Abstract
Stress-related exhaustion has been associated with selective and enduring cognitive impairments. However, little is known about how to address cognitive deficits in stress rehabilitation and how this influences stress recovery over time. The aim of this open-label, parallel randomized controlled trial (ClinicalTrials.gov: NCT03073772) was to investigate the long-term effects of 12 weeks cognitive or aerobic training on cognitive function, psychological health, and work ability for patients diagnosed with exhaustion disorder (ED). One-hundred-and-thirty-two patients (111 women) participating in multimodal stress rehabilitation were randomized to receive additional cognitive training (n = 44), additional aerobic training (n = 47), or no additional training (n = 41). Treatment effects were assessed before, immediately after and one-year post intervention. The primary outcome was global cognitive function. Secondary outcomes included domain-specific cognition, self-reported burnout, depression, anxiety, fatigue and work ability, aerobic capacity, and sick-leave levels. Intention-to-treat analysis revealed a small but lasting improvement in global cognitive functioning for the cognitive training group, paralleled by a large improvement on a trained updating task. The aerobic training group showed improvements in aerobic capacity and episodic memory immediately after training, but no long-term benefits. General improvements in psychological health and work ability were observed, with no difference between interventional groups. Our findings suggest that cognitive training may be a viable method to address cognitive impairments for patients with ED, whereas the effects of aerobic exercise on cognition may be more limited when performed during a restricted time period. The implications for clinical practice in supporting patients with ED to adhere to treatment are discussed.
Introduction
Stress-related illness is one of the primary reasons for sick-leave in Sweden today (Swedish Social Insurance Agency, Citation2017). One of the most widely recognized consequences of prolonged stress exposure is burnout, a condition characterized by emotional exhaustion, depersonalization, and reduced personal accomplishment (Maslach, Schaufeli, & Leiter, Citation2001). Burnout has been studied extensively in the field of work-related stress, however, it is currently not an internationally established clinical diagnosis and various theoretical conceptualizations of the concept exist. Therefore, the Swedish National Board of Health and Welfare in 2003 outlined diagnostic criteria for exhaustion disorder (ED), to improve clinical diagnosis and treatment of patients suffering from stress-related illness. ED has since been included in the Swedish version of the International Classification of Diseases (ICD-10, diagnostic code F43.8A; see Supplementary Table 1 for diagnostic criteria), and the diagnosis has been adopted into healthcare practice. ED can thus be seen as a clinical manifestation of burnout (see Grossi, Perski, Osika, & Savic, Citation2015 for a review) and, in line with this view, patients diagnosed with ED also show high burnout levels (e.g. Glise, Ahlborg, & Jonsdottir, Citation2012).
Clinically, ED is characterized by symptoms of physical and mental exhaustion, attributable to psychosocial stress (either work or non-work related) of minimum six months duration (Grossi et al., Citation2015). Although the prevalence of ED is not well established, population-based studies have classified between 8% (Persson, Österberg, Viborg, Jönsson, & Tenenbaum, Citation2017) and 16% (Glise, Hadzibajramovic, Jonsdottir, & Ahlborg, Citation2010) of participants as possible ED cases, with considerably higher prevalence among patients seeking primary care (Wiegner, Hange, Björkelund, & Ahlborg, Citation2015). The recovery period can be long and elevated levels of burnout (Glise et al., Citation2012; Oosterholt, Maes, Van der Linden, Verbraak, & Kompier, Citation2016; Stenlund, Nordin, & Järvholm, Citation2012), as well as reduced work ability (Stenlund et al., Citation2012) have been observed up to three years post-rehabilitation, implying that the condition can have lasting effects on both an individual and a societal level.
In recent years, several studies have demonstrated concomitant cognitive impairments associated with stress-related exhaustion (e.g. Eskildsen, Andersen, Pedersen, Vandborg, & Andersen, Citation2015; Jonsdottir et al., Citation2013; Ohman, Nordin, Bergdahl, Slunga Birgander, & Stigsdotter Neely, Citation2007). Prospective studies indicate that cognitive deficits, particularly in executive function, working memory, attention and processing speed, can be enduring symptoms with impairments observed when patients are re-examined both immediately (Oosterholt, Van der Linden, Maes, Verbraak, & Kompier, Citation2012) and up to three years after treatment (Eskildsen, Andersen, Pedersen, & Andersen, Citation2016; Jonsdottir et al., Citation2017; Oosterholt et al., Citation2016; Österberg, Skogsliden, & Karlson, Citation2014; van Dam, Keijsers, Eling, & Becker, Citation2012). Furthermore, patients with stress-related exhaustion continue to report a substantial amount of cognitive problems in their everyday life (Eskildsen et al., Citation2016; Oosterholt et al., Citation2012, Citation2016; Österberg et al., Citation2014) and a 3-year follow-up showed that more than 80% of former patients reported problems with mental fatigue after work (Stenlund et al., Citation2012), suggesting that cognitive impairments have long-term consequences for patients’ everyday functioning.
In the Rehabilitation for Improved Cognition (RECO)-study, we have recently demonstrated improvements on a trained working memory updating task, generalizations of training effects (i.e. transfer) to updating and episodic memory and reduced burnout symptoms following computerized cognitive training (Gavelin, Boraxbekk, Stenlund, Järvholm, & Neely, Citation2015), and improved episodic memory performance and aerobic capacity following aerobic training (Eskilsson, Slunga Järvholm, Malmberg Gavelin, Stigsdotter Neely, & Boraxbekk, Citation2017), in relation to a treatment-as-usual control group. Regarding long-term effects of cognitive interventions, previous cognitive training studies in healthy adults have indicated that effects on trained tasks can be maintained over time, but that maintenance of transfer effects may be more limited (Dahlin, Nyberg, Bäckman, & Neely, Citation2008; Sandberg & Stigsdotter Neely, Citation2016; Zinke et al., Citation2014, but see also: Schmiedek, Lövdén, & Lindenberger, Citation2014). For physical exercise, few long-term studies have been conducted, but there have been demonstrations of cognitive improvements also at long-term follow-up (Lautenschlager et al., Citation2008), effects that are likely dependent on maintaining the aerobic fitness levels (Hötting, Schauenburg, & Röder, Citation2012). For patients with ED, no studies have so far investigated the long-term effects of interventions specifically targeting cognitive impairments. Furthermore, the long-term effects of cognitive and aerobic training on variables related to ED recovery, such as psychological health and work ability, remain unclear.
