Abstract
Social stress occurs in all social species, including humans, and shape both mental health and future interactions with conspecifics. Animal models of social stress are used to unravel the precise role of the main stress system – the HPA axis – on the one hand, and the social behavior network on the other, as these are intricately interwoven. The present review aims to summarize the insights gained from three highly useful and clinically relevant animal models of psychosocial stress: the resident-intruder (RI) test, the chronic subordinate colony housing (CSC), and the social fear conditioning (SFC). Each model brings its own focus: the role of the HPA axis in shaping acute social confrontations (RI test), the physiological and behavioral impairments resulting from chronic exposure to negative social experiences (CSC), and the neurobiology underlying social fear and its effects on future social interactions (SFC). Moreover, these models are discussed with special attention to the HPA axis and the neuropeptides vasopressin and oxytocin, which are important messengers in the stress system, in emotion regulation, as well as in the social behavior network. It appears that both nonapeptides balance the relative strength of the stress response, and simultaneously predispose the animal to positive or negative social interactions.
1. Introduction
An appropriate stress response to environmental and homeostatic challenges, called stressors, shapes the health and life quality of organisms. Since the introduction of the concept of stress and homeostasis by Hans Seyle and Walter Cannon in the early twentieth century (Cannon, Citation1929; Selye, Citation1936), significant efforts have been made to gain a better understanding of how individuals cope with distinct stressors (Engelmann, Landgraf, & Wotjak, Citation2004; Herman et al., Citation2016; McEwen et al., Citation2015; Sapolsky, Citation2015). This is of crucial importance for human health, as an imbalance of the stress response has been strongly associated with various mental disorders, such as burnout, major depression and anxiety disorders, but also somatic conditions, e.g. dermatitis, cardiovascular diseases and increased susceptibility to infection or cancerogenesis (Buckley et al., Citation2009; Buske-Kirschbaum, Geiben, & Hellhammer, Citation2001; Cohen, Janicki-Deverts, & Miller, Citation2007; Reiche, Nunes, & Morimoto, Citation2004).
Animal models, predominantly laboratory rodent models, have given significant scientific insights into the activation of the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis, which are known as the key stress response systems (Ulrich-Lai & Herman, Citation2009). Stressors have been classified in different categories, such as physical or physiological (e.g. exercise, cold, salt loading, pain) versus emotional or psychological (open, novel or elevated, i.e. potentially threatening areas) stressors (Bains, Cusulin, & Inoue, Citation2015; Neumann, Citation2007). To avoid confusion with terms used in human research, others have categorized them based on the neural pathways that activate the HPA axis as systemic versus neurogenic stressors (Engelmann et al., Citation2004). However, some stressors comprise aspects of different categories; thus, a more relevant issue is the manner in which the stressors can be delivered: either acutely or chronically, predictably or unpredictably, in adulthood or early in life.
The physiological and behavioral consequences of stressor exposure have been intensively discussedin numerous papers (Apfelbach, Blanchard, Blanchard, Hayes, & McGregor, Citation2005; Armario, Gavaldà, & Martí, Citation1995; Karandrea, Kittas, & Kitraki, Citation2002; Nagaraja & Jeganathan, Citation1999; Paré & Glavin, Citation1986; Sántha et al., Citation2016). More recently, however, it has been recognized that negative or unstable social interactions are among the strongest and enduring sources of stress in humans (Davidson & McEwen, Citation2012; Tost, Champagne, & Meyer-Lindenberg, Citation2015; Wood & Bhatnagar, Citation2015). Prolonged exposure to a psychosocial stressor in adulthood, such as being abused by a partner, living in a socially unstable environment or exposure to bullying or harassment, is a risk factor to develop major depressive , anxiety (e.g. social anxiety disorder, panic disorder) and post-traumatic stress disorders (Arseneault, Citation2017; Campbell, Citation2002; Coker et al., Citation2002; Hansen et al., Citation2006; Modecki, Minchin, Harbaugh, Guerra, & Runions, Citation2014). These effects are even more devastating, if persistent social stress occurs during formative developmental windows, such as early childhood and puberty, as it not only impairs the development of normal social functioning, but also affects the basic layout of the stress system, with severe long-term effects on health and quality of life (Bowlby, Citation1951; Carpenter, Shattuck, Tyrka, Geracioti, & Price, Citation2013; Nelson et al., Citation2007; Nemeroff, Citation2016).
Understanding the causes and effects of social stress becomes increasingly important, as traditional social structures are progressively replaced with technology such as social media, especially among adolescents, with the appearance of novel types of social stress, such as cyberbullying (Hinduja & Patchin, Citation2010; Kowalski, Giumetti, Schroeder, & Lattanner, Citation2014; Tokunaga, Citation2010). Moreover, treatments that specifically alleviate social symptoms, such as social avoidance and social withdrawal up to social fear, are limited partly due to the lack of detailed knowledge about the neuronal pathways involved in the perception of social stressors and the neuronal adaptations as consequence of psychosocial stress. These pathways are likely to be similar to, but also partly different from, those involved in nonsocial stress responses, as complex and reciprocal neuroanatomical links exist between the backbone of the stress system (the HPA axis) and the social behavior network (Baribeau & Anagnostou, Citation2015). Two of the main players modulating HPA axis activity as well as the social behavior network are the nonapeptides oxytocin (OXT) and arginine vasopressin (AVP; see a detailed description in Section 2).
In most mammals, positive social interactions are key to survival and evolutionary fitness starting with the essential parental care to survive the early stages of life to affiliation with peers for communal defense of resources or defense against predators, thermoregulation and social learning, sexual interactions with an opposite-sex partner to attain reproductive success, and pair-bonding (Dunbar & Shultz, Citation2007; Neumann, Citation2009). However, there is a significant “dark side” of social interactions, occurring in unavoidable aversive social encounters seen during the fight for mating partners among males or for dominant positions in a given social hierarchy, referred to as social stressors. Similar to animal models of nonsocial stress, models of acute, repeated or chronic social stress (see ) have been established in order to unravel their neurobiological causes and physiological and behavioral consequences (for review see Blanchard, McKittrick, & Blanchard, Citation2001; DeVries, Glasper, & Detillion, Citation2003; Koolhaas, DeBoer, Buwalda, & Meerlo, Citation2017; Reber, Citation2012; Sandi & Haller, Citation2015; Tamashiro, Nguyen, & Sakai, Citation2005).
