Abstract
Successful coping with stressful events involves adaptive and cognitive processes in the brain that make the individual more resilient to similar stressors in the future. Stressful events result in the secretion of glucocorticoids (GCs) from the adrenal glands into the blood stream. Early work proved instrumental for developing the concept that these hormones act in the brain to coordinate physiological and behavioral responses to stress through binding to two different GC-binding receptors. Once activated these receptors translocate to the nucleus where they act on target genes to facilitate (or sometimes inhibit) transcription. There are two types of receptors in the brain, the mineralocorticoid receptor (MR), and glucocorticoid receptor (GR). This review summarizes recent work which provides new insights regarding the genomic action of these receptors, both under baseline conditions and following exposure to acute stress. This work is discussed alongside the extensive studies undertaken in this field previously and new, and exciting “big data” studies which have generated a wealth of relevant data. The consequence of these new insights will challenge existing assumptions about the role of MRs and GRs and pave the way for the implementation of novel and improved methodologies to identify the role these corticosteroid receptors have in stress-related behavioral adaptation.
Introduction
The biological response to stress involves many systems (nervous, endocrine, immune, etc.) co-operating together to evoke an appropriate reaction. The threshold for the involvement of each of these systems is specific and highly individualized depending on the information received and the genetic, epigenetic, and environmental background of the individual.
A stressor is a situation which an individual perceives as aversive and potentially harmful. In this way, stressors are highly individualized and the impact inflicted by a specific stressor may be different based on prior knowledge, experience, and cognitive abilities. For example, a man waving a gun would most likely be a strong stressor for an adult but for a baby may not constitute a stressor at all. Conversely, a loud unexpected noise may result in a similar response in all subjects, due to a reflex-like, innate realization that the situation could be potentially aversive or dangerous.
The stress response, namely, the collective biological and behavioral outcomes following exposure to a stressor, is equally individualized and has long been an area of interest for scientists. Great progress has been made into understanding the pathways involved in the stress response, especially in responses common to most individuals, such as activation of the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis, and the behavioral fight/freeze/flight responses. Understanding the finer molecular responses occurring in the brain after exposure to a stressor, and how these link to the range of individualized behavioral responses observed in stressed individuals remains a common goal for researchers in the field. In pursuit of this goal, the 1st Munich Winter Conference on Stress was held in Garmisch-Partenkirchen (Germany) in March 2017 to bring together both leaders and young scientists in stress research to discuss their recent advances in the hope of inspiring collaborative research to extend our understanding of the field. This review relates some of these recent advances with existing literature in order to provide a current view regarding how the brain translates the hormonal changes induced by stress into genomic responses via nuclear receptors.
Regulation of the levels of stress hormones accessing the brain
HPA axis activation results in the release of corticotrophin-releasing factor (CRF) from parvocellular neurons residing in the paraventricular nucleus (PVN) of the hypothalamus, which acts on the pituitary to induce the release of adrenocorticotrophic hormone (ACTH) into the circulation. ACTH acts on the adrenal cortex to stimulate the secretion of glucocorticoid hormones (GCs) into the circulation. GCs are synthesized from cholesterol through a series of enzymatic reactions. 11β-hydroxysteroid dehydrogenases (11β-HSDs) regulate the biological activity of glucocorticoids, with 11β-HSD1 catalyzing activation of the steroid into its active forms (cortisol in humans or corticosterone (cort) in rodents). 11β-HSD2 facilitate the opposite reaction converting the active forms cortisol and cort to the inactive forms cortisone and 11-dehydrocorticosterone (Chapman, Holmes, & Seckl, Citation2013). The abundance of 11β-HSD1 and lack of 11β-HSD2 expression in MR-positive brain regions ensures that glucocorticoids are the primary factor driving responses in these areas (Wyrwoll, Holmes, & Seckl, Citation2011). Originally, it was thought that the adult brain completely lacks 11β-HSD2, but indeed more recent studies have shown low to moderate levels of 11β-HSD2 expressed in regions such as the nucleus tractus solitarus and (other) key regions linked to blood pressure control and sodium appetite (Wyrwoll et al., Citation2011).
The HPA axis is also regulated by diurnal cues which serve to impose a circadian rhythm to basal GC secretion. GCs act on a wide range of tissues including the brain to induce physiological responses. The amount of active hormone affecting the brain is regulated by how much hormone enters the brain (Accessibility) and how much is free to exert a biological effect (Availability).
Accessibility
Work in early 2000 looked at how GCs traversed the blood-brain barrier (BBB) by comparing the retention of radiolabeled steroids in wild-type versus mdr1a (multidrug resistance 1a; abcb1a) knockout mice. The abcb1a gene encodes the multidrug transporter P-glycoprotein (Pgp), a key component of the BBB playing a critical role in enforcing selective permeability of the barrier (Schinkel, Citation1999). In rodents, the predominant GC is cort, which appeared to pass freely across the BBB into the brain. Although commonly reported that rats cannot synthesize cortisol due to lack of adrenal 17α-hydroxylase, there is recent evidence that cortisol is present in rodent blood, especially in response to severe acute stress (Gong et al., Citation2015). In humans, cortisol is the predominant GC but, interestingly, is partially excluded from the brain (Karssen et al., Citation2001). This may explain why humans have a higher ratio of cort to cortisol in their brains (∼30%) compared with the ratio in the plasma (∼5%) although the biological implication for such a difference, if any, remains unclear (Karssen, Meijer, & De Kloet, Citation2005). The freely accessible nature of the BBB to cort has since been challenged after a double knockout for both isoforms of the multi-drug resistance gene (mdr1a and mdr1b) was developed and revealed a significant increase in the concentration of cort in the brain and adrenal gland of double knockout mice cf. wild-type animals (Uhr, Holsboer, & Muller, Citation2002). Other studies have shown that the permeability of the BBB to various steroids in vivo, or of model BBB systems in vitro, can be modulated by a number of factors including removal of adrenals by adrenalectomy (ADX) (Long & Holaday, Citation1985) and, potentially, antidepressants (Pariante et al., Citation2001).
Availability
The development of the in vivo microdialysis technique revolutionized the ability of researchers to accurately monitor the free, biologically active, GC levels present in the extracellular fluid surrounding cells in tissues (Jasper & Engeland, Citation1991; Linthorst & Reul, Citation2008; Linthorst, Flachskamm, Holsboer, & Reul, Citation1994). Using this technique, Droste et al. (Citation2008) showed that cort in the extracellular fluid of the rat hippocampus followed a circadian pattern with ultradian pulses in free cort clearly evident over a 24 h period, a pattern that was mirrored in the caudate putamen. Given that the caudate putamen is not significantly involved in HPA axis regulation it is likely that these levels of free cort throughout the brain are derived from circulating plasma levels and not brain structure-specific mechanisms. A later study indeed showed a clear correlation between the levels of free cort in the blood and the corresponding levels of free cort in the periphery and the brain (Qian, Droste, Lightman, Reul, & Linthorst, Citation2012).
Measurement of free cort levels in the rat brain prior to, during and after different stressors confirmed that stress-induced increases in total plasma cort were reflected by the free cort levels in the hippocampus, albeit at a much lower concentration. The duration of the stress-induced elevation in cort depended on the type of stress, but following the return of cort to basal levels after stress the ultradian and circadian pulses were restored (Droste et al., Citation2008). Simultaneous sampling of blood from the jugular vein and microdialysis of the extracellular fluid within the hippocampus during and after exposure of rats to forced swimming (FS) revealed a 20 min lag in the free cort hippocampal peak compared with the peak in total plasma cort. This lag was not evident following a subcutaneous injection of exogenous glucocorticoid, or after a mild stressor such as exposure to the novel environment, indicating that the intensity of the stress was somehow critical in determining the time domain of free cort exposure in the brain (Droste et al., Citation2008). An explanation for this stress-related delay was provided in a follow-up study investigating the role of cort-binding globulin (CBG) after stress (Qian et al., Citation2011). CBG is a transport protein which binds GCs in the blood rendering them inactive for biological action. FS stress caused a substantial and highly significant release of CBG protein from the liver into the bloodstream of rats, which did not occur after exposure of rats to a mild stressor (novel environment) (Qian et al., Citation2011). The function of this rapid release of CBG was most likely to sequester the increase in cort secreted as a result of moderate to severe stress, thus dampening the surge in free GC concentration and resulting in the observed delayed increase in free cort available throughout the body (Qian et al., Citation2011). The consequences of delaying the rise in biologically active GC levels may be to allow time for other stress-induced mechanisms to become established prior to the actions of GC itself, including the strong negative feedback GCs exert on the activated HPA axis.
Functional role of brain GC receptors in behavioral responses following exposure to stress
Brain mineralocorticoid and glucocorticoid receptors
In the brain, GCs exert their biological action via two types of receptors (Reul & de Kloet, Citation1985). The high-affinity mineralocorticoid receptor (MR) is expressed predominantly in limbic areas [i.e. hippocampus, lateral septum, central amygdala (CeA)] whereas the low-affinity glucocorticoid receptor (GR) is ubiquitously expressed throughout the rat brain (Reul & de Kloet, Citation1985, Citation1986; Reul, van den Bosch, & de Kloet, Citation1987). The scientific discovery of the GC receptors and their function in the brain, especially the role of the MR, was recently the focus of a historical review by Joels and de Kloet (Citation2017). Due to their very high affinity for cort, hormone binding to MRs in rats is almost saturated even under conditions of early morning baseline HPA axis activity (Reul & de Kloet, Citation1985; Reul et al., Citation1987). These observations explain why, under any physiological condition, MR immunoreactivity is found in the nucleus, and hardly in the cytoplasm, of hippocampal principal neurons (Gesing et al., Citation2001). As MRs appear to be in a constant activated state, de Kloet and Reul (Citation1987) proposed that these receptors exert tonic actions on brain function. In contrast, GRs display relatively lower binding affinity for natural GCs, which explains their low occupancy by hormone under early morning baseline conditions in the rat hippocampus (Reul & de Kloet, Citation1985; Reul et al., Citation1987). Substantial occupancy of GRs is only observed after a significant rise in circulating GCs, such as that observed after stress or at the circadian peak of GC secretion (Reul & de Kloet, Citation1985; Reul, et al., Citation1987). The occupancy profile of GRs corresponds with this receptor’s envisioned role in exerting negative feedback on enhanced HPA axis activity and in stress-related adaptation and memory formation (Reul, Citation2014; Reul et al., Citation1987).