The aim of this study was to investigate the long-term effects of cognitive training and aerobic training, offered as an addition to a multimodal stress rehabilitation program, on (1) cognitive function; (2) psychological health (levels of burnout, depression, anxiety, and fatigue); and (3) work ability (assessed through sick-leave data and self-report). We hypothesized that for cognitive function, we would observe long-term gains following cognitive training for the trained updating task, whereas for aerobic training, we predicted that long-term effects on episodic memory would be dependent on maintained aerobic fitness levels. For psychological health and work ability, no specific hypotheses were made regarding group differences.
Method
Participants and procedure
The RECO-study was an open-label, parallel, three-armed randomized controlled trial, conducted at the Stress Rehabilitation Clinic at the University Hospital in Umeå, Sweden. The participants and procedure of the trial have previously been described in detail (Eskilsson et al., Citation2017; Gavelin et al., Citation2015). All patients had confirmed diagnosis of ED, according to the diagnostic criteria included in the Swedish version of the ICD-10 (code F43.8A, Table S1).
Table 1. Sample characteristics at baseline.
Participants were recruited from April 2010 until June 2013 and during this period all patients referred to the Stress Rehabilitation Clinic were screened for eligibility. Additionally, eight participants were recruited from the Social Insurance Agency in Umeå, Sweden, in order to speed up the recruitment process. Inclusion criteria were (1) confirmed diagnosis of ED; (2) 18–60 years old; (3) currently employed; (4) considered by a physician and a psychologist to be suitable for group-based stress rehabilitation; (5) no known abuse of alcohol or drugs; (6) not in the need of other treatment; and (7) not participating in other interventional study. All patients were on partial or full-time sick-leave when recruited to the study.
All patients participated in the Stress Rehabilitation Clinic’s usual care, a 24-week multimodal stress rehabilitation program (MMR). The program consisted of 22 weekly three-hour group sessions, based on cognitive behavioral therapy. Each group session started with a relaxation exercise, followed by a specific theme such as psychoeducation regarding stress and recovery, sleep and affect. Additionally, each patient had two individual meetings with the group therapist, to set and evaluate individual goals for behavioral change during rehabilitation. Vocational measures and prescription of physical activity was also included. After 12 weeks of rehabilitation, patients were randomly allocated to one of three study conditions: continued MMR with (1) no additional training; (2) the addition of computerized cognitive training; and (3) the addition of aerobic training. Psychological variables, work ability, and aerobic capacity were assessed before MMR (Baseline), before randomization (T1), after completing the intervention (T2) and at one-year follow-up (T3). At T1, T2, and T3, patients also conducted a two-hour neuropsychological test session to assess cognitive functioning. All participants received a financial compensation of 600 SEK for participating in the study. The study was conducted in accordance with the Declaration of Helsinki and approved by the Umeå Regional Ethical Review Board (Dnr 2010-53-31), and all participants provided written informed consent prior to the start of the study. The trial was retrospectively registered at ClinicalTrials.gov (NCT03073772). The CONSORT 2010 guidelines were used to describe this randomized clinical trial (Schulz, Altman, & Moher, Citation2010).
Randomization, blinding and sample size
Patients were randomized by MMR group in blocks of, at most, eight persons. The randomization was conducted by a person independent to the project, by drawing lots with a 1:1:1 allocation rate. Lots were prepared by the project coordinator and stored safely; the content of a single lot was not revealed until after it was drawn. Due to high drop-out rates in the two intervention groups, randomization was adjusted at the end of the study period, allowing for a doubled possibility of allocation to these groups as compared to the control condition. As the added interventions were embedded in the usual care, neither patients, health care providers nor investigators were masked to group assignment. Sample size was determined based on a pilot study, in which patients with ED conducted cognitive training and improved their performance on the trained updating task Letter memory running span from 3.0 (1.4) pretest to 5.8 (1.9) posttest. Using a two-sided 5% significance level and a power of 80%, 30 participants in each group were required to reach adequate power.
Interventions
Computerized cognitive training
The computerized cognitive training intervention consisted of cognitive tasks chosen to target the specific functions known to be affected by long-term stress. The program has previously been described in detail (Gavelin et al., Citation2015). Briefly, it consisted of six tasks: two tasks targeting the executive functions shifting and updating respectively, one task targeting episodic memory and one task targeting visuospatial short term memory. Participants trained for three times a week during the 12-week intervention period and each session was approximately 15–20 minutes long. Difficulty level was adapted in three steps for all tasks except the shifting tasks, in order to keep the training challenging and minimize the use of task-specific strategies. Feedback on task performance was given after each task. To ensure that all participants understood the training procedure, they were instructed and conducted the training at the Stress Rehabilitation Clinic during the first week of the intervention. Thereafter, they continued training at home, using a Web-based program. During the intervention period, a psychologist contacted participants, either by telephone or e-mail, to provide motivational comments and ensure that there were no technical problems. This was initially done once a week and, if everything worked well for the participant, gradually with sparser time intervals.
Aerobic training
The aerobic training consisted of indoor cycling (see Eskilsson et al., Citation2017). In short, the participants took part of instructor-led group-based training sessions, at an individually chosen training center in the municipality. The aerobic training was conducted three times a week for 12 weeks and each training session was 40 minutes long. A chest belt heart monitor was used to monitor heart rate, and participants were instructed to exercise at a moderate-vigorous intensity, implicating a load of 70–85% of their maximum age-adjusted heart rate (220 – age). Each week, participants received written feedback about their training intensity, as well as motivational comments, by a physiotherapist.