Table 1. Animal models of social stress and their impact on social interactions and nonapeptide systems (OXT/AVP).
In the course of establishing an animal model of social stress it has to be considered that the expected physiological and behavioral consequences are subjected to individual variability. Thus, individuals may be relatively susceptible or resilient to a given stressor. Pronounced heterogeneity within experimental groups can be a challenge in some scientific set-ups; however, more recently investigators have started to embrace individual differences in animal models as a relevant translational tool to identify factors that lead to susceptibility or resilience (Russo, Murrough, Han, Charney, & Nestler, Citation2012).
In the present review, we will summarize what has been learned so far from three important animal models of social stress: the resident-intruder (RI) test (Section 3: The stress of losing…and winning), chronic subordinate colony housing (CSC, Section 4: Who’s the boss?), and social fear conditioning (SFC, Section 5: The shocking truth about social fear). We especially focus on the role of the HPA axis, the neuropeptides AVP and OXT, and how social interactions are affected by social stressors. However, we do not discuss animal models based on the absence of positive social interactions, such as maternal separation (for parental neglect), loss of a bonded partner (for grief), or general social isolation (for loneliness). While social deprivation is indeed an important form of social stress, we refer the reader to excellent reviews on these models (Cacioppo, Cacioppo, Capitanio, & Cole, Citation2015; Koolhaas et al., Citation2011; Nemeroff, Citation2016; Palanza, Gioiosa, & Parmigiani, Citation2001; Pohl, Young, & Bosch, Citation2018; Valzelli, Citation1973; Veenema, Citation2012).
2. Neurobiological substrates underlying social stress
Organisms are exposed to various positive or negative social experiences with the latter resulting in disturbance of the emotional homeostasis and activation of the stress response coordinated by the brain. After perception of the stressor, stress-induced activation of the HPA axis is initiated by neurons of the hypothalamic paraventricular nucleus (PVN) synthesizing corticotropin-releasing hormone (CRH), which reaches the anterior pituitary via portal blood capillaries of the pituitary stalk. It binds to CRH receptors type 1 (CRHR1) expressed by adenohypophysial corticotrope cells (de Kloet, Joëls, & Holsboer, Citation2005). Activation of CRHR1 and subsequent intracellular signaling cascades involving cAMP (Neumann et al., Citation1998) leads to the release of the adrenocorticotropic hormone (ACTH) into systemic circulation. ACTH then binds to melanocortin-2 receptors in the cortex of adrenal glands, and after activating cholesterol based synthesis pathways promotes the release of glucocorticoids (GC). Glucocorticoids, i.e. cortisol in humans and corticosterone (CORT) in rodents, ultimately orchestrate multiple peripheral effects, promote the recruitment of energy resources and modulate the immune system (Bartolomucci, Citation2007; Webster Marketon & Glaser, Citation2008). As steroids, GC can cross the blood-brain barrier and exert a negative feedback on hippocampal, hypothalamic and pituitary structures to terminate the HPA axis response, thus helping the organism to return to basal homeostasis (for review see Herman et al., Citation2016).
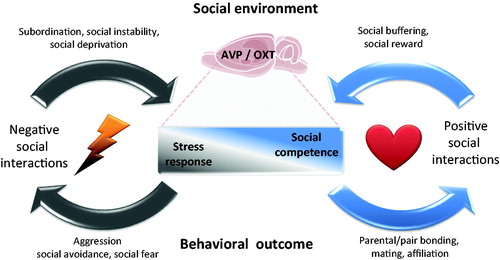
Figure 1. Dynamic interplay between social environment and brain networks mediates stress and social responses. Individual differences in brain oxytocin (OXT) and vasopressin (AVP) systems modulate the levels of stress response and social competence. Negative social interactions, like subordination, social instability or social isolation, trigger a stress response that consequently reinforces negative social behaviors such as aggression or social avoidance, whereas positive social interactions like mating, affiliation and pair bonding, strengthen social competence acting as a social reward and a social stress buffer. A balance between these two circuits is crucial for the wellbeing of social species. Brain image credit: https://openclipart.org/detail/18175/rat-brain..
In parallel, the OXT and AVP systems become activated in a stressor-specific way (Engelmann et al., Citation2004; Jurek & Neumann, Citation2018; Neumann, Citation2007). Magnocellular OXT neurons located in the PVN and the supraoptic nucleus (SON) of the hypothalamus respond with the secretion of OXT from neurohypophysial terminals into the bloodstream in response to nonsocial stressors (exercise, osmotic stress) and reproduction-related social interactions, such as mating and maternal-offspring interacting during nursing (see review in Jurek & Neumann, Citation2018). However, in response to social stressors, OXT secretion into blood remains largely unchanged, but limited data mainly monitored in rodents exist in this context (Neumann, Citation2007). Curiously, AVP is not secreted into the blood during exposure to stressors, except those resulting in increased plasma osmolarity, such as exercise (Landgraf, Häcker, & Buhl, Citation1982; Wotjak et al., Citation1996).
The intracerebral release of these neuropeptides occurs in a strict brain region- and stimulus-dependent manner. OXT and AVP can be released in a somato-dendritic fashion within the PVN and SON, or by axons projecting to limbic areas, such the central amygdala and hippocampus, which are recognized areas for modulating the stress response (Hernández et al., Citation2016; Knobloch et al., Citation2012; Neumann, Citation2007). For instance, exposure to social defeat selectively activates AVP release within the PVN, but not SON (Wotjak et al., Citation1996), while OXT release was found within the SON, but not PVN (Engelmann, Ebner, Landgraf, Holsboer, & Wotjak, Citation1999). Curiously, the intra-PVN release of AVP can be observed mainly in animals with active coping styles (Ebner, Wotjak, Landgraf, & Engelmann, Citation2005). Thus, the context of social interaction is relevant for activating the AVP system.
