EPM replicates a previous study, in which DEP11 males and females in diestrous only display more risk assessment. In addition, males and females were not different in any parameter evaluated in the EPM (Barbosa Neto et al., Citation2012). Therefore, it appears as though maternal deprivation does not alter the fact that expression of anxiety-like behavior is subjected to the phase of the estrous cycle when females are tested.
The CORT reactivity of DEP11 rats to EPM exposure was greater than that of CTL counterparts. As expected, the peak hormonal response was observed at 15 min, followed by a return to basal levels in CTL rats. Although DEP11 animals displayed a similar time-course of CORT release, the magnitude of the response was greater than that of CTL animals at all time-points measured. Moreover, saline injection after MD clearly influenced the adult stress response to EPM in both sexes, inasmuch as variation of CORT levels from basal was higher in DEP11 + SAL animals at 15 and 45 min. Previously, we tested the impact of MD on CORT response to the EPM by taking tail nip serial blood samples before and 15, 30, and 60 min after the test and found that both DEP11 males and females exhibited a progressive increase in the hormone response up to the 60 min time-point (Barbosa Neto et al., Citation2012), suggesting that they could express a deficient negative feedback regulation of the HPA axis. However, comparing these with the current results, it is more plausible to conclude that the former reflects an exaggerated CORT response to the sampling method, especially because DEP11 animals do not exhibit differences from CTL animals in CORT levels following the dexametasone suppression test (Faturi et al., Citation2010).
In the present study, we measured monoamine and their metabolite levels in structures of the limbic system of non-tested animals (i.e. constitutive profile). Although monoamine levels were altered by MD, associated or not to saline injection, no such changes were observed in the turnover rate, likely meaning that alterations in monoamine content did not result from changes in degradation rate, but rather, from changes in synthesis or availability at the target structures. We also observed a clear sex-dependent influence of the neonatal procedures, with males being more affected by the manipulations than females.
In males, MD increased hypothalamic 5-HT levels while in females neonatal SAL had the opposite effect. Hypothalamic 5-HT is involved in the regulation of food intake (Leibowitz & Alexander, Citation1998), so much so that microinjection of even low doses of this amine inhibits feeding (Leibowitz, Weiss, & Shor-Posner, Citation1988). These data are in line with reduced food intake shown by DEP11 males before the NSF, but is in contrast to the behavioral results in females, especially because DEP11 + SAL females ate less in the familiar cage after the NSF test, yet SAL-injected ones (regardless of maternal deprivation) had lower 5-HT hypothalamic levels. Other functions have been attributed to hypothalamic 5-HT, such as activation of the HPA axis, by increasing ACTH levels and its secretagogue, arginine vasopressin in response to restraint stress (J⊘rgensen, Knigge, Kjaer, Vadsholt, & Warberg, Citation1998; J⊘rgensen, Knigge, Kjaer, & Warberg, Citation2002). Decreased 5-HT synthesis is associated with passive coping in mice (De Miguel et al., Citation2011) and maternal separation reduces 5-HT immunoreactivity in the anterior hypothalamus, increases passive coping in the forced swimming test and aggressive behavior in the resident-intruder paradigm (Veenema, Blume, Niederle, Buwalda, & Neumann, Citation2006). Furthermore, MD decreased frontal cortex 5-HT levels in males, which is in agreement with previous studies showing reduced 5-HT levels in the PFC and amygdala of adult males (Rentesi et al., Citation2013). Abundant evidence indicates that the PFC is the main structure mediating behavioral inhibition and impulse control in primates and rodents (Crews & Boettiger, Citation2009; Dalley, Mar, Economidou, & Robbins, Citation2008). This brain area receives 5-HT and NA projections from, respectively, the dorsal raphe nucleus and the locus coeruleus (Fitzgerald, Citation2011) and reduced 5-HT activity in the PFC is a neurobiological underpinning of impulsive behavior (Holmes & Wellman, Citation2009; Roy, Adinoff, & Linnoila, Citation1988; Stein, Hollander, & Liebowitz, Citation1993). In females, MD increased frontal cortex NA, but this monoamine’s role in this behavior is still controversial (Oquendo & Mann, Citation2000), with studies suggesting that increased NA levels induce (Milstein, Dalley, & Robbins, Citation2010; Sun et al., Citation2010) or inhibit impulsive behavior (Robinson et al., Citation2008). The fact that DEP11 males presented lower basal 5-HT levels in the frontal cortex than their CTL counterparts, may suggest that in the NSF, these animals were more impulsive, rather than less anxious. This conclusion is supported by findings that maternal separation in 5-HT deficient male mice leads to shorter latency to initiate feeding in the NSF test without changes in anxiety-like behavior (Sachs et al., Citation2013). The behavior of females is harder to reconcile with the monoaminergic profile; regardless of the neonatal manipulation, females initiated food intake faster in the NSF test and explored more the open areas of the EPM than males, supporting the idea that they were probably less anxious.