Outcomes
The primary outcome evaluated in this study was change in cognitive performance across the three test-sessions (T1, T2, and T3) measured by a global cognitive score, a composite computed as the mean z-score of nine untrained cognitive tests covering the domains executive function, working memory, episodic memory, perceptual speed, and reasoning ability. Secondary outcomes included: domain-specific cognitive functioning, psychological health (i.e. burnout, depression, anxiety, and fatigue), work ability, and aerobic capacity.
Cognitive function
The cognitive test battery used in the RECO-study has previously been described in detail (Gavelin et al., Citation2015) and is therefore only outlined briefly below.
Executive function. The n-back task was used to assess updating ability. In this task, participants were presented with lists of single digits (1–9) and asked to report whether the digit matched the one presented n steps back. Three different conditions were used (1-, 2-, and 3-back), and accuracy (hits – false alarms) in the 3-back condition was used as outcome measure. The Color-word interference test from D-KEFS was used as a measure of inhibition (Delis, Kaplan, & Kramer, Citation2001). The task was administered according to the standardized procedures and an inhibition cost was calculated, i.e. the time taken in seconds to complete incongruent trials as compared to reading color words. Furthermore, the Trail making test from D-KEFS was used as a measure of shifting ability (Delis et al., Citation2001). A shift cost was used as outcome measure, calculated as the time taken (seconds) to complete the shifting condition as compared to the baseline condition (number-sequencing).
Working memory. To assess working memory, Digit span forwards and backwards was adapted from WAIS-R (Wechsler, Citation1981) and Letter-number sequencing from WAIS-III (Wechsler, Citation1997). The tasks were administered according to the standard procedures and the total number of correctly recalled sequences in each condition was used as outcome.
Episodic memory. To assess episodic memory, we used Recall of concrete nouns, a list of 18 concrete nouns that were administered according to the selective reminding procedure (Buschke, Citation1973). Performance was measured as total number of recalled nouns across four presentations.
Perceptual speed. Digit symbol from WAIS-R was used to assess perceptual speed (Wechsler, Citation1981). Total number of correctly drawn symbols during 90 seconds was used as outcome measure.
Reasoning ability. Raven’s advanced progressive matrices were used as a measure of reasoning ability (Raven, Raven, & Court, Citation1998). The test consists of 36 pattern matrices and was split into two parts using odd and even items. Performance was measured in total number of correctly solved matrices in 10 minutes.
Cognitive training criterion task. To assess training gains for the cognitive training intervention, we used Letter memory running span (Miyake et al., Citation2000) as a criterion task. This task targets updating ability and was included in the computerized cognitive training program, as well as in the study assessments. In this task, lists of single letters (A–D) were presented to the participants on a computer screen. After presentation, participants were asked to recall the last four presented letters in the correct order. Total number of correctly recalled four-letter sequences across ten presentations was used as outcome measure.
Psychological variables
The Shirom-Melamed Burnout Questionnaire (SMBQ) was used to measure level of burnout (Melamed, Kushnir, & Shirom, Citation1992). The questionnaire consists of 22 items, rated on a 7-point Likert scale (1 = “almost never”, 7 = “almost always”). The mean of all items was used as outcome measure, with a higher score indicating higher burnout. Cronbach’s alpha for this measure was 0.94. The Hospital Anxiety and Depression scale (HAD) was used to assess levels of depression and anxiety (Zigmond & Snaith, Citation1983). This questionnaire consists of 14 items rated on a 4-point Likert scale (0–3), with seven items targeting depression and anxiety respectively. The total score on each scale (range 0–21) was used as outcome measure, with a higher score indicating more symptoms. Cronbach’s alpha was 0.81 and 0.78 for the depression and anxiety subscale, respectively. The Checklist Individual Strength (CIS) was used to measure level of fatigue (Beurskens et al., Citation2000). The questionnaire consists of 20 items rated on a 7-point Likert scale (1 = “yes, that is true”, 7 = “no, that is not true”). A total score was calculated (range 20–140), with a higher score indicating more fatigue. Cronbach’s alpha for this scale was 0.90. Data for the psychological variables (i.e. SMBQ, HAD, and CIS) were collected from clinical patient records, allowing for data also for patients who discontinued the added intervention but continued with ordinary care.
Aerobic capacity
A submaximal test on a calibrated cycle ergometer was used to assess maximal oxygen uptake (VO2max). The pedaling frequency during the test was 50 revolutions per minute and the work load was adjusted in order to reach a steady state in heart rate of at least 120 beats per minutes. The mean heart rate at the last three minutes of the test was recorded as the steady state heart rate. Åstrands nomogram, adjusted for sex, years of age, body weight, work load, and steady state heart rate was used to estimate VO2max (ml/kg/min) (Astrand & Ryhming, Citation1954).
Work ability
Work ability was measured with sick-leave data from the Swedish Social Insurance Agency. The degree of sick-leave (0%, 25%, 50%, 75%, or 100% of ordinary working time) was used as outcome measure. Furthermore, self-rated work ability was assessed with the Work ability index (WAI) (de Zwart, Frings-Dresen, & van Duivenbooden, Citation2002). The questionnaire consists of ten items covering current work ability in relation to job demands (physical and psychological) and current diseases, as well as items targeting past sickness absence and predicted future work ability. The questionnaire ranges from 7 to 49, with higher values indicating better work ability. Cronbach’s alpha was 0.71.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics, version 24. To investigate if there were any significant differences between participants who completed the intervention and those who dropped out, we used independent samples t-tests for continuous variables and Pearson’s Chi-square tests for categorical variables. Drop-out analyses were conducted separately for each group, with respect to baseline characteristics (age, sex, educational level, and verbal ability) and for the psychological variables at each measurement point (i.e. symptoms of burnout, depression, anxiety and fatigue at T1, T2, and T3). For analysis of sick-leave data, a non-parametric design for repeated measures, with Time as within-subject factors and Group as between-subject factor, was conducted using the %F1_LD_F1 SAS macro in SAS version 9.4.