OXT and AVP are not merely stress-related neuropeptides, however, as they are well known for their important role in social interactions (for reviews see Baribeau & Anagnostou, Citation2015; Bosch & Neumann, Citation2012; Johnson & Young, Citation2017; Lukas & Neumann, Citation2013; Neumann & Landgraf, Citation2012; Jurek & Neumann, Citation2018). Social stimuli may vary from positive (e.g. social support, social reward, and social pleasure) to negative (e.g. social stress) valences (MacDonald & Feifel, Citation2014). As both OXT and AVP systems have been linked with some aspects of sensory cue processing (Albers, Citation2012; Eliava et al., Citation2016; Mitre et al., Citation2016; Tobin et al., Citation2010), they may, therefore, play an important role to integrate sensory social signals, to determine the valence of social stimuli and, thus, the social behavior response.
There is accumulating evidence for the connectivity of OXT and AVP hypothalamic neurons with areas of the social behavior network. The social brain network initially described by Newman (Citation1999) includes the lateral septum (LS), preoptic area, ventromedial hypothalamus, anterior hypothalamus, the periaqueductal gray/central gray, the medial amygdala (MeA) and bed nucleus of the stria terminalis (BNST) (O’Connell & Hofmann, Citation2011). For example, OXT neurons of the SON and PVN innervate the BNST, amygdala and LS (Ingram & Moos, Citation1992; Knobloch, Grinevich, & Dabrowska, Citation2014; Knobloch et al., Citation2012) (for review see Jurek & Neumann, Citation2018). With regard to AVP, AVP neurons project from the BNST and MeA to the LS, in several species except in Syrian hamsters (Dubois-Dauphin, Pevet, Tribollet, & Dreifuss, Citation1990) (reviewed in Albers, Citation2012).
AVP and OXT exert their effects via activation of their respective receptors, i.e. the V1a receptor, the V1b receptor, and the OXT receptor (OXTR), respectively, that are expressed in many brain regions (Johnson & Young, Citation2017; Jurek & Neumann, Citation2018). Due to their widespread expression in the brain, especially in regions that are involved in social behavior, OXT and AVP play a critical role in the output of these brain regions that eventually determine social behavior (for review see Caldwell, Citation2017). Nevertheless, the neuropeptide receptor distribution depends on the age, sex and species (Dumais & Veenema, Citation2016) (for a detailed review see Johnson & Young, Citation2017; Jurek & Neumann, Citation2018), which partly underlie the diverse social behavioral repertoire.
In turn, the social behavior network is also connected to the PVN, which may provide a putative link to explain how social interactions modulate the stress response . Some components of the social network, such as the anterior BNST, have been found to modulate the activity of CRH and OXT neurons of the PVN (Champagne, Beaulieu, & Drolet, Citation1998). Conversely, other components of the social behavior network inhibit the CRH activity via GABAergic inputs, such as the medial preoptic area, the posterior areas of the BNST, the MeA and the LS (reviewed in Ulrich-Lai & Herman, Citation2009). OXT and AVP are, therefore, in a unique position to affect social behavior in response to stress and to influence the stress response in social settings.
In this regard, the OXT system is one of the putative mediators of the so-called “social stress-buffering” effect (Gunnar & Hostinar, Citation2015). OXT is essential for, and activated by, social interactions resulting in elevated OXT release within the brain. Such locally released OXT was found to reduce anxiety levels and the HPA axis responsiveness as found, for example, in mother–offspring interactions (Slattery & Neumann, Citation2008), or in response to sexual activity (Waldherr & Neumann, Citation2007; Waldherr, Nyuyki, Maloumby, Bosch, & Neumann, Citation2010). The same stress dampening effect of OXT has been found in virgin female and male rats (Neumann, Kromer, Toschi, & Ebner, Citation2000; Windle et al., Citation2004). Additionally, female voles that were allowed to have social contact with their male partner did not show an elevated CORT level after being stressed by immobilization compared to single-housed females, an effect which was prevented by application of an OXTR antagonist (Smith & Wang, Citation2014). These findings strongly suggest OXT and AVP as key elements in the bidirectional regulation, in which stress and social circuitries of the brain are continuously shaped by social experiences .
3. The stress of losing and winning: resident-intruder (RI) test
Perhaps the most obvious example of an acute negative and stressful social interaction is fighting with a conspecific. Aggressive behavior is an important part of the social behavior repertoire found throughout the animal kingdom, from solitary living species to species with intricate social hierarchies (Blanchard et al., Citation2001). It is a crucial tactic to obtain and protect essential resources for survival and reproduction such as food, water, group members, offspring, or sexual partners – especially in times of scarcity (Lukas & de Jong, Citation2017; Nelson & Trainor, Citation2007).
Although aggression between conspecifics has been studied in various animal species for more than a century by ethologists, more recently biological psychiatrists and neuroscientists have started to unravel the mechanisms underlying aggression, especially to understand this behavior in humans. These efforts have resulted in a reliable and consistent paradigm in laboratory rats and mice with established face and construct validity: the RI test (Albert, Dyson, & Walsh, Citation1988; Koolhaas et al., Citation2013; Miczek, Maxson, Fish, & Faccidomo, Citation2001; Neumann, Veenema, & Beiderbeck, Citation2010). The RI test relies on the tendency of rodents to protect their territory against unfamiliar intruders and follows the same basic principle in most studies: the release of a smaller and weaker intruder into the home cage of a larger resident for 10–20 min. Observation of the resident allows for the quantification of behaviors, such as threatening postures and (in mice) tail rattles that signal the preparation of attack bites. Residents also show a series of dominant behaviors, such as keep-down, lateral threat, and offensive up-right. Observation of the intruder allows for the quantification of submissive behaviors, such as defensive up-right and freezing as a signal of defeat (Laman-Maharg & Trainor, Citation2017; Sandi & Haller, Citation2015). It is important to notice that the level of aggression displayed by rodents in the RI test not only differs between species (with rats acting relatively gregariously), but also between individuals as a factor of seasonal changes, physiological status, housing condition, genetic background and stress susceptibility (Calcagnoli et al., Citation2014; Neumann et al., Citation2010; Oliva et al., Citation2010; Tulogdi et al., Citation2014; Walker, Sandi, Walker, & Sandi, Citation2018; Walker, Zanoletti, Guillot de Suduiraut, & Sandi, Citation2017; Yang et al., Citation2017).