Under conditions of low GC levels, GRs reside in the cytoplasm in a complex with heat shock protein 90 (hsp90), FK506 binding protein 1 (FKBP51), a co-chaperone protein which stabilizes GR in an inactive state, and other proteins. Upon rising GC levels, GRs shed FKBP51, bind GCs and recruit FK506 binding protein 1 (FKBP52), resulting in translocation to the nucleus to exert biological effects at the genomic level. Classically GRs are thought to dimerize, however, more recent work has shown that GR can interact with DNA as a monomer, dimer, tetramer or in complexes with other transcription factors including MR, opening up additional mechanisms of regulatory control and complexity (Nixon, Andrew, & Chapman, Citation2013; Presman et al., Citation2016). It is presently unclear if oligomerization occurs in the cytoplasm (Savory et al., Citation2001) prior to translocation to the nucleus or at the DNA interface (Luisi et al., Citation1991) where GRs bind to specific glucocorticoid response elements (GREs) that exist within the DNA sequence of GC-inducible genes to elicit their transcriptional effects (Morsink, Joels, et al., Citation2006; Morsink, Steenbergen, et al., Citation2006). It is thought that GRs can interact at several positions within its molecular structure to form oligomers, both in the ligand binding domain (LBD) and DNA binding domain (DBD) (Nixon et al., Citation2013). The generation of a mouse strain with a point mutation within its DBD (GRdim) has been reported to lack the ability for GRs to dimerize and hence prevent GR binding to GREs in target genes (Reichardt et al., Citation1998). Experiments utilizing GRdim mice highlighted the importance of receptor dimerization in the transactivation process, but interestingly not the transrepression of some GC-target genes (Reichardt et al., Citation1998). Subsequent studies, however, have shown that GRdim mice retain at least some transactivation capability (Frijters et al., Citation2010), and replicating the same mutation in cells resulted in some ability to GRs to form dimers (Presman et al., Citation2014), therefore caution is advised when interpreting studies using the GRdim mutation. In addition to their function as nuclear receptors, studies have also shown that both the MR and GR can be present in unique membrane-bound forms with distinct roles in glutamatergic neurotransmission and phosphorylated cAMP response element-binding protein (pCREB) signaling pathways, respectively (Karst et al., Citation2005; Roozendaal et al., Citation2010).
Although MRs and GRs show a remarkably high structural similarity, multiple studies have identified specific properties of the individual receptors in terms of their distribution in the brain, molecular action and ligand specificity (Arriza, Simerly, Swanson, & Evans, Citation1988; Reul & de Kloet, Citation1985, Citation1986; Reul et al., Citation1987). For instance, the synthetic GC dexamethasone (DEX) has been shown to bind to and activate GRs, whereas it can bind to MRs but not activate these receptors (Rafestin-Oblin et al., Citation1986; Reul et al., Citation1987, Citation2000). Researchers have exploited these differences to understand the individual functions of these receptors in response to challenging situations, both in intact animals and in animals which have had their adrenals, and hence their principal endogenous source of GCs, removed by ADX.
Removal of endogenous GCs by adrenalectomy
Early studies showed that ADX significantly impaired hippocampus-dependent learning and adaptive responses in rodents exposed to several behavioral challenges, all of which are known to activate central stress responses including HPA axis activation (Beylin & Shors, Citation2003; Borrell, De Kloet, Versteeg, & Bohus, Citation1983; De Kloet, De Kock, Schild, & Veldhuis, Citation1988; Jefferys, Copolov, Irby, & Funder, Citation1983; Jefferys & Funder, Citation1987; Oitzl & de Kloet, Citation1992; Veldhuis, De Korte, & De Kloet, Citation1985). ADX blocked the stress-induced enhancement in associative learning if trace conditioning was performed within 1 week after surgery (Beylin & Shors, Citation2003). This could not be restored by administration of a low dose of cort or mineralocorticoids sufficient to occupy the MR, but was recoverable if a high level of cort (sufficient to occupy both the MR and GR) was administered 30 min prior to training (Beylin & Shors, Citation2003; Jefferys et al., Citation1983). ADX did not affect initial inhibitory avoidance training but did impair subsequent inhibitory avoidance behavior if the initial training was performed within 120 h after ADX surgery (Borrell et al., Citation1983). Rats trained after 120 h post-ADX showed no deficits in behavior when compared with SHAM-treated animals (Borrell et al., Citation1983). This study highlighted the importance of the timing of the behavioral challenge in relation to ADX and indicates mechanisms may be acting alongside GRs and MRs to compensate for the ongoing loss of endogenous hormones after long-term ADX to recover these essential behavioral processes (Borrell et al., Citation1983; de Kloet & Molendijk, Citation2016; Oitzl & de Kloet, Citation1992). ADX was also shown to impair the behavioral immobility response in the FS retest, a measure of behavioral adaptation (De Kloet et al., Citation1988; Jefferys et al., Citation1983; Reul, Citation2014; Veldhuis & De Kloet, Citation1983; Veldhuis et al., Citation1985). Later studies identified the dentate gyrus of the hippocampus as the neural substrate of the effects of GR activation on this adaptive behavioral response (De Kloet et al., Citation1988). Administration of the GR agonists DEX, RU28362, or cort to ADX rats restored the immobility response and this restoration was blocked by co-administration of the GR antagonist RU486 further implicating a role for the GR in these processes (De Kloet et al., Citation1988; de Kloet & Molendijk, Citation2016; Jefferys & Funder, Citation1987). Interestingly, administration of aldosterone at a concentration to specifically activate the MR only was unable to restore the ADX-induced deficits in the FS-induced behavioral immobility response (De Kloet et al., Citation1988).
Removing the adrenals without replacing endogenous GCs causes extensive neuronal death in the dentate gyrus (DG) of the rat hippocampus whilst simultaneously triggering an increase in progenitor cell proliferation (Cameron & Gould, Citation1994; Sloviter et al., Citation1989). Over time (3–4 months) this disruption leads to degradation of the granular cell layer (Sloviter, Dean, & Neubort, Citation1993; Sloviter, Sollas, Dean, & Neubort, Citation1993). This phenomenon relates to the fact that the hippocampus is one of only a few places in the adult brain which displays constant generation of new granule neurons in the dentate gyrus (Kempermann, Song, & Gage, Citation2015). The result of this widespread cellular destruction post-ADX on learning and memory process is less clear (Armstrong, McIntyre, Neubort, & Sloviter, Citation1993). Providing ADX animals with drinking water containing natural GCs (e.g. cort) protects the integrity and structure of the hippocampus attenuating some of the behavioral deficits induced by long-term ADX (Cameron & Gould, Citation1994; Conrad & Roy, Citation1995). Activation of MRs or GRs after ADX normalized the ADX-induced neuronal proliferation to levels observed in intact rats. Combined activation of both receptors, however, reduced proliferation levels in ADX animals below that of intact animals (Wong & Herbert, Citation2005). Interestingly, when specific agonists were administered to intact animals chronically over 8 days, opposite effects on apoptosis were observed; cort, at a concentration to preferentially activate the MR, reduced apoptosis, however, DEX, the selective GR agonist, dramatically enhanced apoptosis in the DG of these rats (Almeida et al., Citation2000). Chronic stress, resulting in prolonged activation of GRs in intact animals, also leads to enhanced cell death in the DG which can be attenuated by administration of GR antagonist RU486 (Heine, Maslam, Zareno, Joels, & Lucassen, Citation2004; Mayer et al., Citation2006). These findings illustrate an important role for endogenous glucocorticoids in adult neurogenesis and in the structural morphology of the hippocampus, although the interaction of the specific GC receptors in these pathways remains to be fully elucidated. Furthermore, the exact roles of MRs and GRs in the behavioral responses observed after ADX are also still unclear.
GC pharmacology of behavioral adaptation
The use of selective corticosteroid receptor antagonists, including the GR antagonist RU486 developed by the pharmaceutical company Roussel Uclaf (Baulieu, Citation1993) provided further insight into the individual roles of the hippocampal MRs and GRs in adaptive behavioral responses (Oitzl & de Kloet, Citation1992). It is a long-standing finding, that blocking GR, but not MR, function results in an impaired immobility response in rats tested 24 h after an initial FS exposure (Bilang-Bleuel et al., Citation2005; Chandramohan, Droste, Arthur, & Reul, Citation2008; Gutierrez-Mecinas et al., Citation2011; Jefferys & Funder, Citation1987; Veldhuis et al., Citation1985). Recently, we reported that the behavioral immobility response was retained for at least 4 weeks after a single FS event (Gutierrez-Mecinas et al., Citation2011), strongly indicating that the consolidation of this adaptive behavior involves long-term neuroplasticity processes. Our research, spanning approximately 15 years, has substantially contributed to the unraveling of the molecular action of GRs underpinning the behavioral immobility response in the FS test. A key finding was that the consolidation of the immobility response requires, in addition to the activation of GRs, also the N-methyl-D-aspartate receptor (NMDAR)-activated extracellular-signal-regulated kinase-mitogen-activated protein kinase (ERK-MAPK) pathway (Chandramohan et al., Citation2008; Gutierrez-Mecinas et al., Citation2011), a signaling cascade well-known for its prominent role in neuroplasticity processes underlying learning and memory (Roberson et al., Citation1999). Downstream, triggering of this pathway resulted in the activation of the nuclear kinases mitogen- and stress-activated protein kinase 1/2 (MSK1/2) and ETS domain-containing protein (Elk-1)/p300 leading to the prompt phosphorylation and acetylation of histone H3 molecules (H3S10p-K14ac) within the chromatin of dentate gyrus neurons (Chandramohan et al., Citation2008; Gutierrez-Mecinas et al., Citation2011). Collectively, the surge in signaling activity and epigenetic modifications induced the transcription and expression of the immediate-early genes (IEGs) FBJ murine osteosarcoma (c-Fos) and early growth response 1 (Egr-1) in these neurons (Chandramohan et al., Citation2008; Gutierrez-Mecinas et al., Citation2011). The two signaling pathways (GRs and NMDAR-ERK MAPK) were shown to be linked as it was demonstrated that activated GR’s are required for full nuclear kinase activity, H3S10p-K14ac formation, and IEG induction (Gutierrez-Mecinas et al., Citation2011). Moreover, co-immunopreciptation studies revealed a physical protein–protein interaction between GR, ERK1/2, and MSK1/2 following stress indicating a unique non-genomic function of the GR in facilitating the adaptive behavioral response to stress (Gutierrez-Mecinas et al., Citation2011). Recently, we found that the induction of dentate gyrus IEGs associated with the immobility response after FS critically involves DNA demethylation of the promoter and 5′-untranslated regions (5′-UTR) of these genes (Saunderson et al., Citation2016). Presently, it is unknown whether GRs are involved in this epigenetic response.