In order to investigate the effects of the interventions on cognitive functioning, psychological health, work ability, and aerobic capacity, we used linear mixed-effects models (see the Supplementary Material for more details). All cognitive test scores were z-transformed by standardizing them to the baseline mean and standard deviation, with a higher score indicating better performance. The analyses were conducted by the intention-to-treat principle, including all available data, and fitted with full information maximum likelihood estimation. When evaluating treatment effects, we were primarily interested in the Group × Time interaction, representing the difference in change across sessions for the cognitive training and aerobic training group, respectively, relative to the control group. Standardized effect sizes (Cohen’s d) were calculated based on the formula provided by Feingold (equation 7), representing the difference in change between intervention- and control group (i.e. the beta-estimate for the Group × Time interaction), divided by the baseline standard deviation (Feingold, Citation2009). The results from the active intervention phase (i.e. T1 to T2) have previously been reported (Eskilsson et al., Citation2017; Gavelin et al., Citation2015) and are therefore presented in the Supplementary Material. Here, the main focus was on evaluating the long-term training effects at the one-year follow-up.
Results
Baseline characteristics and missing data
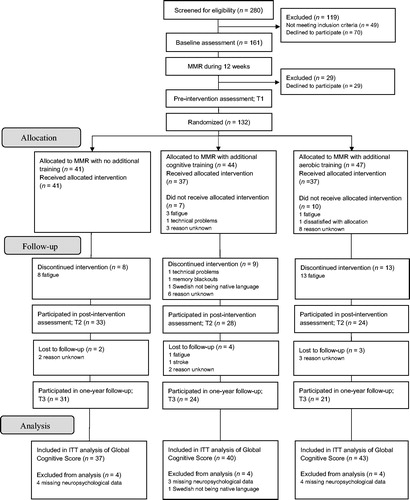
displays the flow of patients through the study. In total, 231 patients fulfilled the inclusion criteria. Of these, 161 agreed to participate and completed the baseline assessment, before entering the 24-week multimodal stress rehabilitation program. As outlined above, all patients initially participated in 12 weeks of MMR, without any additional treatment. During this pre-intervention phase, 29 patients discontinued the study. Thus, a total of 132 patients remained to participate in the pre-intervention assessment and were thereafter randomized to the control group (n = 41), the cognitive training group (n = 44), and the aerobic training group (n = 47). Baseline characteristics are displayed in . Seventeen patients (seven in the cognitive training group and ten in the aerobic training group) were randomized but never started training and thirty patients (eight control group; nine cognitive training group; and 13 aerobic training group) discontinued study participation during the active intervention phase. An additional nine patients did not complete the one-year assessment. This resulted in a total drop-out rate of ten patients (24%) in the control group, 20 patients (45%) in the cognitive training group, and 26 patients (55%) in the aerobic training group. Drop-out rates differed significantly between the groups, with more patients dropping out from the two intervention groups relative to the control group. The most common reason for dropping-out was that participating in MMR took a lot of effort and that the added interventions, as well as the extensive assessments associated with study participation, was experienced as too demanding.
There were no significant differences between patients who completed the one-year follow-up and those who dropped out with respect to age, sex, educational level, or verbal ability. When comparing levels of burnout, depression, anxiety, and fatigue between those patients who completed the one-year follow-up and those who had discontinued study participation, no significant differences were found at T1. However, for the cognitive training group, completers showed lower symptoms of burnout (p = .03) and fatigue (p = .01) than drop-outs at T3. Additionally, for the control group, patients who discontinued study participation showed lower symptoms of fatigue at T2 than those completing the one-year assessment (p = .01). For all other psychological measures and time-points, completers were not significantly different from drop-outs.
Cognitive function
Group means and standard deviations for the cognitive variables at each assessment occasion are displayed in (see Supplementary Table 2 for the results from the full models). There were no significant differences between the intervention groups and the control group in performance at T1 on any of the cognitive variables (Table S2). The results from the tests of the Group × Time interactions from T1 to T3 are displayed in , showing the estimated difference in change from pretest to one-year follow-up for the respective intervention groups, using the control group as a reference.
Table 2. Means and standard deviations on the cognitive variables for each group and time-point.
Table 3. Long-term training-related effects on the cognitive variables.
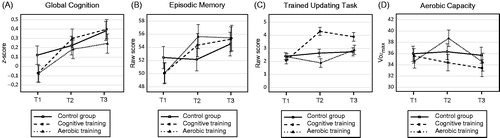
There was a significant difference in average change across time between the cognitive training group and the control group for the global cognitive score from T1 to T3 (p = .02, Cohen’s d = 0.35), showing that the cognitive training group improved more from pretest to one-year follow-up than the control group. No significant difference in change in global cognition was found between the aerobic training group and the control group. Furthermore, although a training-related improvement was found for episodic memory immediately after training for both the aerobic training and the cognitive training group (Table S2), no significant differences in change from T1 to T3 were found (). Finally, for the cognitive training criterion updating task, the cognitive training group improved more than the control group from T1 to T3 (p = .004, Cohen’s d = 0.87). No significant long-term effects were found for the remaining cognitive measures, i.e. executive function, working memory, perceptual speed, or reasoning ability. shows the average change across the three time-points for each group on (a) the global cognitive score, (b) episodic memory, and (c) the trained updating task, based on the estimated means of the model.
Figure 2. Changes across time for each group based on the estimated means from the model in (A) performance on the global cognitive score, based on the mean z-score of nine cognitive tests covering the domains executive function, working memory, episodic memory, processing speed, and reasoning ability; (B) performance in episodic memory; (C) performance on the cognitive training criterion updating task and; (D) aerobic capacity. Error bars indicate SEM. T1: pre intervention; T2: post intervention; T3: one-year follow-up; VO2max: maximal oxygen uptake.