Although the RI paradigm shows a great translational capacity, the fact that conflicts in humans partially consist of verbal aggression is undeniable and, unfortunately, this cannot be addressed in animal models. Nevertheless, the border between verbal and physical aggression is not well defined, as verbal aggression may escalate to physical aggression. In addition, some papers have already shown that physical aggression is the main trigger to generate severe levels of psychopathology in the victims (Caspi et al., Citation2002; Dackis et al., Citation2015). Another issue, which still needs to be addressed, is the translational value of pathological aggression models. Both the criteria used to define abnormal aggression in animal models as well as the neurobiology underlying this type of aggression may differ in rodents and humans (for review see Haller, Citation2013, Citation2016).
The spontaneous and easily elicited behaviors displayed during the RI test enable the detailed investigation of the neurobiological underpinnings of the phenomenon of social defeat and social aggression. Several studies have focused on HPA axis activation, especially in defeated individuals, and found that acute social defeat by the dominant defeater is a stressor resulting in markedly increased plasma CORT and ACTH levels as well as an up-regulation of CRH mRNA in the PVN (Huhman, Bunnell, Mougey, & Meyerhoff, Citation1990; Keeney et al., Citation2006). In rats, social defeat triggered increased c-fos expression in brain regions involved in the stress response, such as the PVN, central amygdala and brainstem, but also in regions of the social behavior network, like the BNST, LS, MeA and lateral hypothalamus (Martinez, Phillips, & Herbert, Citation1998). As discussed in Section 2, these regions are well known to express OXTR and V1a receptors. In fact, exposure to an aggressive resident leads to OXT, but not AVP, release in the medial part of the LS in male rats (Ebner, Wotjak, Landgraf, & Engelmann, Citation2000), a pivotal region for the expression of social behaviors, such as social memory (Lukas, Bredewold, Landgraf, Neumann, & Veenema, Citation2011), aggression (Wong et al., Citation2016) and social avoidance (Zoicas, Slattery, & Neumann, Citation2014). A single defeat also activated magnocellular OXT neurons in the PVN of male Peromyscus californicus mice, which might be the source of septal OXT release (Steinman et al., Citation2016). Furthermore, acute exposure to a dominant aggressive encounter generated social avoidance of the defeated animal in both male and female rats; treatment with intracerebroventricular (i.c.v.) OXT was able to rescue social defeat induced social avoidance at least in males (Lukas, Toth, et al., Citation2011; Lukas & Neumann, Citation2014).
A more complex link between the HPA axis and aggression emerges in the dominant residents. In humans, goal-oriented instrumental aggression as seen in patients with conduct disorder (in children) and antisocial personality disorder (in adults) is associated with hypo-arousal features, such as low cortisol levels, low heart rate and low skin conductance (Haller, Citation2016; Nelson & Trainor, Citation2007). Abnormal reactive aggression, expressed on impulse in individuals with intermittent explosive disorder is associated with hyper-arousal features (Haller, Citation2013, Citation2016; Nelson & Trainor, Citation2007).
Using the RI test, evidence for pro-aggressive effects of both stress hypo- and hyper-responsivity has been confirmed in rodents. A striking finding in this context is that surgical removal of the adrenals by adrenalectomy (ADX) generated abnormal aggression and decreased social interaction in male Wistar rats reminiscent of hypo-arousal-associated instrumental aggression in humans (Haller, Halász, Mikics, & Kruk, Citation2004). In contrast, acute inhibition of GC synthesis with metyrapone (which stimulates ACTH secretion) decreased aggressiveness, and intraperitoneal and i.c.v. treatment with CORT increased aggression in male rats (Haller et al., Citation2004). These data show that a normal functioning of the HPA axis is necessary for aggression in rats, whereas the total absence of CORT leads to abnormal aggression.
Manipulating the stress system during adolescence, an important developmental window defined by the maturation of several hormonal systems including the HPA axis as well as the transition from play-fighting to serious offensive behavior (Bredewold, Smith, Dumais, & Veenema, Citation2014; Veenema, Bredewold, & De Vries, Citation2013; Wommack, Salinas, & Delville, Citation2005), profoundly affects aggression in adulthood. For example, preventing the normal surge in plasma CORT by chronic dexamethasone treatment during adolescence reduced play-fighting and increased aggression in young adult male golden hamsters (Wommack et al., Citation2005). In addition, repeated exposure to peripubertal stress (PPS) resulted in exaggerated and abnormal aggression in both male and female rats (Cordero, Ansermet, & Sandi, Citation2013; Marquez et al., Citation2013; Tzanoulinou, Riccio, de Boer, & Sandi, Citation2014). PPS-rats also showed a higher CORT to testosterone ratio after exposure to the RI test. This leads to the hypothesis that hyperactivity (or perhaps: hyperstimulation) of the HPA axis during adolescence generates pathological aggressive behavior in adulthood. Indeed, rats treated with CORT during the peripubertal period also presented exacerbated aggressiveness (Veenit, Cordero, Tzanoulinou, & Sandi, Citation2013). Similarly, when juvenile rats were assigned to three groups based on their endogenous low, intermediate and high CORT responses to stress and then outbreed for at least four generations, the resulting offspring not only still displayed the difference in CORT levels to restraint stress, but high CORT offspring also showed increased offensive behavior in the RI test (Walker et al., Citation2017).
These results indicate that manipulating the HPA axis may lead to changes in aggressive behavior. In addition, rodents showing abnormal aggression as a trait also appear to have abnormal HPA axis responsivity. For example, rats selectively bred for low anxiety-related behaviors (LAB) display not only exacerbated, but also abnormal inter-male aggressive behavior, which included the attack of non-receptive females and narcotized male intruders, and of vulnerable targets (throat, head, belly, and paws) (Beiderbeck, Neumann, & Veenema, Citation2007; Beiderbeck et al., Citation2012; Veenema, Torner, Blume, Beiderbeck, & Neumann, Citation2007) (for review see Neumann et al., Citation2010). Compared with non-selected rats LAB rats also showed higher CORT levels under basal conditions and higher ACTH, but not CORT levels in response to the RI test, and this was associated with a greater activation of medial parvocellular neurons in the PVN (Veenema et al., Citation2007). It is possible that increased hypothalamic and pituitary activation combined with a blunted adrenal CORT response during an RI may underlie the abnormal aggression seen in LAB rats. Similarly, mice with a normal circadian CORT release, but a reduced CORT response to stress presented a shorter latency to attack (Touma et al., Citation2008). Mice selectively bred for high aggression, such as the Turku Aggressive and the Short Attack Latency mice, did not show abnormal basal CORT levels, but they had lower heart rates under basal conditions, reminiscent of the hypoarousal phenotype (Caramaschi, de Boer, & Koolhaas, Citation2008).