In the Morris water maze (MWM) paradigm, blocking GR function during the acquisition and consolidation phases of spatial learning causes significant deficits in behavioral performance (Oitzl & de Kloet, Citation1992; Oitzl, de Kloet, Joels, Schmid, & Cole, Citation1997). These deficits were not observed if GR blockade was restricted to the retrieval phase of testing. The effect of blocking the MR during spatial water maze learning resulted in a change in behavioral strategy leading to the suggestion that the GRs are playing a role in the consolidation of spatial information whereas MRs are involved in evaluation and response selection (Oitzl & de Kloet, Citation1992). MR, but not GR, expression in the hippocampus was later shown to correlate with anxiety state of individuals (Herrero, Sandi, & Venero, Citation2006). Individuals displaying low levels of anxiety had higher hippocampal MR expression when compared with highly anxious individuals who had relatively lower MR expression (Herrero et al., Citation2006). Furthermore, highly anxious individuals showed impaired acquisition and memory formation when subjected to MWM training (Herrer, et al., Citation2006)
A role for GCs in determining learning strategy was supported by later studies in which Kim et al. (Kim, Lee, Han, & Packard, Citation2001) investigated the effect of stress on learning in a MWM task. Acquisition of memory between stressed and non-stressed rats did not differ, however, rats which had been stressed by exposure to 60 tailshocks prior to acquisition displayed an altered search strategy in the 24 h probe test with a higher percentage of rats adopting a stimulus (dorsal-striatal) based learning strategy as opposed to a spatial (hippocampus-) based learning strategy. In the unstressed group, all rats used a spatial learning strategy in the 24 h probe test (Kim et al., Citation2001). These findings were also observed in an analogous study on human subjects using a similar approach (Schwabe et al., Citation2007). Subsequent studies have investigated the role of MRs and GRs in mediating the stress-induced switch in learning strategy and found that blocking the MR, in both rodents and humans, prevented the stress-induced preference for adopting a stimulus-response strategy (Schwabe, Schachinger, de Kloet, & Oitzl, Citation2010; Vogel, Fernandez, Joels, & Schwabe, Citation2016; Vogel, Klumpers, Kroes, et al., Citation2015; Vogel, Klumpers, Krugers, et al., Citation2015). Interestingly, blocking MRs in a non-stressful manner i.e. by administration of the MR antagonist RU28318 in rats, resulted in a significantly impaired spatial performance in the circular hole board learning paradigm and also prevented mice switching to a stimulus-response strategy when exposed to stress or following an injection of cort leading to impaired performance (Schwabe et al., Citation2010). The authors argued that by blocking MR under conditions in which GR is activated prevents the switch in strategy resulting in the behavioral deficits observed in RU28318-treated mice compared with controls (Schwabe et al., Citation2010). This would imply that the MR is solely responsible for the switch in strategy as other mechanisms failed to compensate when the MR was blocked by its antagonist.
Use of genetically modified rodent strains to discern GC receptor function
The development of genetically modified receptor expression in rodents provided another route in which to decipher GC receptor function, however, complete loss of either the MR or GR transcription from the early embryonic stages results in death shortly after birth due to dehydration and respiratory failure, respectively (Berger et al., Citation1998; Cole et al., Citation1995). In mice only expressing one functional GR allele (GR ± mice), GR expression in the hippocampus was significantly reduced compared with wild-type mice (Ridder et al., Citation2005). Despite showing normal plasma cort levels under basal conditions, these GR ± mice had an elevated and prolonged cort response to immobilization stress, potentially indicative of impaired feedback onto the HPA axis. GR ± mice also showed a significant impairment in stress coping during a three-day learned helplessness challenge when compared with wild-type mice. Interestingly, GR ± mice did not show any deficits in acute challenges designed to measure locomotion and exploration (open field and novel environment), anxiety (elevated plus maze (EPM) and light/dark preference test), fear conditioning (context or cued), or acute adaptive responses (single FS test) (Ridder et al., Citation2005). In another study, transgenic mice expressing antisense RNA complementary to that of the GR mRNA to reduce GR expression exhibited a similarly elevated HPA axis response to stress, dexamethasone non-suppression as well as cognitive impairments (Montkowski et al., Citation1995; Stec, Barden, Reul, & Holsboer, Citation1994). These mice spent more time in the open arms of the elevated plus maze than wild-type animals (Montkowski et al., Citation1995), which is typically interpreted as displaying reduced anxiety-like behavior and was not observed in GR ± mice. This interpretation, however, needs caution as the parallel impaired performance in the MWM is indicative of major cognitive impairments, therefore an inability to properly assess the situation when exposed to the elevated plus maze may also account for this “less anxious” observation (Montkowski et al., Citation1995). Furthermore, transgenic mice lacking GR throughout the entire nervous system (Grl1LoxP mice) expressed a severe phenotype indicative of Cushing’s syndrome (reduced size, abnormal fat distribution, and lower bone density than controls) (Tronche et al., Citation1999). These mice had significantly increased plasma levels of cort under both basal conditions and after 40 min restraint stress compared with wild-type mice. The behavioral immobility response in the 24 h-latency FS test was reduced in Grl1LoxP mice indicative of impaired cognition when compared with wild-type controls, and anxiety-like behavior in the elevated zero maze and light/dark box was reduced (Tronche et al., Citation1999).
Later studies utilized regional disruption of GR in the brain in attempts to determine function. Mice were generated with a forebrain-specific reduction in GR expression (FBGRKO), which became active at ∼3 weeks of age and lead to 90–100% loss in immunoreactivity throughout the hippocampus, basolateral amygdala but not the central amygdala (CeA) and most of the cortex at 4–6 months (Boyle et al., Citation2005). Diurnal HPA axis regulation was maintained in FKGRKO mice, however, these mice showed significantly higher levels of cort than wild-type animals at various stages of the circadian cycle (Boyle et al., Citation2005; Boyle, Kolber, Vogt, Wozniak, & Muglia, Citation2006; Furay, Bruestle, & Herman, Citation2008). These observations were made despite there being no difference in the expression of GR in the PVN and anterior pituitary, the major sites of negative feedback to the HPA axis, between FBGRKO and wild-type mice indicating a role for forebrain-specific GRs in this negative regulation of HPA function (Boyle et al., Citation2005). Interestingly, although no compensatory increase in MR mRNA/protein expression was observed in unmanipulated FBGRKO mice compared with wild-type controls (Boyle et al., Citation2005, 2006), hippocampal MR mRNA expression was increased by handling and mild stress compared with controls (Boyle et al., Citation2005). Restraint stress (30 min), however, caused a significant decrease in MR mRNA expression in the cornu ammonis 1 (CA1) and DG regions compared with controls (Boyle et al., Citation2006).
In terms of behavior, FBGRKO mice showed reduced activity in the initial FS test and in the tail suspension test, and sucrose preference (increased anhedonia) (Boyle et al., Citation2005). Further studies into the effect of the FBGRKO on anxiety-like behaviors revealed an increased locomotor/impulsivity observed in the EPM and light/dark preference test (Boyle et al., Citation2006), but these observations were not replicated in a later study in which no difference in the performance of FBGRKO and wild-type mice in the EPM was observed (Furay et al., Citation2008). Inclusion of females FBGRKO mice into the analysis revealed surprising gender differences in resultant behavior despite female subjects showing the same pattern of brain GR disruption as male FBGRKO mice (Solomon et al., Citation2012). In contrast to male FBGRKO mice, baseline cort secretion in female FBGRKO mice did not differ from that in wild-type mice, equally, there were no differences in stress-induced cort secretion between FBGRKO and control females following 30 min restraint stress (Solomon et al., Citation2012). The increase in behavioral immobility observed in the FS test and the reduction in the sucrose preference test, which were observed in male FBGRKO mice, was not apparent in female FBGRKO mice leading to the conclusion that differences in the central feedback response on the HPA axis between male and female mice could explain the lack of behavioral phenotype in female FBGRKO mice (Solomon et al., Citation2012).
Meanwhile, generation of another GR mutant mouse with GR deleted in all POMC expressing cells (GRPOMCCre) resulted in a lack of GR immunoreactivity in the pituitary at 3 months whilst maintaining GR expression in all other regions relevant for HPA axis regulation including hippocampus and PVN (Schmidt et al., Citation2009). Comparing the responses of GRPOMCCre and wild-type mice exposed to chronic social defeat stress (CSDS) revealed a resilience in GRPOMCCre mice since the CSDS-induced increases in anxiety-like behavior and emotionality and CDSD-induced decreases in exploratory behavior were not observed in GRPOMCCre mice (Wagner et al., Citation2011).
Attempts to address the function of GRs in the brain from an alternative angle resulted in the creation of a mouse strain overexpressing GR by ∼78% (GRov), primarily in the forebrain (prefrontal cortex, nucleus accumbens, bed nucleus of stria terminalis, CeA, PVN and hippocampus) without affecting expression in the midbrain or pituitary (Wei et al., Citation2004). GRov mice displayed normal HPA axis activity, both under basal conditions and following mild stress (EPM, 5 min). Locomotor activity did not differ significantly between GRov and control mice; however, GRov mice spent significantly less time and made less entries into open arms of the EPM, thus exhibiting an enhanced anxiety response which was also observed during the light–dark preference test (Wei et al., Citation2004). Overexpression of forbrain GR increased levels of immobility observed in the FS compared to controls (Wei et al., Citation2004); the opposite response to that observed when GR was deleted from this region (Boyle et al., Citation2005). A later study from the same group investigated the consequences of this mutation when induced at different stages of development (Wei et al., Citation2012). Even if GR was only overexpressed in early life it was capable of causing lifelong disruption of stress vulnerability, in part by transcriptome-wide changes in specific brain regions linked to the stress response. Conversely, delaying overexpression of GR in this region until mice reached adulthood did not result in the previously observed increases in anxiety-like behavior compared with control supporting the presence of critical periods for developing stress vulnerabilities (Wei et al., Citation2012).
Together these studies support a functional role for the GR in HPA function [reviewed in Laryea, Muglia, Arnett, & Muglia (Citation2015)], cognition and anxiety modulation. That said, the different genetic approaches used to create these transgenic strains, behavioral methodology, gender, and timing of these manipulations appear to be confounding factors and make widespread interpretation of these studies difficult.