Psychological variables
Group means and standard deviations for the psychological variables, aerobic capacity, and work ability are presented in (see Supplementary Table 3 for the full results from the models). shows the estimates for the Group × Time interactions, indicating the difference in change from T1 to T3 for the respective intervention groups, with the control group as a reference. Results showed no significant differences in change across sessions between the intervention groups and the control group in levels of burnout, depression, anxiety, or fatigue. However, all groups improved significantly across time on the psychological measures, indicating a general improvement in psychological well-being directly following the MMR and at one-year follow-up (Table S4).
Table 4. Means and standard deviations on psychological variables, work ability, and aerobic capacity for each group and time-point.
Table 5. Long-term training-related effects on psychological variables, work ability, and aerobic capacity.
Aerobic capacity
There were no significant long-term effects on aerobic capacity in either of the two intervention groups (). Thus, although the aerobic training group showed improvements in aerobic capacity immediately following the intervention (Table S3), no evidence of long-term gains was found at one-year follow-up. shows the change in aerobic capacity across the three time-points for each group, based on the estimated means of the model.
Work ability
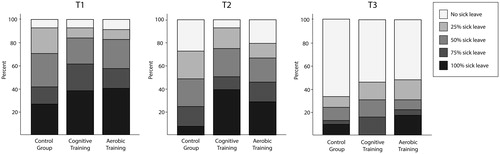
No significant differences were found between the groups in rates of change in self-rated work ability (WAI; ). Across all groups, self-rated work ability improved significantly across time (Table S4). There were no significant differences between the groups in change in sick-leave levels (p = .19), however, for all groups the degree of sick leave decreased across time (p < .001). displays the distribution of sick leave rates across the groups at each time-point.
Post-hoc analyses
As described above, drop-out analyses revealed that for the cognitive training group, patients who completed the one-year assessment showed significantly lower symptoms of burnout and fatigue at T3 than those who had dropped-out. We therefore conducted a post hoc analysis, in which we investigated changes in levels of burnout and fatigue by per-protocol, i.e. only including patients who had completed the intervention period. Results showed a greater decrease in burnout symptoms for the cognitive training group relative the control group from T1 to T3 (β = −0.54, 95% CI [−1.07, −0.01], p = .047, Cohen’s d = 0.51). No significant Group × Time interaction was found for level of fatigue. Additionally, since we observed immediate gains, but no long-term effects, for the aerobic training group in both aerobic capacity and episodic memory, we were interested in whether changes in these variables during the follow-up period were related. Therefore, we investigated the relation between change in aerobic capacity and change in episodic memory performance for the aerobic training group between T2 and T3, using Pearson correlation. Although there were indications of a positive relationship between the variables, the effect did not reach statistical significance (r = 0.49, p = .06, n = 15).
Adverse events
There were no reported adverse events in any of the groups.
Discussion
The aim of this randomized clinical trial was to investigate the long-term effects of cognitive training and aerobic training, offered as an addition to a multimodal stress rehabilitation program, on (1) cognitive function; (2) psychological health; and (3) work ability, for patients diagnosed with stress-related exhaustion disorder. The main finding of the study was that the addition of cognitive training to stress rehabilitation yielded a small but lasting improvement in cognitive performance, relative to treatment-as-usual. The addition of aerobic training led to an increase in episodic memory performance directly following the intervention, however, no long-term improvement was observed. For psychological health and work ability, no additional effects of cognitive or aerobic training were found relative to multimodal stress rehabilitation alone.
For the cognitive training group, a long-term training gain was observed for the criterion updating task and the effect size indicated a large improvement. Previous studies on healthy adults have suggested that improvements on trained tasks are quite stable, at least up to 1.5 years after training (Dahlin et al., Citation2008; Sandberg & Stigsdotter Neely, Citation2016; Zinke et al., Citation2014). Our results add to these findings, demonstrating long-term robustness of training gains also for patients with ED.
Our results further showed that cognitive training led to a small improvement on the global cognitive score which, importantly, remained one year after training. Notably, the long-term effect on the global cognitive score was found in the absence of any significant domain-specific cognitive changes. This may suggest that the training-related effect on global cognition was not driven by any specific cognitive domain, but rather reflects a general improvement across several different cognitive abilities. These results are somewhat at odds with previous studies, in which the long-term benefits following cognitive training have been restricted to tasks similar to those trained (Dahlin et al., Citation2008; Sandberg & Stigsdotter Neely, Citation2016; Zinke et al., Citation2014). Given the generally quite narrow transfer effects following cognitive training, its utility in clinical settings has been questioned (Melby-Lervåg, Redick, & Hulme, Citation2016). However, one previous study demonstrated that 100 days of training on tasks targeting perceptual speed, episodic memory, and working memory led to reliable transfer effects in a group of healthy young adults two years after the end of training (Schmiedek et al., Citation2014). Thus, more extensive training targeting different cognitive abilities may be a key to reaching broader and more lasting effects. Here, we demonstrate that for patients with ED, a multifactorial training program can be beneficial in strengthening cognitive functioning, seemingly beyond the acquisition of task-specific strategies. Although the effect size of the observed training-related improvement was small, previous studies have indicated that the cognitive impairments observed in patients with ED are generally of a small-moderate magnitude (e.g. Eskildsen et al., Citation2015; Jonsdottir et al., Citation2013), but that they nevertheless have a profound impact on everyday cognitive functioning (Eskildsen et al., Citation2015; Oosterholt, Maes, Van der Linden, Verbraak, & Kompier, Citation2014). Thus, the training-related effect observed in our study may well be of clinical relevance in the rehabilitation of ED, and this is further supported by the fact that our post hoc per-protocol analysis showed additional positive effects of cognitive training relative to MMR alone on burnout levels (see also Gavelin et al., Citation2015).