The neurobiological mechanisms involved in the regulation of aggressive behavior by the HPA axis are still largely unknown. GC receptor (GR) is widely expressed in brain regions involved in aggression such as LS, amygdala, BNST, prefrontal cortex, ventromedial hypothalamus, and the anterior hypothalamus (Pryce, Citation2008; van Eekelen, Bohn, & de Kloet, Citation1991). It is possible that CORT, after being released by the adrenal glands during aggression binds to regional GR to change neuronal activation associated with aggression. Conversely, prolonged absence or abnormal binding of CORT to GR in these brain areas, especially in sensitive periods such as puberty, may alter the neuronal architecture and, thereby, affect aggressive tendencies in adulthood. Indeed, early life stress and ADX are both known to change neuronal activity and plasticity in brain regions associated with aggression (Biro et al., Citation2016; Halász, Tóth, Kalló, Liposits, & Haller, Citation2006; Haller, Harold, Sandi, & Neumann, Citation2014; Kohl et al., Citation2015; Marquez et al., Citation2013; Tzanoulinou et al., Citation2014).
Although both AVP and OXT affect aggressive behavior in various ways (de Jong & Neumann, Citation2017; Lukas & de Jong, Citation2017; Neumann et al., Citation2010) only circumstantial evidence for their role linking the HPA axis and aggression can be found. In high-aggressive LAB rats, the elevated basal CORT levels were accompanied by a reduction in AVP release within the LS during the RI test (Beiderbeck et al., Citation2007; Veenema et al., Citation2007). Also, maternally separated rats showed increased aggressiveness and enhanced CORT response to stress, combined with a blunted release of AVP in the LS during social interactions (Lukas, Bredewold, et al., Citation2011; Veenema, Blume, Niederle, Buwalda, & Neumann, Citation2006). These data point to the LS as a potential brain region to integrate CORT and AVP messages to influence aggressive output.
In addition, central OXT release, as shown within the PVN in response to stress, is inhibited in ADX rats (Torner, Plotsky, Neumann, & de Jong, Citation2017). Since ADX induces abnormal aggression in male rats (as discussed above) and OXT may reduce aggression under some circumstances (Calcagnoli, De Boer, Althaus, De Boer, & Koolhaas, Citation2013; Calcagnoli et al., Citation2014; de Jong, Beiderbeck, & Neumann, Citation2014), a tentative circuit of blunted OXT release during the RI test in ADX rats can be drawn. Clearly, these potential pathways need to be proven in experiments involving all components together.
4. Who’s the boss? The chronic subordinate colony housing (CSC) paradigm
Animal models of chronic stress have traditionally focused on nonsocial challenges such as exposure to light, noise, cold, shaking, or predator cues (reviewed in Willner, Citation1997). However, as the majority of disease-promoting stressful stimuli in humans are of psychosocial nature, chronic stress models, with a psychosocial component such as submission or social exclusion are of higher clinical relevance (Björkqvist, Citation2001). A reliable animal model for studying the consequences of chronic psychosocial stress should, therefore, combine psychological and social aspects to mimic the human situation more accurately. solely Nevertheless, social stress models in rodents are almost exclusively mediated by social defeat that includes physical attacks and injuries. As already mentioned in the previous chapter, it can therefore not be distinguished to which extend the behavioral and physiological consequences of chronic psychosocial stress are due to physical harm or psychosocial stress. Thus, almost all animal models of chronic psychosocial stress bear certain limitations in their translational potential.
In recent years several of such models have been developed, including chronic social defeat in adulthood (Berton et al., Citation2006) or during adolescence (Schmidt et al., Citation2007), the combination of social defeat and overcrowding (Reber, Obermeier, Straub, Falk, & Neumann, Citation2006), as well as CSC (Reber et al., Citation2007). All of these models present face and predictive validity. Despite the fact that they differ in their detailed stress procedure, and in the duration and frequency of stress exposure, the outcome is largely comparable. Thus, exposure to chronic social stress primarily results in reduced body weight gain, thymus atrophy, adrenal hypertrophy, HPA axis dysregulation as well as elevated levels of anxiety-related or depressive-like behavior (Berton, Aguerre, Sarrieau, Mormede, & Chaouloff, Citation1998; Gruver & Sempowski, Citation2008; Heinrichs, Pich, Miczek, Britton, & Koob, Citation1992; Keeney & Hogg, Citation1999; Reber et al., Citation2007; Stefanski & Engler, Citation1999).
The CSC paradigm was developed in our lab as a clinically relevant model of chronic psychosocial stress and gained increasing attention over the last years (Reber et al., Citation2007). It mimics the type of health-compromising and disease-promoting stressors that humans potentially face on a daily basis. The murine model is based on the natural drive of male mice to establish a hierarchical order within their colony. The largest male will emerge as dominant and consequently force the remaining mice into a subordinate position by repeated aggressive attacks and threats especially seen during the first hour of establishing social hierarchies. In detail, in the CSC model, four C57BL/6 mice are introduced into the home cage of a slightly bigger and, consequently, dominant CD1 resident for 19 consecutive days. To counteract habituation to the resident, the experimental mice are transferred to the cage of an unknown dominant resident on day 8 and again on day 15, which adds an unpredictable and uncontrollable component to the model. The behavioral interactions within the colony are analyzed during the first 30 min of each initial confrontation with the resident (i.e. on days 1, 8 and 15) to ensure the hierarchical position of each mouse (Reber & Neumann, Citation2008).