Mice lacking MR (MRCaMKCre) in the forebrain were indistinguishable from wild-type mice in terms of life expectancy, sensory, and motor function but did display behavioral deficits, however, the nature of these deficits was hard to characterize (Berger et al., Citation2006). A gross survey of the hippocampus in MRCaMKCre mice showed abnormal projections between the DG and CA regions, and GR expression was increased compared with wild-type mice throughout the CA regions (Berger et al., Citation2006). Interestingly, when MR was overexpressed in the forebrain of mice (MRov) the opposite effect, a significant reduction in GR expression compared to wild-type mice, was observed (Rozeboom, Akil, & Seasholtz, Citation2007). MRov mice also displayed reduced anxiety/increased risk-taking behaviors in classic tests for anxiety (open field test and EPM) (Rozeboom et al., Citation2007). No differential effect was observed on basal plasma cort levels, a readout of basal HPA activity, when these mice strains were compared with wild-types, irrespective of whether MR was over- or under-expressed (Berger et al., Citation2006; Rozeboom et al., Citation2007). MR may therefore be acting to regulate GR expression and prevent the development of an anxious state.
Genomic action of GC receptors under baseline conditions and after stress
GC receptor trafficking
Brain GC receptors have been extensively characterized already back in the 1980s and 1990s in terms of their ligand binding properties, occupancy profile, topography, and molecular structure. Therefore, it is surprising that it is still largely unknown how they interact with the genome in vivo, as well as what the consequences for gene expression, physiology, and behavior are. Using sophisticated fluorescent microscopy techniques, the intracellular dynamics of GRs, particularly their trafficking between the cytoplasm and the nucleus, could be assessed in vitro in real time (Paakinaho et al., Citation2017). This work revealed an intra-nuclear dwell time of seconds for a single GR molecule, highlighting the highly dynamic nature of transcription factor trafficking (Paakinaho et al., Citation2017). Dwell time was significantly increased in the presence of DEX and affected by the presence of certain cofactors (Paakinaho et al., Citation2017). Studies of GR trafficking in a GrH2 rat hepatoma cell line have shown that once released from the chromatin unligated GR is sequestered into a subnuclear compartment (Yang, Liu, & DeFranco, Citation1997). From this “nuclear export staging area” GR can either be reactivated upon rebinding its ligand, hence bypassing travel back through the cytoplasm, or it can be exported out of the nucleus in an adenosine triphosphate-independent manner, most likely through a phosphotyrosine-associated mechanism (Yang et al., Citation1997). Further work and substantial advances in technology are required to determine if these molecules follow similar dynamics in vivo. Notably, the presence of GRs or any other transcription factor in the nucleus does not necessarily mean that the factor is genomically and/or transcriptionally active.
Genomic action
Once it had been clarified that there are two types of GC receptors, i.e. MRs and GRs, the quest was on to understand their function; a quest which is still ongoing. A key role for these receptors in genomic regulation became apparent with numerous studies showing roles for the GR in chromatin remodeling and transcriptional regulation by direct binding of GC receptors to GREs (Becker, Gloss, Schmid, Strahle, & Schutz, Citation1986; Burd et al., Citation2012; Carlstedt-Duke et al., Citation1988; Chakravarti et al., Citation1996; Evans, Birnberg, & Rosenfeld, Citation1982; Fryer & Archer, Citation1998; Rupprecht, Arriza, et al., Citation1993; Schauer, Chalepakis, Willmann, & Beato, Citation1989; Scheidereit, Geisse, Westphal, & Beato, Citation1983; Umesono & Evans, Citation1989). Mainly using ligand-binding assays and luciferase reporter assays in vitro, the transactivation potential of ligands as well as structure–activity relationships could be studied (Rupprecht, Arriza, et al., Citation1993; Rupprecht, Reul, et al., Citation1993). These studies provide insight into the pharmacological profile of both GRs and MRs and the structural requirements of the receptors for ligand binding, DNA binding, and transactivation. Based on this body of work, two underlying assumptions are often made; firstly, that the relationship between increasing GC levels and the molecular action of GC receptors is linear, and secondly that the occupancy of GC receptors by ligand is correlated with the genomic action of GCs. Yet, although these studies in vitro have been highly valuable in our understanding of the molecular structure of GC receptors, it should be noted that these assumptions are largely based on observations made in vitro (e.g. by transfected receptor genes and luciferase reporter assays). Presently, it is still unclear to which extent the observations made in cell cultures in vitro can be transposed to the action of GCs via brain GC receptors in vivo. We are only beginning to understand how GRs and MRs interact with the genome in brain tissue. Moreover, until recently, genomic interactions of MRs and GRs had only been studied in pharmacological models (e.g. ADX and GC administration; (Datson et al., Citation2011; Polman, de Kloet, & Datson, Citation2013)), but not under physiological conditions (e.g. stress). The investigation of interactions between GC receptors and target DNA has been made possible due to advances in molecular techniques, including the chromatin immunoprecipitation (ChIP) technique, which can be applied on cultured cells as well as tissues.
Recent advances
Applying ChIP on chromatin prepared from the hippocampus of intact rats, we showed for the first time that binding of both MRs and GRs to GREs located within or in close vicinity of GC target genes was very low under early morning baseline conditions but increased significantly after exposure to acute stress or at the peak of baseline GC secretion in the early evening (baseline PM) (Mifsud & Reul, Citation2016). Three well-studied GC-inducible genes were investigated as part of this study, namely FK506 binding protein 5 (Fkbp5), period circadian clock 1 (Per1) and serum and glucocorticoid regulated kinase 1 (Sgk1), which are involved in GR regulation, circadian activity, and neuronal plasticity, respectively (Akiyama et al., Citation1999; Anacker et al., Citation2013; Conway-Campbell et al., Citation2010; Denny, Valentine, Reynolds, Smith, & Scammell, Citation2000; Polman et al., Citation2012; Webster, Goya, Ge, Maiyar, & Firestone, Citation1993). These genes carry GRE sites either within intronic sequences (Fkbp5) or within their promoter region. Whilst the strong increase in GR to GRE binding after stress (Mifsud & Reul, Citation2016) was expected based on earlier receptor binding studies (Reul & de Kloet, Citation1985; Reul et al., Citation1987), the relatively low MR binding to GREs under baseline AM conditions was surprising given the high levels of MR occupancy by ligand shown previously (Mifsud & Reul, Citation2016; Reul & de Kloet, Citation1985; Reul et al., Citation1987). Previous work had shown that MRs are highly occupied by the extremely low GC levels that circulate in the early morning (De Kloet & Reul, Citation1987; Mifsud & Reul, Citation2016; Reul & de Kloet, Citation1985), and that under such conditions these receptors are located in the nucleus of hippocampal neurons (Gesing et al., Citation2001; Reul et al., Citation2000). Together, these early observations indicate that although MRs are bound by ligand and located in the nucleus in the early morning, it appears that access and binding of the receptor to GRE sites is tightly controlled as only relatively low levels of binding were measured under baseline conditions (Mifsud & Reul, Citation2016). At present, we can only speculate about the molecular mechanisms enforcing the low binding of MRs to GREs under baseline AM conditions, as outlined in . Possibly, access to GREs is controlled by local epigenetic modifications of the chromatin which are subject to change after stress or under baseline PM conditions permitting access of MRs (and GRs) to such GREs. This is indeed the case in human lymphoblastoid cells where it has been shown that access of GRs to Fkbp5 GRE is controlled by the local DNA methylation status (Klengel et al., Citation2013). Alternatively, steroid receptor co-regulators can restrict or facilitate access of GC receptors to their recognition sites, therefore, context-dependent selectivity may be conferred by co-regulators, forming another layer of regulatory control. Nuclear receptor coactivator 1 (NCoA1) is a key steroid receptor coactivator known to modulate the transcriptional response after MR and GR activation (Grenier et al., Citation2004; Meijer et al., Citation2005; Meijer, van der Laan, Lachize, Steenbergen, & de Kloet, Citation2006). Other coregulators known to interact with GC receptors are extensively reviewed elsewhere (Fuller, Yang, & Young, Citation2017; Mahfouz et al., Citation2016). An additional mechanism explaining the enhanced MR binding to GREs after stress may be heterodimerization with GRs (see below). One may argue that these conclusions and concepts are based on just three GC target genes, but our ongoing MR (and GR) ChIP studies combined with next-generation sequencing (Seq) technology show that this binding profile (baseline AM versus stress/baseline PM) appears to exist in hundreds of genes across the genome. Nevertheless, at present, the existence of genes that are under constitutive control by MRs cannot be excluded, i.e. such genes would be under constant regulation by MRs interacting directly or indirectly with the DNA template and/or other chromatin constituents, and presumably not through classic GREs. Indeed, our preliminary MR ChIP-Seq indicates that some genes present substantial binding of MRs irrespective of the physiological state (baseline AM/PM, stress) of the animal (Mifsud, Kennedy, Salatino, Engledow, Lockstone & Reul, unpublished).
Figure 1. MR and GR interaction with the genome. This diagram presents an overview of the different modes of interaction of MRs and GRs with certain genes, including Fkbp5, Sgk1, and Per1, in the hippocampus genome under baseline AM (panel A) and stress conditions (B). (A) Under baseline AM conditions (low-cort), MRs are highly occupied and reside in the nucleus but are not able to bind or bind only weakly. This low accessibility may be due to co-regulators and/or local epigenetic modifications (e.g. DNA methylation, repressive histone modifications) and/or inherent low affinity of the receptor for GREs. The consequence is that such genes present only very low transcriptional activity under baseline AM condition. (B) Under stress/baseline PM conditions (high-cort), accessibility of GREs is enhanced (potentially resulting from expunge of inhibitory co-regulators and/or altered epigenetic status (DNA demethylation, pro-access histone modifications)). Activated GRs form homodimers or heterodimers with MRs and bind to GREs resulting in regulation (activation or in some cases inhibition) of gene transcription. Assisted loading mechanisms may provide MR homodimers access to GREs as well. Other genes are likely to have other mechanisms of regulation by these glucocorticoid receptors but these are not covered in this diagram.
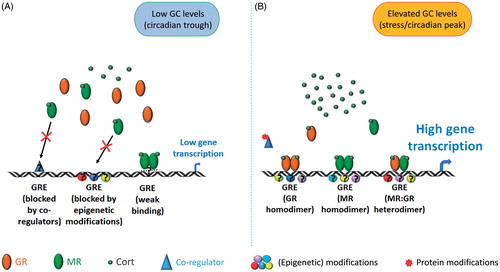
There is further evidence for the intricate way access of GC receptors to the genome is regulated. For instance, the rat Fkbp5 gene contains more than one GRE site, e.g. within intron 5 there are two GREs. One GRE site, as mentioned above, readily binds MRs and GRs after stress or under baseline PM conditions; the other one, located just approximately 4 kb upstream of the former one, however, does not show enhanced binding under these conditions (Mifsud & Reul, Citation2016). Our observations made in vivo correspond with previous Fkbp5 GRE-specific findings shown in vitro (Hubler & Scammell, Citation2004). Furthermore, the level of MR or GR binding (enrichment) after stress was found to be highly gene dependent (Mifsud & Reul, Citation2016). We found that, under conditions of elevated circulating GCs (stress, baseline PM), the levels of MR and GR binding to (active) GREs within Fkbp5 and Per1 was consistently higher than those at the GRE within Sgk1. This distinction may relate to the number of cells in the hippocampus in which particular GREs within genes may be accessible by MRs and GRs and, as mentioned, this regulation may be controlled by the local chromatin’s epigenetic status, steroid co-regulator actions, and possibly the likelihood of MR/GR heterodimer formation (; also see below). In addition, it is currently unknown if such control is defined by the GRE’s nucleotide sequence and/or local modulators of the chromatin structure mediating access and stability of receptor-chromatin binding. These findings indicate that multiple processes other than ligand-receptor binding exist, likely through distinct mechanisms, controlling both MR and GR access to GREs within the DNA. Clearly, in GC target genes, these mechanisms are facilitated by exposure to stress/circadian factors/elevated GC levels.