As we have previously reported (Eskilsson et al., Citation2017), the aerobic training group showed improvements in episodic memory immediately after the intervention period. However, the results presented here revealed no long-term effects on episodic memory performance following aerobic training. This could be interpreted in light of the fact that the improvement in aerobic capacity observed for the aerobic training group immediately following the intervention did not remain at the one-year follow-up. Thus, it appears that participants in the aerobic training group did not maintain aerobic exercise levels after the intervention was completed. Improvements in episodic memory following physical exercise have been found to positively correlate with changes in cardiovascular fitness (Hötting et al., Citation2012), with increased neurogenesis in the hippocampus as one proposed mechanism (Pereira et al., Citation2007). Although few previous studies have investigated the long-term effects of physical exercise on cognition, one study found that improvements in episodic memory following two different exercise interventions were sustained only for those individuals who had maintained their cardiovascular fitness levels at one-year follow-up (Hötting, Schauenburg, & Röder, Citation2012). In our study, no significant relationship was observed between change in aerobic capacity and episodic memory performance for the aerobic training group during follow-up. However, this analysis was restricted to only 15 individuals and the size of the correlation coefficient nevertheless suggested a moderate positive association between the variables. Taken together, our results tentatively add to previous findings, suggesting that benefits from aerobic training on episodic memory may be dependent on whether exercise levels are sustained or not.
For the psychological variables, no additional benefits were found from the added interventions on levels of burnout, depression, anxiety, or fatigue. However, across all groups, a general improvement in psychological well-being was observed. This suggests that the ordinary care (i.e. MMR based on cognitive behavioral therapy) may be effective in giving lasting improvements in psychological health for this patient group, in line with what has previously been indicated (Glise et al., Citation2012; Sonntag-Ostrom et al., Citation2015; Stenlund et al., Citation2012), although this needs to be interpreted with caution since our study did not include a no-treatment control group. Similarly, we found no additional effects of the added interventions on self-rated work-ability or sick-leave levels. This is perhaps not surprising, considering that workplace involvement has been proposed to be an important interventional element when attempting to enhance return-to-work after sick-leave (Cullen et al., Citation2017) and none of the added interventions in our study directly targeted work ability through adjustments at the workplace. Instead, all patients received vocational rehabilitative measures as part of the MMR and we observed a general improvement across time for all three groups. However, it is noteworthy that a little over 40 percent of patients were still on some degree of sick leave (part- or full-time) at the one-year follow-up. This highlights the fact that ED recovery can take a long time and that more research is needed on how to best support work resumption for this patient group.
Our study suffered from high drop-out rates, which may raise questions regarding the feasibility of the interventions in a clinical setting. Therefore, adherence to treatment is important to consider. Previous interventional studies involving this patient group have struggled with similar issues (Sonntag-Ostrom et al., Citation2015; Stenlund et al., Citation2012) and since the core symptom of ED is a severe lack of energy, adding extra elements to stress rehabilitation may be too strenuous for some patients. Additionally, the MMR program includes the acquisition of strategies for prioritizing activities, which may have counteracted study participation. As stated above, the most common reason for dropping out was that participating in the MMR took a lot of effort and that the added interventions, as well as the extensive study assessments, was experienced as too demanding. Importantly, our drop-out analysis revealed few differences between those who completed the one-year follow-up and those who dropped out, suggesting that discontinued study participation was not related to symptom levels. Furthermore, relatively high drop-out rates were seen also in the control group, indicating that withdrawn study involvement was not exclusively related to the added interventions per se. Nevertheless, careful consideration needs to be taken to the timing of added interventions in relation to other rehabilitative measures and in explicitly supporting patients to adhere to treatment. Adherence to behavioral change is often a challenge (Middleton, Anton, & Perri, Citation2013) and it is possible that the participants in our study may have benefitted from support in incorporating the added training into their everyday life. An interesting venue for future research may be to investigate motivational factors and predictors for adherence, as well as the possibility to provide more individually tailored interventions.
Some limitations of this study need to be addressed. As discussed above, the study suffered from high drop-out rates, which may limit the generalizability of our results. However, a strength of the study is the randomized controlled design, only including patients with confirmed diagnosis of ED in a clinical setting, speaking in favor of the applicability of the results in the treatment of this patient group. In this context, it is also worth noting that the majority of the participants in our study were female; however, this is in line with demonstrations that women are over-represented in clinical ED populations (e.g. Glise et al., Citation2012). Additionally, our sample size was small and the study suffered from low power, especially considering the well-controlled study design (all patients participated in stress rehabilitation as their primary treatment during the study period). The power calculation was based on immediate training gains for the cognitive training criterion task; however, effects on non-trained tasks at one year follow-up would arguably be expected to be smaller. Also, no power calculation was conducted in relation to aerobic training. In order to avoid making a Type II error, we chose not to correct our results for multiple comparisons. In light of these limitations, the results from this initial study need to be replicated in a larger study group. Finally, our study did not use a placebo control and blinding of participants to group assignment was not possible. Thus, we cannot exclude the possibility that motivational and expectancy effects may have influenced the results. However, all groups participated in MMR during the intervention period, making the beneficial effects observed in our study less likely to be related to factors such as stress recovery or social contact.
Conclusions
In conclusion, our results provide evidence that cognitive training has long-term effects on cognitive functioning in patients with ED, whereas the effects of a limited time period of aerobic exercise on cognition may be more restricted. Considering that relatively mild cognitive impairments are a prominent concern in this patient group, with impact on everyday life, the fact that cognitive training was successful in giving maintained effects on cognition, albeit small, is encouraging. However, careful consideration of the timing of added interventions in relation to other stress rehabilitative measures is warranted, as are rehabilitative strategies for supporting patients with ED to adhere to training and to incorporate activities that may benefit cognitive functioning into their daily life.