As appropriate same-aged controls, both single housed (SH) as well as group housed (GH) mice have to be considered. We found that GH mice display symptoms of stress similar to CSC mice after 3 weeks indicating that the formation of a social hierarchy is likely even in a same-age, same-weight group of male mice, which is per se stressful in male mice (Singewald, Nguyen, Neumann, Singewald, & Reber, Citation2009). As social isolation does not seem to evoke alterations in stress-related immunological and endocrine parameters in C57BL/6 mice (Kamakura, Kovalainen, Leppäluoto, Herzig, & Mäkelä, Citation2016; Võikar, Polus, Vasar, & Rauvala, Citation2005), but see (Ieraci, Mallei, & Popoli, Citation2016), SH mice were chosen as adequate controls (SHC). After 19 days of exposure to CSC housing, multiple profound and long-lasting physiological and behavioral alterations were found compared to SHC.
Of note, the CSC model has also been established in male Wistar rats. However, the results are far less consistent and reliable compared with mice with respect to physiological, behavioral, and immunological parameters (Nyuyki, Beiderbeck, Lukas, Neumann, & Reber, Citation2012). Moreover, the choice of appropriate controls, i.e. SH or GH rats, is less clear (Ahmed, Stinus, Lemoal, & Cador, Citation1995; Brown & Grunberg, Citation1995; Wallace et al., Citation2009).
Mice subjected to 19 days of CSC show consistent signs of chronic stress such as enlarged adrenals and a disrupted HPA axis activity. While plasma morning ACTH levels are elevated, basal CORT concentrations of CSC mice remain comparable to those of SHC mice (Füchsl, Langgartner, & Reber, Citation2013; Reber et al., Citation2007; Uschold-Schmidt, Nyuyki, Füchsl, Neumann, & Reber, Citation2012; Veenema, Reber, Selch, Obermeier, & Neumann, Citation2008). In contrast, CSC mice are incapable to mount the circadian rise in plasma CORT seen in the evening hours (Reber et al., Citation2007). Also, in response to a mild heterotypic stressor, CSC mice react with exaggerated plasma CORT levels and an increased adrenal CORT content, whereas only a severe acute heterotypic stressor such as 6 min of forced swimming is able to further elevate plasma ACTH levels (Füchsl, Langgartner, et al., Citation2013; Uschold-Schmidt et al., Citation2012).
The enlargement of the adrenals seems to be mediated by cell hyperplasia potentially caused by an enhanced adrenal availability and/or mobilization capacity of cholesterol (Füchsl, Uschold-Schmidt, & Reber, Citation2013). However, stimulation of adrenal explants from CSC mice with ACTH revealed a reduced in vitro CORT response suggesting a reduced ACTH sensitivity of cortical adrenal cells (Reber et al., Citation2007; Uschold-Schmidt et al., Citation2012). Since CSC mice display elevated plasma ACTH levels, and because the decreased adrenal sensitivity for ACTH cannot prevent an exaggerated CORT response to an acute heterotypic stressor, the involvement of additional mechanisms resulting in activated CORT synthesis and/or secretion is likely. In addition to the increased size and functionality of the adrenals, a hyperplasia-mediated elevated capability of the pituitary gland to produce and secrete ACTH after 19 days of CSC was shown. A compromised pituitary negative feedback inhibition seems unlikely, as shown by the dexamethasone suppression test (Füchsl, Langgartner, et al., Citation2013).
Exposure to CSC also alters neuropeptide systems, which may contribute to stress-induced HPA axis adaptations. Thus, decreased AVP mRNA levels in the PVN were found, but the numbers of AVP-positive magnocellular and parvocellular neurons in the PVN as well as the relative expression of V1b in the pituitary remained unchanged (Füchsl, Langgartner, et al., Citation2013; Reber & Neumann, Citation2008). Although the levels of OXT mRNA in the PVN and SON remained comparable between SHC and CSC mice, OXTR binding in the median raphe nucleus was diminished after CSC exposure (Peters, Slattery, Uschold-Schmidt, Reber, & Neumann, Citation2014; Reber & Neumann, Citation2008). Furthermore, CRH mRNA in the PVN remained unchanged after 3 weeks of CSC exposure, while pituitary CRHR1 protein levels were found to be decreased, and the amount of corticotroph cells were increased (Reber et al., Citation2007). The increased amount of corticotroph cells combined with reduced CRHR1, but not V1b expression suggests that these newly formed cells rather express V1b, thereby shifting their sensitivity from CRH to AVP as the main pituitary ACTH secretagogue (Füchsl, Langgartner, et al., Citation2013; Rotondo et al., Citation2016). The detailed involvement of neuropeptides in CSC-induced physiological changes remains to be studied.
Exposure to CSC also leads to a robust and long-lasting (at least 7 days after stressor termination) increase in general anxiety-related behavior (Reber et al., Citation2007; Reber & Neumann, Citation2008; Slattery et al., Citation2012; Uschold-Schmidt et al., Citation2012; Veenema et al., Citation2008). Importantly, the increase in state anxiety was not found to be accompanied by an increase in depressive-like behaviors as assessed in the sucrose preference or forced swim test (Slattery et al., Citation2012). However, other behaviors are also affected. One week after termination of the CSC paradigm, CSC mice display hyperactivity together with elevated ethanol consumption, symptoms that are often found in patients with post-traumatic stress disorder (Peters, Slattery, Flor, Neumann, & Reber, Citation2013; Reber et al., Citation2016; Slattery et al., Citation2012). Another notable behavioral alteration was seen with respect to naturally occurring social preference behavior, as CSC mice showed a lack of preference for a social stimulus (non-familiar conspecific) over a neutral stimulus (empty cage) in the social preference/avoidance test. However, robust symptoms of social anxiety, such as social avoidance, could not be found (Slattery et al., Citation2012). Recently, the importance of brain OXT in social preference behavior of mice and rats has been demonstrated, as a social approach in the social preference/avoidance test was blocked by i.c.v. infusion of an OXT receptor antagonist. Further, i.c.v. OXT facilitated social approach and reversed social defeat-induced lack of social preference (see above) (Lukas, Toth, et al., Citation2011; Lukas & Neumann, Citation2014). Together with the CSC-induced decrease in OXTR binding in the median raphe nucleus, this finding point towards the assumption that (i) OXT signaling is impaired in CSC mice and (ii) OXT may exert a potential role in reversing CSC-induced symptoms. Indeed, chronic i.c.v. infusion of OXT at lower dose during 19 days of CSC using osmotic minipumps partly prevented CSC-induced physiological and behavioral consequences including the CSC-induced increase in anxiety (Peters et al., Citation2014). OXT has been repeatedly shown to exert anxiolytic effects (Neumann, Torner, & Wigger, Citation2000; Neumann & Slattery, Citation2016) also within the median raphe nucleus (Yoshida et al., Citation2009). Although untested, a diminished regional release of OXT as a result of CSC exposure might explain the lack of social preference and increased anxiety-like behavior, as well as the compensatory effects of chronic OXT.