So far, the stressor mentioned in this section concerned FS. In our recent study (Mifsud & Reul, Citation2016), we compared the effects of different stressors, i.e. novelty exposure, restraint, which, as previously shown, evoke significantly different increases in plasma cort levels (Qian et al., Citation2011). To our surprise, after exposure to these acute stressors, the observed MR binding levels, as well as the GR binding levels to GREs associated with Fkbp5, Per1, and Sgk1, were largely similar (Mifsud & Reul, Citation2016). Thus, although FS-evoked plasma cort levels were approximately 5-fold higher than those after novelty exposure, binding of MRs and GRs to GREs was comparable. Stress-induced differences in the level of MR/GR binding between different genes was maintained regardless of the type of stress applied. These findings may indicate that above a certain threshold of cort these GC receptor-specific genomic responses in the hippocampus are independent of GC levels (Mifsud & Reul, Citation2016). The discrepancy between post-stress plasma cort levels and MR/GR binding could also be due to the different nature of the stressors (evoking differential MR/GR accessibility through distinct epigenetic mechanisms) and/or stressor-specific influences on circulating CBG levels and their effect on the time course of the free cort response after stress (Qian et al., Citation2011). Regardless, our data demonstrate that caution should be taken when drawing conclusions on genomic effects of GC receptors based on circulating cort values.
GC receptor dimerization and its implications for GC receptor-mediated gene transcription
GC receptor dimerization
As mentioned previously, one possible explanation for the increased MR binding after exposure to stress is that MRs may require dimerization with GRs to facilitate binding to GREs in GC target genes. Traditionally, GR has been thought to be the primary mediator of the genomic effects of elevated cort levels after stress as it only becomes substantially occupied after such GC levels due to its lower binding affinity for cort compared with MR (Reul et al., Citation1987, Citation2015; Trollope, Mifsud, Saunderson, & Reul, Citation2017; Zalachoras, Houtman, & Meijer, Citation2013). Evidence does exist, however, showing that MRs and GRs can form heterodimers, in addition to the respective homodimers, in cell-free systems and in cells in vitro (Liu, Wang, Sauter, & Pearce, Citation1995; Savory et al., Citation2001; Trapp, Rupprecht, Castren, Reul, & Holsboer, Citation1994). Trapp et al. (Citation1994) were able to show using hormone binding assays, electrophoretic shift assays and luciferase reporter assays in vitro that MR:GR heterodimers were capable of forming and exert a higher affinity to recognition sites as well as elicit stronger gene transcriptional effects on the genome than the respective homodimers. Albeit a major contribution to the molecular GC receptor field, this work, conducted on cell cultures in vitro, did not involve ChIP analysis, thus heterodimerization could only be inferred indirectly based mainly on (co-)transfection of MRs and/or GRs and analysis of DNA binding and transcriptional outcomes (Trapp et al., Citation1994). Our recent work applied novel sequential and tandem ChIP technologies to rat hippocampal tissues to provide strong evidence that MR:GR heterodimers are binding to target genes in vivo (Mifsud & Reul, Citation2016). Furthermore, this work showed that exposure to acute stress enhanced MR:GR heterodimer formation at GREs in a gene-specific manner, providing strong evidence that such heterodimers are partly responsible for the stress-induced transcription of Fkbp5 and Per1, but most likely not Sgk1 (Mifsud & Reul, Citation2016). This technical advancement in the ChIP technology will allow the study of the mechanisms determining the formation of MR-GR heterodimers at certain GREs and genes as well as the significance of such heterodimers for transcriptional regulation.
It is widely accepted that GRdim mice lack the ability to form conventional GR homodimers due to their mutated DBD but this region may not be required for MR:GR heterodimer formation in vitro (Savory et al., Citation2001), which may explain the lack of effect of this mutation on GC-induced Fkbp5 in GRdim mice (Frijters et al., Citation2010). Interestingly, studies have shown that, in GRdim mice in vivo, Fkbp5 gene expression retains the ability to be upregulated (>50%) by prednisolone, a synthetic GC that binds to both GRs and MRs (Frijters et al., Citation2010). This would support our finding that MR:GR heterodimers are binding to the Fkbp5 gene under high GC conditions, i.e. after FS stress (Mifsud & Reul, Citation2016).
Work performed in vitro has shown that GR is capable of assisting binding of other transcription factors by a mechanism referred to as “assisted loading”. Assisted loading occurs when the binding of one receptor, in this case GR, effectively recruits cofactors involved with the remodeling of chromatin, which remain in place after the first receptor is released, usually within seconds of binding (Voss et al., Citation2011). The remodeled chromatin makes it easier for binding of a second receptor, in this case MR, which on its own would not be as capable of recruiting the cofactors required for successful binding to the GRE. This model has been used to explain non-competitive binding of GR and estrogen receptor (ER), as well as other transcription factors including AP1 and pBox, to the same GRE in cell lines in vitro (Voss et al., Citation2011) but no direct evidence exists that this can occur with GRs and MRs in vivo.
Genome-wide sequencing
The dawn of next-generation sequencing (NGS) technology to assess DNA sequences across the entire genome is revealing the true extent of MR and GR binding to DNA under various conditions in the rat hippocampus (Polman et al., Citation2013; van Weert et al., Citation2017). In early GR ChIP-sequencing (Seq) studies in ADX rats, the possible existence of two distinct populations of GR binding sites in the hippocampus was proposed, those that were bound by GRs across a range of cort injections (3–3000 µg/kg) and a second set that only bound GRs if the highest concentration of cort was administered (3000 µg/kg) (Polman et al., Citation2013). More than 99.9% of significant GR binding peaks contained a GRE and motif analysis revealed that these GREs commonly (58%) occurred together with zinc finger and BTB domain containing 3 (Zbtb3) elements. Of the 14 non-GREs containing significant GR binding peaks, all contained a motif targeted by CG11181 gene product from transcript CG11181-RB (CUP) which has been reported to be involved in the regulation of oocyte mRNA transcripts (Broyer, Monfort, & Wilhelm, Citation2017; Polman et al., Citation2013); its role in the hippocampus is unknown. Comparing GR ChIP-Seq data from this study with MR ChIP-Seq data generated from the same samples in parallel revealed 918 MR-exclusive sites, 475 MR-GR overlapping sites, and 1450 GR exclusive sites (van Weert et al., Citation2017). All sites contained GRE-like motifs, however, an Atonal BHLH Transcription Factor 1 (Atoh1) binding sequence was uniquely present in close proximity to those GREs bound only by MRs (van Weert et al., Citation2017). Atoh1 protein is not expressed in the adult mouse hippocampus but subsequent analysis both in vitro and in vivo implicated the basic helix–loop–helix (bHLH) family member NeuroD as the likely transcription factor interacting with this site, potentially responsible for driving specificity for MR binding over GR at these MR exclusive sites (van Weert et al., Citation2017). ChIP-seq analysis is currently lacking regarding the interaction of MRs and GRs with the genome under physiological conditions like stress and circadian variation. Moreover, such data are also entirely lacking for brain structures other than the hippocampus as well as relevant endocrine tissues like the pituitary and adrenal gland. On a positive note, various research groups are presently working hard to fill these gaps in knowledge thus a wealth of whole genome data can be expected to emerge over the coming years.
Concluding remarks
This review shows how far the field has advanced, in terms of both knowledge and development of more refined techniques. Clearly, GC action upon the central nervous system is very tightly controlled by multiple physiological, cellular, and molecular mechanisms. Synthesis, availability, and access of ligand and receptors and access to target DNA is regulated through a complex network of intertwined signaling and epigenetic pathways and other nuclear factors (e.g. co-regulators). This network is designed to ensure that the effects of such a powerful, ubiquitous ligand are constrained in terms of magnitude and time, and specific in molecular and cellular terms.
Indeed, the advent of new technologies such as novel ChIP protocols (Mifsud & Reul, Citation2016) has challenged some existing assumptions and indicated the existence of additional levels of regulatory control. Although it is still early days, NGS studies (Polman et al., Citation2013; van Weert et al., Citation2017) have produced an overwhelming amount of data, much of which is still awaiting the development of the computational pipelines and mathematical models required to reveal its full potential. To date, NGS studies have been performed on pharmacological models (ADX and GC administration) to study the effects of graded doses of GCs on MR and GR binding to the genome. We are keenly anticipating the revelation of MR and GR interaction with the genome in the context of physiological rises in GC levels due to stress or circadian drive. It will be interesting to see whether these data correspond with those obtained in pharmacological models or indicate that additional stress/circadian-related regulatory factors are in force.
These are exciting times for GC receptor research, mainly as a result of the developments in ChIP and NGS technologies. While the effects of stress on the brain may be becoming clearer, the functional implications of such effects in terms of physiology and behavior still remains, in part, a mystery. A key challenge is an individuality in physiological and behavioral responses which needs to be recognized in our experimental designs and analyzes (Ardi, Albrecht, Richter-Levin, Saha, & Richter-Levin, Citation2016). Such approaches in combination with molecular analyses (MR, GR ChIP, and NGS) may provide key insights into the genomic basis of resilience and stress coping.