The most striking result in males was the reduction of DA levels in all brain areas of DEP11 + SAL rats, except in the hypothalamus. These results are in line with findings in DEP3 males, which exhibit increased behavioral response to apomorphine, a D2 dopaminergic receptor agonist, possibly reflecting reduced dopaminergic transmission (Rots et al., Citation1996). The dopaminergic system is intrinsically related to motivation and reward (Wauquier, Citation1980), but current evidence implies its involvement in reinforced learning and habit formation (reviewed in Salamone & Correa, Citation2012). Importantly for the context of the present study is the literature concerning the involvement of dopaminergic system in fear and anxiety, mediated by projections from substantia nigra and ventral tegmental area, the main dopaminergic nuclei, to the central nucleus of the amygdala (reviewed in Björklund & Dunnett, Citation2007); antagonism of D1 dopaminergic receptor (De la Mora, Gallegos-Cari, Arizmendi-Garcia, Marcellino, & Fuxe, Citation2010); and stimulation of the D2 receptor produces anxiolysis (Garcia, Martinez, Brandão, & Morato, Citation2005). Evidence confirming this role comes from studies showing that highly aggressive rats display greater anxiety levels in the EPM and low basal DA levels in the PFC and hippocampus (Patki, Atrooz, Alkadhi, Solanki, & Salim, Citation2015), micro-injection of DA in the mPFC lowers anxiety-like behavior in the EPM (Dent & Neill, Citation2012) and knockout mice for the DA transporter are less anxious in the EPM and the light–dark transition test (Carpenter, Saborido, & Stanwood, Citation2012). The fact that DEP11 + SAL males displayed lower DA levels in all regions evaluated, except the HPT, suggests that association of both neonatal manipulations reduced the availability of this monoamine in the mesolimbic/mesocortical pathways, possibly including the nucleus accumbens. Nonetheless, these animals did not show greater anxiety levels than the other groups, so it is tempting to speculate that they could be more vulnerable to develop drug-related behaviors.
In conclusion, the results of this study refuted the hypothesis that saline injection in neonates made stress responsive would lead to enduring negative consequences on emotional behaviors. Nonetheless, maternal deprivation by itself or in association with saline injection had an impact in biological parameters, increasing CORT reactivity to the EPM. Particularly in males, association of the two neonatal stressors reduced DA levels in most brain areas assessed.
Acknowledgements
The authors would like to express their gratitude to Marcus ViníciusBunscheit for technical assistance in this study.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Allsop, S., Vander Weele, C.M., Wichmann, R., & Tye, K.M. (2014). Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Frontiers in Behavioral Neuroscience, 8, 241. doi:10.3389/fnbeh.2014.00241
- Applegate, E.A., Upton, D.E., & Stern, J.S. (1982). Food intake, body composition and blood lipids following treadmill exercise in male and female rats. Physiology & Behavior, 28, 917–920.
- Asarian, L., & Geary, N. (2013). Sex differences in the physiology of eating. American Journal of Physiology, 305, R1215–R1267. doi:10.1152/ajpregu.00446.2012
- Barbosa Neto, J.B., Tiba, P.A., Faturi, C.B., de Castro-Neto, E.F., da Graça Naffah-Mazacoratti, M., de Jesus Mari, J., …, Suchecki, D. (2012). Stress during development alters anxiety-like behavior and hippocampal neurotransmission in male and female rats. Neuropharmacology, 62, 518–526. doi:10.1016/j.neuropharm.2011.09.011
- Beck, K.D., & Luine, V.N. (1999). Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Research, 830, 56–71.
- Björklund, A., & Dunnett, S.B. (2007). Dopamine neuron systems in the brain: An update. Trends in Neuroscience, 30, 194–202. doi:10.1016/j.tins.2007.03.006
- Bodnoff, S.R., Suranyi-Cadotte, B., Aitken, D.H., Quirion, R., & Meaney, M.J. (1988). The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berlin), 95, 298–302.
- Bohn, M.C. (1980). Granule cell genesis in the hippocampus of rats treated neonatally with hydrocortisone. Neuroscience, 5, 2003–2012.
- Caldji, C., Tannenbaum, B., Sharma, S., Francis, D., Plotsky, P.M., & Meaney, M.J. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Science United States of America, 95, 5335–5340.