The effects of CSC also seem to depend on genetic and environmental factors. Thus, whereas mice selected for high anxiety-like behavior were comparable to NAB mice in behavioral, physiological, and neuroendocrine parameters measured after CSC exposure, LAB mice appeared rather stress resilient (Füchsl, Neumann, & Reber, Citation2014). Further, early life stress induced by maternal separation enhanced the vulnerability to CSC as indicated by exaggerated negative consequences (Veenema et al., Citation2008). Resilience and susceptibility to the detrimental consequences of chronic stress have drawn increasing attention during the last decades. Various studies have shown a major impact of the individual predisposition on physiological and behavioral responses to stress. It was shown that social hierarchy alone can predict behavioral vulnerability to chronic social defeat (Larrieu, Cherix, Lei, & Gruetter, Citation2017). In the learned helplessness paradigm, Berton et al. (Citation2007) could identify two distinct mouse subpopulations that show either a deficit in escape behavior or a response similar to unstressed mice, therefore termed resilient. Similarly, it was shown that chronic social defeat-induced social avoidance is only displayed in about 50–60% of all tested mice (Krishnan et al., Citation2007) and, in a mouse model of chronic stress during adolescence, persistent alterations in behavior and basal CORT levels were only found in a subset of these mice in adulthood (Schmidt et al., Citation2010). Therefore, in the evaluation and interpretation of chronic stress-induced behavioral and physiological consequences resilience and susceptibility of individuals have to be taken into account (for review see Russo et al., Citation2012; Schmidt, Sterlemann, & Müller, Citation2008).
However, each of these chronic psychosocial stress models has its own limitations with respect to the translational value. Thus, mice subjected to 19 days of CSC are constantly exposed to the dominating resident, forcing the mice into an inescapable situation that cannot, other than in humans, potentially be avoided. Further, as mentioned before, the role of physical injuries remains to be discussed. As in other models of chronic social defeat, the occurrence of bite wounds is uncontrollable, but has a major impact on the consequences of chronic stress. Indeed, it was recently shown that physical injuries appear to be required for specific symptoms of the CSC (Foertsch et al., Citation2017).
Taken together, exposure of male mice to CSC housing as an ethological and clinically relevant model of chronic psychosocial stress provides a powerful experimental tool to study the mechanisms underlying the pathophysiology of stress-induced somatic and psychiatric disorders. For a detailed discussion of the CSC model we refer to an excellent review by Langgartner, Füchsl, Uschold-Schmidt, Slattery, & Reber (Citation2015).
5. The shocking truth about social fear: the social fear conditioning (SFC) paradigm
Social fear can be considered as an adaptive response to stressful conspecific encounters (Gilbert, Citation2001). However, in some cases, a maladaptive response develops in the shape of pathologies, as seen in social anxiety disorder (SAD) patients. SAD is defined by a persistent and exacerbated social fear and avoidance of social situations (American Psychiatric Association, Citation2013). Patients with SAD show a strong physiological stress response, i.e. increased heart rate, in social situations (Elzinga, Spinhoven, Berretty, de Jong, & Roelofs, Citation2010), and a greater neuronal activation of fear-related areas (e.g. amygdala), when exposed to harsh facial expressions (Goldin, Manber, Hakimi, Canli, & Gross, Citation2009).
Various animal models of stress are used to induce lack of social preference or social avoidance, such as chronic social defeat (Berton et al., Citation2006; Slattery et al., Citation2012; Yan, Cao, Das, Zhu, & Gao, Citation2010), foot shock exposure (Haller & Bakos, Citation2002) and maternal separation (Franklin, Linder, Russig, Thony, & Mansuy, Citation2011). However, these models have considerable limitations to study SAD, as the exposed animal also typically displays increased general anxiety- and/or depression-related behaviors and impaired locomotion (Hollis, Wang, Dietz, Gunjan, & Kabbaj, Citation2010), which interfere with the selective investigation of social fear.
Recently, a reliable animal model for SAD with a better face and predictive validity has been established in our lab, the SFC paradigm (Toth, Neumann, & Slattery, Citation2012). The SFC model is based on the classic principle of operant conditioning (Thorndike, Citation1933) using negative reinforcement to enable the formation of an association between a specific behavior and its consequences. The SFC paradigm consists of three phases: during the social fear acquisition phase (day 1), mice are habituated to a conditioning chamber followed by the free exploration of a nonsocial stimulus (a small empty wire-mesh cage). The empty cage is then replaced by an identical wire-mesh cage containing a social stimulus, typically a mouse of the same strain, size and sex. Whereas unconditioned mice are allowed to freely explore the social stimulus, conditioned mice receive a mild electric foot shock (0.7 mA) every time they approach and investigate the social stimulus.
During the extinction phase (day 2), the successful association between the shock and the social stimulus expressed as low social investigation interpreted as social fear is assessed. Social fear extinction is performed in the home cage of the experimental mouse to exclude any unspecific fear of the conditioning box or a novel arena. In detail, first three nonsocial stimuli (empty wire-mesh cages) are consecutively presented to exclude fear of these cages and to provide insights into the fear specificity (e.g. nonsocial vs. social). Thereafter, six different social stimuli are consecutively presented. Although social fear is mainly defined by reduced social investigation time, different types of behaviors can be observed: fear-related behavior (e.g. freezing, defensive burying), social motivation (stretched approaches), and nonsocial behaviors (e.g. exploration, resting, and feeding). Typically, repeated exposure of SFC mice to a series of unknown conspecifics leads to a gradual decline in the fear response.