Acknowledgements
We are grateful to the 1st Munich winter conference on Stress for providing the opportunity to discuss our new work and concepts.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akiyama, M., Kouzu, Y., Takahashi, S., Wakamatsu, H., Moriya, T., Maetani, M., … Shibata, S. (1999). Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. Journal of Neuroscience, 19, 1115–1121. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9920673
- Almeida, O.F., Conde, G.L., Crochemore, C., Demeneix, B.A., Fischer, D., Hassan, A.H., … Michaelidis, T.M. (2000). Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB Journal, 14, 779–790. doi:10.1096/fasebj.14.5.779
- Anacker, C., Cattaneo, A., Musaelyan, K., Zunszain, P.A., Horowitz, M., Molteni, R., … Pariante, C.M. (2013). Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 110, 8708–8713. doi:10.1073/pnas.1300886110
- Ardi, Z., Albrecht, A., Richter-Levin, A., Saha, R., & Richter-Levin, G. (2016). Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiology of Disease, 88, 139–147. doi:10.1016/j.nbd.2016.01.012
- Armstrong, J.N., McIntyre, D.C., Neubort, S., & Sloviter, R.S. (1993). Learning and memory after adrenalectomy-induced hippocampal dentate granule cell degeneration in the rat. Hippocampus, 3, 359–371. doi:10.1002/hipo.450030310
- Arriza, J.L., Simerly, R.B., Swanson, L.W., & Evans, R.M. (1988). The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron, 1, 887–900. doi:10.1016/0896-6273(88)90136-5
- Baulieu, E.E. (1993). RU 486 – A decade on today and tomorrow. In M.S. Donaldson, L. Dorflinger, S.S. Brown & L.Z. Benet (Eds.), Clinical applications of mifepristone (RU 486) and other antiprogestins: Assessing the science and recommending a research agenda (pp. 71–119). Washington (DC): The National Academies Press.
- Becker, P.B., Gloss, B., Schmid, W., Strahle, U., & Schutz, G. (1986). In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature, 324, 686–688. doi:10.1038/324686a0
- Berger, S., Bleich, M., Schmid, W., Cole, T.J., Peters, J., Watanabe, H., … Schutz, G. (1998). Mineralocorticoid receptor knockout mice: pathophysiology of Na + metabolism. Proceedings of the National Academy of Sciences of the United States of America, 95, 9424–9429. doi:10.1073/pnas.95.16.9424
- Berger, S., Wolfer, D.P., Selbach, O., Alter, H., Erdmann, G., Reichardt, H.M., … Schutz, G. (2006). Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proceedings of the National Academy of Sciences of the United States of America, 103, 195–200. doi:10.1073/pnas.0503878102
- Beylin, A.V., & Shors, T.J. (2003). Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Hormones and Behavior, 43, 124–131. doi:10.1016/S0018-506X(02)00025-9
- Bilang-Bleuel, A., Ulbricht, S., Chandramohan, Y., De Carli, S., Droste, S.K., & Reul, J.M.H.M. (2005). Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: Involvement in a glucocorticoid receptor-dependent behavioural response. European Journal of Neuroscience, 22, 1691–1700. doi:10.1111/j.1460-9568.2005.04358.x
- Borrell, J., De Kloet, E.R., Versteeg, D.H., & Bohus, B. (1983). Inhibitory avoidance deficit following short-term adrenalectomy in the rat: The role of adrenal catecholamines. Behavioral and Neural Biology, 39, 241–258. doi:10.1016/S0163-1047(83)90910-X
- Boyle, M.P., Brewer, J.A., Funatsu, M., Wozniak, D.F., Tsien, J.Z., Izumi, Y., & Muglia, L.J. (2005). Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proceedings of the National Academy of Sciences of the United States of America, 102, 473–478. doi:10.1073/pnas.0406458102
- Boyle, M.P., Kolber, B.J., Vogt, S.K., Wozniak, D.F., & Muglia, L.J. (2006). Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. Journal of Neuroscience, 26, 1971–1978. doi:10.1523/JNEUROSCI.2173-05.2006
- Broyer, R.M., Monfort, E., & Wilhelm, J.E. (2017). Cup regulates oskar mRNA stability during oogenesis. Developmental Biology, 421, 77–85. doi:10.1016/j.ydbio.2016.06.040
- Burd, C.J., Ward, J.M., Crusselle-Davis, V.J., Kissling, G.E., Phadke, D., Shah, R.R., & Archer, T.K. (2012). Analysis of chromatin dynamics during glucocorticoid receptor activation. Molecular and Cellular Biology, 32, 1805–1817. doi:10.1128/MCB.06206-11
- Cameron, H.A., & Gould, E. (1994). Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience, 61, 203–209. doi:10.1016/0306-4522(94)90224-0
- Carlstedt-Duke, J., Stromstedt, P.E., Persson, B., Cederlund, E., Gustafsson, J.A., & Jornvall, H. (1988). Identification of hormone-interacting amino acid residues within the steroid-binding domain of the glucocorticoid receptor in relation to other steroid hormone receptors. The Journal of Biological Chemistry, 263, 6842–6846. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3360809
- Chakravarti, D., LaMorte, V.J., Nelson, M.C., Nakajima, T., Schulman, I.G., Juguilon, H., … Evans, R.M. (1996). Role of CBP/P300 in nuclear receptor signalling. Nature, 383, 99–103. doi:10.1038/383099a0
- Chandramohan, Y., Droste, S.K., Arthur, J.S., & Reul, J.M.H.M. (2008). The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. European Journal of Neuroscience, 27, 2701–2713. doi:10.1111/j.1460-9568.2008.06230.x
- Chapman, K., Holmes, M., & Seckl, J. (2013). 11beta-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiological Reviews, 93, 1139–1206. doi:10.1152/physrev.00020.2012
- Cole, T.J., Blendy, J.A., Monaghan, A.P., Krieglstein, K., Schmid, W., Aguzzi, A., … Schutz, G. (1995). Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Development, 9, 1608–1621. doi:10.1101/gad.9.13.1608
- Conrad, C.D., & Roy, E.J. (1995). Dentate gyrus destruction and spatial learning impairment after corticosteroid removal in young and middle-aged rats. Hippocampus, 5, 1–15. doi:10.1002/hipo.450050103
- Conway-Campbell, B.L., Sarabdjitsingh, R.A., McKenna, M.A., Pooley, J.R., Kershaw, Y.M., Meijer, O.C., … Lightman, S.L. (2010). Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. Journal of Neuroendocrinology, 22, 1093–1100. doi:10.1111/j.1365-2826.2010.02051.x
- Datson, N.A., Polman, J.A., de Jonge, R.T., van Boheemen, P.T., van Maanen, E.M., Welten, J., … Meijer, O.C. (2011). Specific regulatory motifs predict glucocorticoid responsiveness of hippocampal gene expression. Endocrinology, 152, 3749–3757. doi:10.1210/en.2011-0287
- De Kloet, E.R., De Kock, S., Schild, V., & Veldhuis, H.D. (1988). Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology, 47, 109–115. doi:10.1159/000124900
- de Kloet, E.R., & Molendijk, M.L. (2016). Coping with the forced swim stressor: Towards understanding an adaptive mechanism. Neural Plasticity, 2016, 6503162. doi:10.1155/2016/6503162
- De Kloet, E.R., & Reul, J.M.H.M. (1987). Feedback action and tonic influence of corticosteroids on brain function: A concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology, 12, 83–105. doi:10.1016/0306-4530(87)90040-0
- Denny, W.B., Valentine, D.L., Reynolds, P.D., Smith, D.F., & Scammell, J.G. (2000). Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology, 141, 4107–4113. doi:10.1210/endo.141.11.7785
- Droste, S.K., de Groote, L., Atkinson, H.C., Lightman, S.L., Reul, J.M.H.M., & Linthorst, A.C.E. (2008). Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology, 149, 3244–3253. doi:10.1210/en.2008-0103
- Evans, R.M., Birnberg, N.C., & Rosenfeld, M.G. (1982). Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proceedings of the National Academy of Sciences of the United States of America, 79, 7659–7663. doi:10.1073/pnas.79.24.7659
- Frijters, R., Fleuren, W., Toonen, E.J., Tuckermann, J.P., Reichardt, H.M., van der Maaden, H., … Alkema, W. (2010). Prednisolone-induced differential gene expression in mouse liver carrying wild type or a dimerization-defective glucocorticoid receptor. BMC Genomics, 11, 359. doi:10.1186/1471-2164-11-359
- Fryer, C.J., & Archer, T.K. (1998). Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature, 393, 88–91. doi:10.1038/30032
- Fuller, P.J., Yang, J., & Young, M.J. (2017). 30 Years of the mineralocorticoid receptor: coregulators as mediators of mineralocorticoid receptor signalling diversity. Journal of Endocrinology, 234, T23–T34. doi:10.1530/JOE-17-0060
- Furay, A.R., Bruestle, A.E., & Herman, J.P. (2008). The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology, 149, 5482–5490. doi:10.1210/en.2008-0642
- Gesing, A., Bilang-Bleuel, A., Droste, S.K., Linthorst, A.C., Holsboer, F., & Reul, J.M. (2001). Psychological stress increases hippocampal mineralocorticoid receptor levels: Involvement of corticotropin-releasing hormone. Journal of Neuroscience, 21, 4822–4829. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11425909
- Gong, S., Miao, Y.L., Jiao, G.Z., Sun, M.J., Li, H., Lin, J., … Tan, J.H. (2015). Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One, 10, e0117503. doi:10.1371/journal.pone.0117503
- Grenier, J., Trousson, A., Chauchereau, A., Amazit, L., Lamirand, A., Leclerc, P., … Massaad, C. (2004). Selective recruitment of p160 coactivators on glucocorticoid-regulated promoters in Schwann cells. Molecular Endocrinology, 18, 2866–2879. doi:10.1210/me.2004-0241
- Gutierrez-Mecinas, M., Trollope, A.F., Collins, A., Morfett, H., Hesketh, S.A., Kersante, F., & Reul, J.M.H.M. (2011). Long-lasting behavioral responses to stress involve a direct interaction of glucocorticoid receptors with ERK1/2-MSK1-Elk-1 signaling. Proceedings of the National Academy of Sciences of the United States of America, 108, 13806–13811. doi:10.1073/pnas.1104383108
- Heine, V.M., Maslam, S., Zareno, J., Joels, M., & Lucassen, P.J. (2004). Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. European Journal of Neuroscience, 19, 131–144. doi:10.1046/j.1460-9568.2003.03100.x
- Herrero, A.I., Sandi, C., & Venero, C. (2006). Individual differences in anxiety trait are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors. Neurobiology of Learning and Memory, 86, 150–159. doi:10.1016/j.nlm.2006.02.001
- Hubler, T.R., & Scammell, J.G. (2004). Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress & Chaperones, 9, 243–252. doi:10.1379/CSC-32R.1
- Jasper, M.S., & Engeland, W.C. (1991). Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. American Journal of Physiology, 261(5 Pt 2), R1257–R1268. doi:10.1152/ajpregu.1991.261.5.R1257
- Jefferys, D., Copolov, D., Irby, D., & Funder, J. (1983). Behavioural effect of adrenalectomy: Reversal by glucocorticoids or [D-Ala2,Met5]enkephalinamide. European Journal of Pharmacology, 92, 99–103. doi:10.1016/0014-2999(83)90113-9
- Jefferys, D., & Funder, J.W. (1987). Glucocorticoids, adrenal medullary opioids, and the retention of a behavioral response after stress. Endocrinology, 121, 1006–1009. doi:10.1210/endo-121-3-1006
- Joels, M., & de Kloet, E.R. (2017). 30 years of the mineralocorticoid receptor: the brain mineralocorticoid receptor: a saga in three episodes. Journal of Endocrinology, 234, T49–T66. doi:10.1530/JOE-16-0660
- Karssen, A.M., Meijer, O.C., & De Kloet, E.R. (2005). Corticosteriods and the blood-brain barrier. In T. Steckler, N. H. Kalin & J. M. H. M. Reul (Eds.), Handbook of stress and the brian (First ed., Vol. 1, pp. 329–340). The Netherlands: Elsevier.