- Carpenter, A.C., Saborido, T.P., & Stanwood, G.D. (2012). Development of hyperactivity and anxiety responses in dopamine transporter-deficient mice. Developmental Neuroscience, 34, 250–257. doi:10.1159/000336824
- Cohen, H., Zohar, J., & Matar, M. (2003). The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biological Psychiatry, 53, 463–473.
- Crews, F.T., & Boettiger, C.A. (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry and Behavior, 93, 237–247. doi:10.1016/j.pbb.2009.04.018
- Dalley, J.W., Mar, A.C., Economidou, D., & Robbins, T.W. (2008). Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacology, Biochemistry and Behavior, 90, 250–260. doi:10.1016/j.pbb.2007.12.021
- De la Mora, M.P., Gallegos-Cari, A., Arizmendi-Garcia, Y., Marcellino, D., & Fuxe, K. (2010). Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Progress in Neurobiology, 90, 198–216. doi:10.1016/j.pneurobio.2009.10.010
- De Miguel, Z., Vegas, O., Garmendia, L., Arregi, A., Beitia, G., & Azpiroz, A. (2011). Behavioral coping strategies in response to social stress are associated with distinct neuroendocrine, monoaminergic and immune response profiles in mice. Behavioral Brain Research, 225, 554–561. doi:10.1016/j.bbr.2011.08.011
- Dent, M.F., & Neill, D.B. (2012). Dose-dependent effects of prefrontal dopamine on behavioral state in rats. Behavioral Neuroscience, 126, 620–639. doi:10.1037/a0029640
- Faturi, C.B., Tiba, P.A., Kawakami, S.E., Catallani, B., Kerstens, M., & Suchecki, D. (2010). Disruptions of the mother-infant relationship and stress-related behaviours: Altered corticosterone secretion does not explain everything. Neuroscience and Biobehavioral Reviews, 34, 821–834. doi:10.1016/j.neubiorev.2009.09.002
- File, S.E., & Seth, P. (2003). A review of 25 years of the social interaction test. European Journal of Pharmacology, 463, 35–53.
- Fitzgerald, P.J. (2011). A neurochemical yin and yang: Does serotonin activate and norepinephrine deactivate the prefrontal cortex? Psychopharmacology (Berlin), 213, 171–182. doi:10.1007/s00213-010-1856-1
- Garcia, A.M., Martinez, R., Brandão, M.L., & Morato, S. (2005). Effects of apomorphine on rat behavior in the elevated plus-maze. Physiology & Behavior, 85, 440–447. doi:10.1016/j.physbeh.2005.04.027
- Girardi, C.E., Zanta, N.C., & Suchecki, D. (2014). Neonatal stress-induced affective changes in adolescent Wistar rats: Early signs of schizophrenia-like behavior. Frontiers in Behavioral Neuroscience, 8, 319. doi:10.3389/fnbeh.2014.00319
- Hofer, M.A. (1994). Early relationships as regulators of infant physiology and behavior. Acta Paediatrica, Suppl. 397, 9–18.
- Holmes, A., & Wellman, C.L. (2009). Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews, 33, 773–783. doi:10.1016/j.neubiorev.2008.11.005
- Huang, W.L., Harper, C.G., Evans, S.F., Newnham, J.P., & Dunlop, S.A. (2001). Repeated prenatal corticosteroid administration delays astrocyte and capillary tight junction maturation in fetal sheep. International Journal of Developmental Neuroscience, 19, 487–493.
- Imhof, J.T., Coelho, Z.M., Schmitt, M.L., Morato, G.S., & Carobrez, A.P. (1993). Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behavioral Brain Research, 56, 177–180.
- Johnston, A.L., & File, S.E. (1991). Sex differences in animal tests of anxiety. Physiology & Behavior, 49, 245–250.
- Jørgensen, H., Knigge, U., Kjaer, A., Vadsholt, T., & Warberg, J. (1998). Serotonergic involvement in stress-induced ACTH release. Brain Research, 811, 10–20.
- Jørgensen, H., Knigge, U., Kjaer, A., & Warberg, J. (2002). Serotonergic involvement in stress-induced vasopressin and oxytocin secretion. European Journal of Endocrinology, 147, 815–824.
- Jupp, B., Caprioli, D., & Dalley, J.W. (2013). Highly impulsive rats: Modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction. Disease Models & Mechanisms, 6, 302–311. doi:10.1242/dmm.010934
- Kennedy, D.P., & Adolphs, R. (2012). The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences, 16, 559–572. doi:10.1016/j.tics.2012.09.006
- Landauer, M.R., & Balster, R.L. (1982). A new test for social investigation in mice: Effects of d-amphetamine. Psychopharmacology (Berlin), 78, 322–325.