Gavelin_et_al._Supplementary_Material_revised.docx
Download MS Word (28.1 KB)Acknowledgements
We thank Petra Sandberg for her valuable contribution to the development of the tasks included in the cognitive training program used in this study, Tony Qwillbard for programming the Internet-based program and Leif Nilsson for statistical consultation. Also, we thank Maja Agnemo, Ingela Aronsson, Erik Karlsson, Sofia Levin, Sofia Nording and Maud Steneberg for their excellent help in data collection. And finally we sincerely thank all the patients for devoting time and effort to participate in the RECO-study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Astrand, P.O., & Ryhming, I. (1954). A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. Journal of Applied Physiology, 7, 218–221. doi: 10.1152/jappl.1954.7.2.218
- Beurskens, A.J.H.M., Bültmann, U., Kant, I., Vercoulen, J.H.M.M., Bleijenberg, G., & Swaen, G.M.H. (2000). Fatigue among working people: Validity of a questionnaire measure. Occupational and Environmental Medicine, 57, 353–357. doi: 10.1136/oem.57.5.353
- Buschke, H. (1973). Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior, 12, 543–550. doi: 10.1016/S0022-5371(73)80034-9
- Cullen, K.L., Irvin, E., Collie, A., Clay, F., Gensby, U., Jennings, P.A., … Amick, B.C. (2017). Effectiveness of workplace interventions in return-to-work for musculoskeletal, pain-related and mental health conditions: An update of the evidence and messages for practitioners. Journal of Occupational Rehabilitation, 28, 1–15. doi: 10.1007/s10926-016-9690-x
- Dahlin, E., Nyberg, L., Bäckman, L., & Neely, A.S. (2008). Plasticity of executive functioning in young and older adults: Immediate training gains, transfer, and long-term maintenance. Psychology and Aging, 23, 720–730. doi: 10.1037/a0014296
- de Zwart, B.C.H., Frings-Dresen, M.H.W., & van Duivenbooden, J.C. (2002). Test-retest reliability of the Work Ability Index questionnaire. Occupational Medicine, 52, 177–181. doi: 10.1093/occmed/52.4.177
- Delis, D.C., Kaplan, E., & Kramer, J.H. (2001). Delis-Kaplan Executive Functioning System (D-KEFS). San Antonio: The Psychological Corporation.
- Dureman, I., Kebbon, L., & Österberg, E. (1971). Manual till DS-batteriet [Manual for the DS-battery]. Stockholm: Psykologiförlaget.
- Eskildsen, A., Andersen, L.P., Pedersen, A.D., & Andersen, J.H. (2016). Cognitive impairments in former patients with work-related stress complaints – one year later. Stress, 19, 559–566. doi: 10.1080/10253890.2016.1222370
- Eskildsen, A., Andersen, L.P., Pedersen, A.D., Vandborg, S.K., & Andersen, J.H. (2015). Work-related stress is associated with impaired neuropsychological test performance: A clinical cross-sectional study. Stress, 18, 198–207. doi: 10.3109/10253890.2015.1004629
- Eskilsson, T., Slunga Järvholm, L., Malmberg Gavelin, H., Stigsdotter Neely, A., & Boraxbekk, C.-J. (2017). Aerobic training for improved memory in patients with stress-related exhaustion: A randomized controlled trial. BMC Psychiatry, 17, 322. doi: 10.1186/s12888-017-1457-1
- Feingold, A. (2009). Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods, 14, 43–53. doi: 10.1037/a0014699
- Gavelin, H.M., Boraxbekk, C.-J., Stenlund, T., Järvholm, L.S., & Neely, A.S. (2015). Effects of a process-based cognitive training intervention for patients with stress-related exhaustion. Stress, 18, 578–588. doi: 10.3109/10253890.2015.1064892
- Glise, K., Ahlborg, G., & Jonsdottir, I.H. (2012). Course of mental symptoms in patients with stress-related exhaustion: Does sex or age make a difference? BMC Psychiatry, 12, 18. doi: 10.1186/1471-244X-12-18
- Glise, K., Hadzibajramovic, E., Jonsdottir, I.H., & Ahlborg, G. (2010). Self-reported exhaustion: A possible indicator of reduced work ability and increased risk of sickness absence among human service workers. International Archives of Occupational and Environmental Health, 83, 511–520. doi: 10.1007/s00420-009-0490-x
- Grossi, G., Perski, A., Osika, W., & Savic, I. (2015). Stress-related exhaustion disorder – clinical manifestation of burnout? A review of assessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scandinavian Journal of Psychology, 56, 626–636. doi: 10.1111/sjop.12251
- Hötting, K., Reich, B., Holzschneider, K., Kauschke, K., Schmidt, T., Reer, R., … Röder, B. (2012). Differential cognitive effects of cycling versus stretching/coordination training in middle-aged adults. Health Psychology, 31, 145–155. doi: 10.1037/a0025371
- Hötting, K., Schauenburg, G., & Röder, B. (2012). Long-term effects of physical exercise on verbal learning and memory in middle-aged adults: Results of a one-year follow-up study. Brain Sciences, 2, 332–346. doi: 10.3390/brainsci2030332
- Jonsdottir, I.H., Nordlund, A., Ellbin, S., Ljung, T., Glise, K., Währborg, P., & Wallin, A. (2013). Cognitive impairment in patients with stress-related exhaustion. Stress, 16, 181–190. doi: 10.3109/10253890.2012.708950
- Jonsdottir, I.H., Nordlund, A., Ellbin, S., Ljung, T., Glise, K., Währborg, P., … Wallin, A. (2017). Working memory and attention are still impaired after three years in patients with stress-related exhaustion. Scandinavian Journal of Psychology, 58, 504–509. doi: 10.1111/sjop.12394
- Lautenschlager, N.T., Cox, K.L., Flicker, L., Foster, J.K., van Bockxmeer, F.M., Xiao, J., … Almeida, O.P. (2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease. JAMA, 300, 1027–1037. doi: 10.1001/jama.300.9.1027