- Karssen, A.M., Meijer, O.C., van der Sandt, I.C., Lucassen, P.J., de Lange, E.C., de Boer, A.G., & de Kloet, E.R. (2001). Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology, 142, 2686–2694. doi:10.1210/endo.142.6.8213
- Karst, H., Berger, S., Turiault, M., Tronche, F., Schutz, G., & Joels, M. (2005). Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America,, 102, 19204–19207. doi:10.1073/pnas.0507572102
- Kempermann, G., Song, H., & Gage, F.H. (2015). Neurogenesis in the Adult Hippocampus. Cold Spring Harbor Perspectives in Biology, 7, a018812. doi:10.1101/cshperspect.a018812
- Kim, J.J., Lee, H.J., Han, J.S., & Packard, M.G. (2001). Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. Journal of Neuroscience, 21, 5222–5228. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11438597
- Klengel, T., Mehta, D., Anacker, C., Rex-Haffner, M., Pruessner, J.C., Pariante, C.M., … Binder, E.B. (2013). Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience, 16, 33–41. doi:10.1038/nn.3275
- Laryea, G., Muglia, L., Arnett, M., & Muglia, L.J. (2015). Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis by gene targeting in mice. Frontiers in Neuroendocrinology, 36, 150–164. doi:10.1016/j.yfrne.2014.09.002
- Linthorst, A.C., Flachskamm, C., Holsboer, F., & Reul, J.M. (1994). Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenocortical axis activity, and body temperature. Endocrinology, 135, 520–532. doi:10.1210/endo.135.2.7518383
- Linthorst, A.C., & Reul, J.M.H.M. (2008). Stress and the brain: solving the puzzle using microdialysis. Pharmacology Biochemistry and Behavior, 90, 163–173. doi:10.1016/j.pbb.2007.09.019
- Liu, W., Wang, J., Sauter, N.K., & Pearce, D. (1995). Steroid receptor heterodimerization demonstrated in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America,, 92, 12480–12484. doi:10.1073/pnas.92.26.12480
- Long, J.B., & Holaday, J.W. (1985). Blood-brain barrier: endogenous modulation by adrenal-cortical function. Science, 227, 1580–1583. doi:10.1126/science.3975627
- Luisi, B.F., Xu, W.X., Otwinowski, Z., Freedman, L.P., Yamamoto, K.R., & Sigler, P.B. (1991). Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature, 352, 497–505. doi:10.1038/352497a0
- Mahfouz, A., Lelieveldt, B.P., Grefhorst, A., van Weert, L.T., Mol, I.M., Sips, H.C., … Meijer, O.C. (2016). Genome-wide coexpression of steroid receptors in the mouse brain: Identifying signaling pathways and functionally coordinated regions. Proceedings of the National Academy of Sciences of the United States of America, 113, 2738–2743. doi:10.1073/pnas.1520376113
- Mayer, J.L., Klumpers, L., Maslam, S., de Kloet, E.R., Joels, M., & Lucassen, P.J. (2006). Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. Journal of Neuroendocrinology, 18, 629–631. doi:10.1111/j.1365-2826.2006.01455.x
- Meijer, O.C., Kalkhoven, E., van der Laan, S., Steenbergen, P.J., Houtman, S.H., Dijkmans, T.F., … de Kloet, E.R. (2005). Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology, 146, 1438–1448. doi:10.1210/en.2004-0411
- Meijer, O.C., van der Laan, S., Lachize, S., Steenbergen, P.J., & de Kloet, E.R. (2006). Steroid receptor coregulator diversity: What can it mean for the stressed brain? Neuroscience, 138, 891–899. doi:10.1016/j.neuroscience.2005.07.004
- Mifsud, K.R., & Reul, J.M.H.M. (2016). Acute stress enhances heterodimerization and binding of corticosteroid receptors at glucocorticoid target genes in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 113, 11336–11341. doi:10.1073/pnas.1605246113
- Montkowski, A., Barden, N., Wotjak, C., Stec, I., Ganster, J., Meaney, M., … Holsboer, F. (1995). Long-term antidepressant treatment reduces behavioural deficits in transgenic mice with impaired glucocorticoid receptor function. Journal of Neuroendocrinology, 7, 841–845. doi:10.1111/j.1365-2826.1995.tb00724.x
- Morsink, M.C., Joels, M., Sarabdjitsingh, R.A., Meijer, O.C., De Kloet, E. R., & Datson, N.A. (2006). The dynamic pattern of glucocorticoid receptor-mediated transcriptional responses in neuronal PC12 cells. Journal of Neurochemistry, 99, 1282–1298. doi:10.1111/j.1471-4159.2006.04187.x
- Morsink, M.C., Steenbergen, P.J., Vos, J.B., Karst, H., Joels, M., de Kloet, E. R., & Datson, N.A. (2006). Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. Journal of Neuroendocrinology, 18, 239–252. doi:10.1111/j.1365-2826.2006.01413.x
- Nixon, M., Andrew, R., & Chapman, K.E. (2013). It takes two to tango: Dimerisation of glucocorticoid receptor and its anti-inflammatory functions. Steroids, 78, 59–68. doi:10.1016/j.steroids.2012.09.013
- Oitzl, M.S., & de Kloet, E.R. (1992). Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neurosciences, 106, 62–71. doi:10.1037/0735-7044.106.1.62
- Oitzl, M.S., de Kloet, E.R., Joels, M., Schmid, W., & Cole, T.J. (1997). Spatial learning deficits in mice with a targeted glucocorticoid receptor gene disruption. European Journal of Neuroscience, 9, 2284–2296. doi:10.1111/j.1460-9568.1997.tb01646.x
- Paakinaho, V., Presman, D.M., Ball, D.A., Johnson, T.A., Schiltz, R.L., Levitt, P., … Hager, G.L. (2017). Single-molecule analysis of steroid receptor and cofactor action in living cells. Nature Communications, 8, 15896. doi:10.1038/ncomms15896
- Pariante, C.M., Makoff, A., Lovestone, S., Feroli, S., Heyden, A., Miller, A.H., & Kerwin, R.W. (2001). Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. British Journal of Pharmacology, 134, 1335–1343. doi:10.1038/sj.bjp.0704368
- Polman, J.A., de Kloet, E.R., & Datson, N.A. (2013). Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome. Endocrinology, 154, 1832–1844. doi:10.1210/en.2012-2187
- Polman, J.A., Hunter, R.G., Speksnijder, N., van den Oever, J.M., Korobko, O.B., McEwen, B.S., … Datson, N.A. (2012). Glucocorticoids modulate the mTOR pathway in the hippocampus: Differential effects depending on stress history. Endocrinology, 153, 4317–4327. doi:10.1210/en.2012-1255
- Presman, D.M., Ganguly, S., Schiltz, R.L., Johnson, T.A., Karpova, T.S., & Hager, G.L. (2016). DNA binding triggers tetramerization of the glucocorticoid receptor in live cells. Proceedings of the National Academy of Sciences of the United States of America,, 113, 8236–8241. doi:10.1073/pnas.1606774113
- Presman, D.M., Ogara, M.F., Stortz, M., Alvarez, L.D., Pooley, J.R., Schiltz, R.L., … Pecci, A. (2014). Live cell imaging unveils multiple domain requirements for in vivo dimerization of the glucocorticoid receptor. PLoS Biology, 12, e1001813. doi:10.1371/journal.pbio.1001813
- Qian, X., Droste, S.K., Gutierrez-Mecinas, M., Collins, A., Kersante, F., Reul, J.M.H.M., & Linthorst, A.C. (2011). A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology, 152, 3738–3748. doi:10.1210/en.2011-1008
- Qian, X., Droste, S.K., Lightman, S.L., Reul, J.M.H.M., & Linthorst, A.C. (2012). Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology, 153, 4346–4353. doi:10.1210/en.2012-1484
- Rafestin-Oblin, M.E., Lombes, M., Lustenberger, P., Blanchardie, P., Michaud, A., Cornu, G., & Claire, M. (1986). Affinity of corticosteroids for mineralocorticoid and glucocorticoid receptors of the rabbit kidney: Effect of steroid substitution. Journal of Steroid Biochemistry, 25, 527–534. doi:10.1016/0022-4731(86)90398-5
- Reichardt, H.M., Kaestner, K.H., Tuckermann, J., Kretz, O., Wessely, O., Bock, R., … Schutz, G. (1998). DNA binding of the glucocorticoid receptor is not essential for survival. Cell, 93, 531–541. doi:10.1016/S0092-8674(00)81183-6
- Reul, J.M.H.M. (2014). Making memories of stressful events: a journey along epigenetic, gene transcription, and signaling pathways. Frontiers in Psychiatry, 5, 5. doi:10.3389/fpsyt.2014.00005
- Reul, J.M.H.M., & de Kloet, E.R. (1985). Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology, 117, 2505–2511. doi:10.1210/endo-117-6-2505
- Reul, J.M.H.M., & de Kloet, E.R. (1986). Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. Journal of Steroid Biochemistry, 24, 269–272. doi:10.1016/0022-4731(86)90063-4
- Reul, J.M.H.M., Gesing, A., Droste, S., Stec, I.S., Weber, A., Bachmann, C., … Linthorst, A.C.E. (2000). The brain mineralocorticoid receptor: Greedy for ligand, mysterious in function. European Journal of Pharmacology, 405, 235–249. doi:10.1016/S0014-2999(00)00677-4
- Reul, J.M.H.M., van den Bosch, F.R., & de Kloet, E.R. (1987). Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: Functional implications. Journal of Endocrinology, 115, 459–467. doi:10.1677/joe.0.1150459
- Reul, J.M.H.M., Collins, A., Saliba, R.S., Mifsud, K.R., Carter, S.D., Gutierrez-Mecinas, M., … Linthorst, A.C.E. (2015). Glucocorticoids, epigenetic control and stress resilience. Neurobiology of Stress, 1, 44–59. doi:10.1016/j.ynstr.2014.10.001
- Ridder, S., Chourbaji, S., Hellweg, R., Urani, A., Zacher, C., Schmid, W., … Gass, P. (2005). Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. Journal of Neuroscience, 25, 6243–6250. doi:10.1523/JNEUROSCI.0736-05.2005