- Leibowitz, S.F., & Alexander, J.T. (1998). Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biological Psychiatry, 44, 851–864.
- Leibowitz, S.F., Weiss, G.F., & Shor-Posner, G. (1988). Hypothalamic serotonin: Pharmacological, biochemical and behavioral analyses of its feeding-suppressive action. Clinical Neuropharmacology, 11, S51–S71.
- Levine, S., Huchton, D.M., Wiener, S.G., & Rosenfeld, P. (1991). Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Developmental Psychobiology, 24, 547–558. doi:10.1002/dev.420240803
- Li, H., Liu, X., Poh, Y., Wu, L., Zhou, Q.G., & Cai, B.C. (2014). Rapid determination of corticosterone in mouse plasma by ultra fast liquid chromatography-tandem mass spectrometry. Biomedical Chromatography, 28, 1860–1863. doi:10.1002/bmc.3232
- Lovick, T.A. (2012). Estrous cycle and stress: Influence of progesterone on the female brain. Brazilian Journal of Medical and Biological Research, 45, 314–320. doi:10.1590/S0100-879X2012007500044
- Machado, R.B., Tufik, S., & Suchecki, D. (2008). Chronic stress during paradoxical sleep deprivation increases paradoxical sleep rebound: association with prolactin plasma levels and brain serotonin content. Psychoneuroendocrinology, 33, 1211–1224. doi:10.1016/j.psyneuen.2008.06.007
- Matthews, S.G. (2000). Antenatal glucocorticoids and programming of the CNS. Pediatric Research, 47, 291–300.
- Matthews, K., Wilkinson, L.S., & Robins, T.W. (1996). Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiology & Behavior, 59, 99–107.
- Mazor, A., Matar, M.A., Kaplan, Z., Kozlovsky, N., Zohar, J., & Cohen, H. (2009). Gender-related qualitative differences in baseline and post-stress anxiety responses are not reflected in the incidence of criterion-based PTSD-like behaviour patterns. World Journal of Biological Psychiatry, 10, 856–869. doi:10.1080/15622970701561383
- McGhee, N.K., Jefferson, L.S., & Kimball, S.R. (2009). Elevated corticosterone associated with food deprivation upregulares expression in rat skeletal muscle of the mTORC1 repressor, REDD1. The Journal of Nutrition, 139, 828–834. doi:10.3945/jn.108.099846
- Meyer, J.S. (1983). Early adrenalectomy stimulates subsequent growth and development of the rat brain. Experimental Neurology, 82, 432–446.
- Milstein, J.A., Dalley, J.W., & Robbins, T.W. (2010). Methylphenidate-induced impulsivity: Pharmacological antagonism by beta-adrenoreceptor blockade. Journal of Psychopharmacology, 24, 309–321. doi:10.1177/0269881108098146
- Oquendo, M.A., & Mann, J.J. (2000). The biology of impulsivity and suicidality. Psychiatric Clinics of North America, 23, 11–25.
- Patki, G., Atrooz, F., Alkadhi, I., Solanki, N., & Salim, S. (2015). High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neuroscience Letters, 584, 308–313. doi:10.1016/j.neulet.2014.10.051
- Pellow, S., Chopin, P., File, S.E., & Briley, M. (1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods, 14, 149–167.
- Penke, Z., Felszeghy, K., Fernette, B., Sage, D., Nyakas, C., & Burlet, A. (2001). Postnatal maternal deprivation produces long-lasting modifications of the stress response, feeding and stress-related behaviour in the rat. European Journal of Neuroscience, 14, 747–755.
- Pothos, E.N., Hernandez, L., & Hoebel, B.G. (1995). Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: Implications for a possible neurochemical link between weight loss and drug abuse. Obesity Research, 3, 525S–529S.
- Powell, T.R., Fernandes, C., & Schalkwyk, L.C. (2012). Depression-related behavioral tests. Current Protocols in Mouse Biology, 2, 119–127. doi:10.1002/9780470942390.mo110176
- Rentesi, G., Antoniou, K., Marselos, M., Syrrou, M., Papadopoulou-Daifoti, Z., & Konstandi, M. (2013). Early maternal deprivation-induced modifications in the neurobiological, neurochemical and behavioral profile of adult rats. Behavioral Brain Research, 244, 29–37. doi:10.1016/j.bbr.2013.01.040
- Robinson, E.S., Eagle, D.M., Mar, A.C., Bari, A., Banerjee, G., Jiang, X., … Robbins, T.W. (2008). Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology, 33, 1028–1037. doi:10.1038/sj.npp.1301487
- Rosenfeld, P., Gutierrez, Y.A., Martin, A.M., Mallett, H.A., Alleva, E., & Levine, S. (1991). Maternal regulation of the adrenocortical response in preweanling rats. Physiology & Behavior, 50, 661–671.