- Maslach, C., Schaufeli, W.B., & Leiter, M.P. (2001). Job Burnout. Annual Review of Psychology, 52, 397–422. doi: 10.1146/annurev.psych.52.1.397
- Melamed, S., Kushnir, T., & Shirom, A. (1992). Burnout and risk factors for cardiovascular diseases. Behavioral Medicine (Washington, D.C.), 18, 53–60. doi: 10.1080/08964289.1992.9935172
- Melby-Lervåg, M., Redick, T.S., & Hulme, C. (2016). Working memory training does not improve performance on measures of intelligence or other measures of “far transfer.” Perspectives on Psychological Science, 11, 512–534. doi: 10.1177/1745691616635612
- Middleton, K.R., Anton, S.D., & Perri, M.G. (2013). Long-term adherence to health behavior change. American Journal of Lifestyle Medicine, 7, 395–404. doi: 10.1177/1559827613488867
- Miyake, A., Friedman, N.P., Emerson, M.J., Witzki, A.H., Howerter, A., & Wager, T.D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi: 10.1006/cogp.1999.0734
- Ohman, L., Nordin, S., Bergdahl, J., Slunga Birgander, L., & Stigsdotter Neely, A. (2007). Cognitive function in outpatients with perceived chronic stress. Scandinavian Journal of Work, Environment & Health, 33, 223–232. doi: 10.5271/sjweh.1131
- Oosterholt, B.G., Maes, J.H.R., Van der Linden, D., Verbraak, M.J.P.M., & Kompier, M.A.J. (2014). Cognitive performance in both clinical and non-clinical burnout. Stress (Amsterdam, Netherlands), 17, 400–409. doi: 10.3109/10253890.2014.949668
- Oosterholt, B.G., Maes, J.H.R., Van der Linden, D., Verbraak, M.J.P.M., & Kompier, M.A.J. (2016). Getting better, but not well: A 1.5 year follow-up of cognitive performance and cortisol levels in clinical and non-Clinical burnout. Biological Psychology, 117, 89–99. doi: 10.1016/j.biopsycho.2016.02.009
- Oosterholt, B.G., Van der Linden, D., Maes, J.H., Verbraak, M.J., & Kompier, M.A. (2012). Burned out cognition – cognitive functioning of burnout patients before and after a period with psychological treatment. Scandinavian Journal of Work, Environment & Health, 38, 358–369. doi: 10.5271/sjweh.3256
- Österberg, K., Skogsliden, S., & Karlson, B. (2014). Neuropsychological sequelae of work-stress-related exhaustion. Stress, 17, 59–69. doi: 10.3109/10253890.2013.862615
- Pereira, A.C., Huddleston, D.E., Brickman, A.M., Sosunov, A.A., Hen, R., McKhann, G.M., … Small, S.A. (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences, 104, 5638–5643. doi: 10.1073/pnas.0611721104
- Persson, R., Österberg, K., Viborg, N., Jönsson, P., & Tenenbaum, A. (2017). Two Swedish screening instruments for exhaustion disorder: Cross-sectional associations with burnout, work stress, private life stress, and personality traits. Scandinavian Journal of Public Health, 45, 381–388. doi: 10.1177/1403494817696182
- Raven, J., Raven, J.C., & Court, J.H. (1998). Manual for Raven’s progressive matrices and vocabulary scales. Oxford: Oxford Psychologists Press.
- Sandberg, P., & Stigsdotter Neely, A. (2016). Long-term effects of executive process training in young and old adults. Neuropsychological Rehabilitation, 26, 761–782. doi: 10.1080/09602011.2015.1108205
- Schmiedek, F., Lövdén, M., & Lindenberger, U. (2014). Younger adults show long-term effects of cognitive training on broad cognitive abilities over 2 years. Developmental Psychology, 50, 2304–2310. doi: 10.1037/a0037388
- Schulz, K.F., Altman, D.G., & Moher, D. (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Medicine, 8, 18. doi: 10.1186/1741-7015-8-18
- Sonntag-Ostrom, E., Nordin, M., Dolling, A., Lundell, Y., Nilsson, L., & Jarvholm, L.S. (2015). Can rehabilitation in boreal forests help recovery from exhaustion disorder? The randomised clinical trial ForRest. Scandinavian Journal of Forest Research, 30, 732–748. doi: 10.1080/02827581.2015.1046482
- Stenlund, T., Nordin, M., & Järvholm, L. (2012). Effects of rehabilitation programmes for patients on long-term sick leave for burnout: A 3-year follow-up of the REST study. Journal of Rehabilitation Medicine, 44, 684–690. doi: 10.2340/16501977-1003
- Swedish Social Insurance Agency. (2017). Psykiatriska diagnoser. Korta analyser 2017:1 [Psychiatric diagnoses. Short analyses 2017:1] Stockholm: Försäkringskassan. 6 p. [In Swedish].
- van Dam, A., Keijsers, G.P.J., Eling, P.A.T.M., & Becker, E.S. (2012). Impaired cognitive performance and responsiveness to reward in burnout patients: Two years later. Work & Stress, 26, 333–346. doi: 10.1080/02678373.2012.737550
- Wechsler, D. (1981). Wechsler Adult Intelligence Scale – revised manual. San Antonio: The Psychological Corporation.
- Wechsler, D. (1997). Wechsler Adult Intelligence Scale – third edition. San Antonio: The Psychological Corporation.
- Wiegner, L., Hange, D., Björkelund, C., & Ahlborg, G. (2015). Prevalence of perceived stress and associations to symptoms of exhaustion, depression and anxiety in a working age population seeking primary care – An observational study. BMC Family Practice, 16, 8. https://doi.org/10.1186/s12875-015-0252-7
- Zigmond, A.S., & Snaith, R.P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67, 361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x
- Zinke, K., Zeintl, M., Rose, N.S., Putzmann, J., Pydde, A., & Kliegel, M. (2014). Working memory training and transfer in older adults: Effects of age, baseline performance, and training gains. Developmental Psychology, 50, 304–315. doi: 10.1037/a0032982