- Roberson, E.D., English, J.D., Adams, J.P., Selcher, J.C., Kondratick, C., & Sweatt, J.D. (1999). The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. Journal of Neuroscience, 19, 4337–4348. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10341237
- Roozendaal, B., Hernandez, A., Cabrera, S.M., Hagewoud, R., Malvaez, M., Stefanko, D.P., … Wood, M.A. (2010). Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. Journal of Neuroscience, 30, 5037–5046. doi:10.1523/JNEUROSCI.5717-09.2010
- Rozeboom, A.M., Akil, H., & Seasholtz, A.F. (2007). Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proceedings of the National Academy of Sciences of the United States of America, 104, 4688–4693. doi:10.1073/pnas.0606067104
- Rupprecht, R., Arriza, J.L., Spengler, D., Reul, J.M.H.M., Evans, R.M., Holsboer, F., & Damm, K. (1993a). Transactivation and synergistic properties of the mineralocorticoid receptor: Relationship to the glucocorticoid receptor. Molecular Endocrinology, 7, 597–603. doi:10.1210/mend.7.4.8388999
- Rupprecht, R., Reul, J.M.H.M., van Steensel, B., Spengler, D., Soder, M., Berning, B., … Damm, K. (1993b). Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. European Journal of Pharmacology, 247, 145–154. doi:10.1016/0922-4106(93)90072-H
- Saunderson, E.A., Spiers, H., Mifsud, K.R., Gutierrez-Mecinas, M., Trollope, A.F., Shaikh, A., … Reul, J.M.H.M. (2016). Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America, 113, 4830–4835. doi:10.1073/pnas.1524857113
- Savory, J.G., Prefontaine, G.G., Lamprecht, C., Liao, M., Walther, R.F., Lefebvre, Y.A., & Hache, R.J. (2001). Glucocorticoid receptor homodimers and glucocorticoid-mineralocorticoid receptor heterodimers form in the cytoplasm through alternative dimerization interfaces. Molecular and Cellular Biology, 21, 781–793. doi:10.1128/MCB.21.3.781-793.2001
- Schauer, M., Chalepakis, G., Willmann, T., & Beato, M. (1989). Binding of hormone accelerates the kinetics of glucocorticoid and progesterone receptor binding to DNA. Proceedings of the National Academy of Sciences of the United States of America,, 86, 1123–1127. doi:10.1073/pnas.86.4.1123
- Scheidereit, C., Geisse, S., Westphal, H.M., & Beato, M. (1983). The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature, 304, 749–752. doi:10.1038/304749a0
- Schinkel, A.H. (1999). P-Glycoprotein, a gatekeeper in the blood-brain barrier. Advanced Drug Delivery Reviews, 36, 179–194. doi:10.1016/S0169-409X(98)00085-4
- Schmidt, M.V., Sterlemann, V., Wagner, K., Niederleitner, B., Ganea, K., Liebl, C., … Muller, M.B. (2009). Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short- and long-term consequences. Endocrinology, 150, 2709–2716. doi:10.1210/en.2008-1211
- Schwabe, L., Oitzl, M.S., Philippsen, C., Richter, S., Bohringer, A., Wippich, W., & Schachinger, H. (2007). Stress modulates the use of spatial versus stimulus-response learning strategies in humans. Learning & Memory, 14, 109–116. doi:10.1101/lm.435807
- Schwabe, L., Schachinger, H., de Kloet, E.R., & Oitzl, M.S. (2010). Corticosteroids operate as a switch between memory systems. Journal of Cognitive Neuroscience, 22, 1362–1372. doi:10.1162/jocn.2009.21278
- Sloviter, R.S., Dean, E., & Neubort, S. (1993a). Electron microscopic analysis of adrenalectomy-induced hippocampal granule cell degeneration in the rat: Apoptosis in the adult central nervous system. The Journal of Comparative Neurology, 330, 337–351. doi:10.1002/cne.903300305
- Sloviter, R.S., Sollas, A.L., Dean, E., & Neubort, S. (1993). Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: Characterization of an in vivo model of controlled neuronal death. The Journal of Comparative Neurology, 330, 324–336. doi:10.1002/cne.903300304
- Sloviter, R.S., Valiquette, G., Abrams, G.M., Ronk, E.C., Sollas, A.L., Paul, L.A., & Neubort, S. (1989). Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science, 243, 535–538. doi:10.1126/science.2911756
- Solomon, M.B., Furay, A.R., Jones, K., Packard, A.E., Packard, B.A., Wulsin, A.C., & Herman, J.P. (2012). Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience, 203, 135–143. doi:10.1016/j.neuroscience.2011.12.014
- Stec, I., Barden, N., Reul, J.M.H.M., & Holsboer, F. (1994). Dexamethasone nonsuppression in transgenic mice expressing antisense RNA to the glucocorticoid receptor. Journal of Psychiatric Research, 28, 1–5. doi:10.1016/0022-3956(94)90031-0
- Trapp, T., Rupprecht, R., Castren, M., Reul, J.M.H.M., & Holsboer, F. (1994). Heterodimerization between mineralocorticoid and glucocorticoid receptor: A new principle of glucocorticoid action in the CNS. Neuron, 13, 1457–1462. doi:10.1016/0896-6273(94)90431-6
- Trollope, A.F., Mifsud, K.R., Saunderson, E.A., & Reul, J.M.H.M. (2017). Molecular and epigenetic mechanisms underlying cognitive and adaptive responses to stress [Review]. Epigenomes, 1, 17. doi:10.3390/epigenomes1030017
- Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P.C., … Schutz, G. (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nature Genetics, 23, 99–103. doi:10.1038/12703.
- Uhr, M., Holsboer, F., & Muller, M.B. (2002). Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. Journal of Neuroendocrinology, 14, 753–759. doi:10.1046/j.1365-2826.2002.00836.x
- Umesono, K., & Evans, R.M. (1989). Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell, 57, 1139–1146. doi:10.1016/0092-8674(89)90051-2
- van Weert, L., Buurstede, J.C., Mahfouz, A., Braakhuis, P.S.M., Polman, J.A.E., Sips, H.C.M., … Meijer, O.C. (2017). NeuroD factors discriminate mineralocorticoid from glucocorticoid receptor DNA binding in the male rat brain. Endocrinology, 158, 1511–1522. doi:10.1210/en.2016-1422
- Veldhuis, H.D., & De Kloet, E.R. (1983). Antagonistic effects of aldosterone on corticosterone-mediated changes in exploratory behavior of adrenalectomized rats. Hormone Behavior, 17, 225–232. doi:10.1016/0018-506X(83)90009-0
- Veldhuis, H.D., De Korte, C.C., & De Kloet, E.R. (1985). Glucocorticoids facilitate the retention of acquired immobility during forced swimming. European Journal of Pharmacology, 115, 211–217. doi:10.1016/0014-2999(85)90693-4
- Veldhuis, H.D., Van Koppen, C., Van Ittersum, M., & De Kloet, E.R. (1982). Specificity of the adrenal steroid receptor system in rat hippocampus. Endocrinology, 110, 2044–2051. doi:10.1210/endo-110-6-2044
- Vogel, S., Fernandez, G., Joels, M., & Schwabe, L. (2016). Cognitive adaptation under stress: A case for the mineralocorticoid receptor. Trends in Cognitive Sciences, 20, 192–203. doi:10.1016/j.tics.2015.12.003
- Vogel, S., Klumpers, F., Kroes, M.C., Oplaat, K.T., Krugers, H.J., Oitzl, M.S., … Fernandez, G. (2015). A stress-induced shift from trace to delay conditioning depends on the mineralocorticoid receptor. Biological Psychiatry, 78, 830–839. doi:10.1016/j.biopsych.2015.02.014
- Vogel, S., Klumpers, F., Krugers, H.J., Fang, Z., Oplaat, K.T., Oitzl, M.S., … Fernandez, G. (2015). Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology, 40, 947–956. doi:10.1038/npp.2014.271
- Voss, T.C., Schiltz, R.L., Sung, M.H., Yen, P.M., Stamatoyannopoulos, J.A., Biddie, S.C., … Hager, G.L. (2011). Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism. Cell, 146, 544–554. doi:10.1016/j.cell.2011.07.006
- Wagner, K.V., Wang, X.D., Liebl, C., Scharf, S.H., Muller, M.B., & Schmidt, M.V. (2011). Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology, 36, 579–587. doi:10.1016/j.psyneuen.2010.09.007
- Webster, M.K., Goya, L., Ge, Y., Maiyar, A.C., & Firestone, G.L. (1993). Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Molecular and Cellular Biology, 13, 2031–2040. doi:10.1128/MCB.13.4.2031
- Wei, Q., Fentress, H.M., Hoversten, M.T., Zhang, L., Hebda-Bauer, E.K., Watson, S.J., … Akil, H. (2012). Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biological Psychiatry, 71, 224–231. doi:10.1016/j.biopsych.2011.07.009
- Wei, Q., Lu, X.Y., Liu, L., Schafer, G., Shieh, K.R., Burke, S., … Akil, H. (2004). Glucocorticoid receptor overexpression in forebrain: A mouse model of increased emotional lability. Proceedings of the National Academy of Sciences of the United States of America,, 101, 11851–11856. doi:10.1073/pnas.0402208101
- Wong, E.Y., & Herbert, J. (2005). Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. European Journal of Neuroscience, 22, 785–792. doi:10.1111/j.1460-9568.2005.04277.x
- Wyrwoll, C.S., Holmes, M.C., & Seckl, J.R. (2011). 11beta-hydroxysteroid dehydrogenases and the brain: From zero to hero, a decade of progress. Frontiers in Neuroendocrinology, 32, 265–286. doi:10.1016/j.yfrne.2010.12.001
- Yang, J., Liu, J., & DeFranco, D.B. (1997). Subnuclear trafficking of glucocorticoid receptors in vitro: Chromatin recycling and nuclear export. The Journal of Cell Biology, 137, 523–538. doi:10.1083/jcb.137.3.523
- Zalachoras, I., Houtman, R., & Meijer, O.C. (2013). Understanding stress-effects in the brain via transcriptional signal transduction pathways. Neuroscience, 242, 97–109. doi:10.1016/j.neuroscience.2013.03.038