- Rots, N.Y., de Jong, J., Workel, J.O., Levine, S., Cools, A.R., & De Kloet, E.R. (1996). Neonatal maternally deprived rats have as adults elevated basal pituitary-adrenal activity and enhanced susceptibility to apomorphine. Journal of Neuroendocrinology, 8, 501–506.
- Roy, A., Adinoff, B., & Linnoila, M. (1988). Acting out hostility in normal volunteers: Negative correlation with levels of 5HIAA in cerebrospinal fluid. Psychiatry Research, 24, 187–194.
- Sachs, B.D., Rodriguiz, R.M., Siesser, W.B., Kenan, A., Royer, E.L., Jacobsen, J.P., … Caron, M.G. (2013). The effects of brain serotonin deficiency on behavioural disinhibition and anxiety-like behaviour following mild early life stress. The International Journal of Neuropsychopharmacology, 16, 2081–2094. doi:10.1017/S1461145713000321
- Salamone, J.D., & Correa, M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76, 470–485. doi:10.1016/j.neuron.2012.10.021
- Schoenfeld, N.M., Leathem, J.H., & Rabii, J. (1980). Maturation of adrenal stress responsiveness in the rat. Neuroendocrinology, 31, 101–105. doi:10.1159/000123058
- Stein, D.J., Hollander, E., & Liebowitz, M.R. (1993). Neurobiology of impulsivity and the impulse control disorders. Journal of Neuropsychiatry and Clinical Neuroscience, 5, 9–17. doi:10.1176/jnp.5.1.9
- Suchecki, D., Nelson, D.Y., Van Oers, H., & Levine, S. (1995). Activation and inhibition of the hypothalamic–pituitary–adrenal axis of the neonatal rat: Effects of maternal deprivation. Psychoneuroendocrinology, 20, 169–182.
- Sullivan, R.M., & Holman, P.J. (2010). Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neuroscience and Biobehavioral Reviews, 34, 835–844. doi:10.1016/j.neubiorev.2009.11.010
- Sun, H., Green, T.A., Theobald, D.E., Birnbaum, S.G., Graham, D.L., Zeeb, F.D., … Winstanley, C.A. (2010). Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biological Psychiatry, 67, 649–656. doi:10.1016/j.biopsych.2009.11.030
- Veenema, A.H., Blume, A., Niederle, D., Buwalda, B., & Neumann, I.D. (2006). Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. European Journal of Neuroscience, 24, 1711–1720. doi:10.1111/j.1460-9568.2006.05045.x
- Verma, P., Hellemans, K.G., Choi, F.Y., Yu, W., & Weinberg, J. (2010). Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiology & Behavior, 99, 276–285. doi:10.1016/j.physbeh.2009.11.002
- Viveros, M.P., Díaz, F., Mateos, B., Rodríguez, N., & Chowen, J.A. (2010). Maternal deprivation induces a rapid decline in circulating leptin levels and sexually dimorphic modifications in hypothalamic trophic factors and cell turnover. Hormones and Behavior, 57, 405–414. doi:10.1016/j.yhbeh.2010.01.009
- Viveros, M.P., Llorente, R., Díaz, F., Romero-Zerbo, S.Y., Bermudez-Silva, F.J., Rodríguez de Fonseca, F., … Chowen, J.A. (2010). Maternal deprivation has sexually dimorphic long-term effects on hypothalamic cell-turnover, body weight and circulating hormone levels. Hormones and Behavior, 57, 808–819. doi:10.1016/j.yhbeh.2010.08.003
- Wauquier, A. (1980). The pharmacology of catecholamine involvement in the neural mechanisms of reward. Acta Neurobiologiae Experimentalis), 40, 665–686.
- Wertheimer, G.S., Girardi, C.E., de Oliveira, A.S., Monteiro Longo, B., & Suchecki, D. (2016). Maternal deprivation alters growth, food intake, and neuropeptide Y in the hypothalamus of adolescent male and female rats. Developmental Psychobiology, 58, 1066–1075. doi:10.1002/dev.21440
- Witek-Janusek, L. (1988). Pituitary-adrenal response to bacterial endotoxin in developing rats. American Journal of Physiology, 255, E525–E530. doi:10.1152/ajpendo.1988.255.4.E525