The extinction of social fear depends on a shift in the balance between social motivation and social fear, a higher social motivation to explore the social stimulus should facilitate social fear extinction. Vice versa, high levels of social fear and low levels of social motivation should delay extinction. Differences in this balance between social motivation and social fear due to genetic and epigenetic factors, early life experience (e.g. maternal separation) and physiological state (e.g. lactation) determine an individual’s social fear resilience or susceptibility (Menon et al., Citation2018; Zoicas & Neumann, Citation2016). Finally, during the extinction-recall phase (day 3), mice are again exposed to another set of six social stimuli. Therefore, the recall phase helps to evaluate the strength of the extinction learning (for a detailed description see Toth, Neumann, & Slattery, Citation2013).
The SFC paradigm allows the specific induction of social fear in mice without affecting any other type of behaviors like general anxiety, depressive-like behavior or locomotion. The SFC-induced social fear can be seen both acutely, i.e. after 24 h, but also up to 15 days after acquisition. Moreover, social fear is sensitive to pharmacotherapy and can be reversed by administration of anxiolytic or antidepressant drugs, such as diazepam or paroxetine (Toth et al., Citation2012), and by OXT (see below). Taken together, the SFC paradigm resembles the main behavioral outcome of SAD and fulfills the criteria of face and predictive validity.
As a model of SAD, however, the SFC paradigm has some limitations. For example, modeling psychosocial symptoms, such as negative self-evaluation, is impossible in rodents. Moreover, the social interactions between the experimental and the stimulus mouse are intentionally simplified, as the stimulus mouse is always enclosed in a small cage. On the one hand, this excludes the possibility to assess active avoidance, whereas, on the other, prevents active interference of the stimulus mouse with the social behavior of the experimental mouse. In addition, the limitation of the physical interaction offers the advantage that social fear is induced without the risk of fight- or attack-induced injuries produced by more naturalistic models, such as social defeat paradigms.
Due to the relatively recent establishment of the SFC paradigm, the contribution of the stress system has not been studied in this model. Specifically, it is still unknown, whether SFC results in elevated activity of hypothalamic CRH neurons and, subsequently, increased plasma ACTH and GC concentrations. Since electric foot shocks strongly increase plasma ACTH and CORT levels in rats and mice (Anisman, Lacosta, Kent, Mcintyre, & Merali, Citation1998; Citation2001), it can be assumed that a similar rise also occurs during social fear acquisition. A similar hormonal stress response may occur in the extinction phase, as conditioned mice are known to be capable to elicit a HPA axis response in anticipation of the stressor, as shown in cued fear conditioning studies (Sarro et al., Citation2014; Ulrich-Lai & Herman, Citation2009). SFC mice indeed show signs of stress including freezing and defensive burying towards the first social stimuli presented during the social fear extinction phase. The stress response may decrease gradually during the repeated presentation of social stimuli (Bhatnagar et al., Citation2002), coinciding with a more relaxed behavioral pattern by the animal.
There is evidence from SAD patients that this psychopathology is accompanied by disturbances in the HPA axis activity (Elzinga et al., Citation2010; Faucher et al., Citation2016; Roelofs et al., Citation2009). In patients with post-traumatic stress disorder, a role of GC as enhancers of fear memory extinction has been described (Singewald et al., Citation2015), and it is likely that a similar pathway underlies SAD. Indeed, a clinical trial with oral cortisone treatment before social stressor exposure improved the outcome of repeated exposure therapy in patients who suffered from social phobia (Soravia et al., Citation2006).
With respect to neuroendocrine mechanisms underlying social fear, the involvement of the OXT system has been investigated in detail. Indeed, OXT was found to play a prominent role in the regulation of social fear in the SFC paradigm. Zoicas et al., (Citation2014) and Menon et al., 2018 showed that i.c.v. OXT infusion before social fear extinction phase increased social investigation of conditioned male and virgin female mice, respectively. Interestingly, this is similar to the rescue of social investigation in socially defeated male rats (Lukas, Toth, et al., Citation2011). So far, an important brain area of OXT-mediated actions appears to be the LS. Notably, increasing the availability of OXT in the LS by local infusions of OXT reversed social fear (Zoicas et al., Citation2014). Moreover, high activation of the septal OXT system, as seen in lactation, results in efficient resilience against social fear conditioning (Menon et al., 2018) However, SFC mice showed an attenuated OXT release within the LS, but an elevated OXTR binding. Social avoidance induced by social defeat was also found to be accompanied by an up-regulated OXTR mRNA in the LS (Litvin et al., Citation2011), again suggesting a common neurobiological pathway of social avoidance observed in both paradigms. In this line, Guzmán et al. (Citation2013) showed that overexpression and knockdown of the OXTR in the LS enhanced and reduced fear-induced freezing responses, respectively, after social defeat.
Altogether, the evidence suggests that the LS region might be an important integrative center during social stress events. Since the LS heavily expresses GR and is involved in social memory (Morimoto et al., Citation1996), it is possible that the elevated CORT levels triggered by electric foot shock (s) during social fear acquisition or by the exposure to the social stimulus during extinction cause local CORT binding and potentiation of social fear memory. The putative role of septal CORT and GR together with interactions with local OXTR needs, however, further exploration. Although evidence for an impaired OXT system in SAD patients is emerging (Ziegler et al., Citation2015) (reviewed in Neumann & Slattery, Citation2016), similar evidence for an impaired HPA axis is still ambiguous (Elzinga et al., Citation2010). Therefore, the SFC paradigm would be a good model to investigate this aspect of the SAD.
6. Concluding remarks
Social stress is an intrinsic aspect of social interactions that shape the well-being of the individual. Whereas positive social interactions serve as a protective factor buffering against the negative consequences of stressor exposure (, right side), psychosocial stress, especially in a chronic manner may result in HPA axis dysregulation (, left side).
In animal research, the choice of the animal model needs careful consideration, as each model is specific with respect to the nature of the stressor(s). Besides, social stressors can vary in the duration ranging from acute, as mimicked by the RI or SFC, to chronic, as in the CSC model. Further, the stress in the RI and the CSC model originates from a natural but, due to spontaneous social interaction, uncontrollable stressor, whereas in the SFC social fear is induced by a controlled painful, but harmless electric foot shock. Therefore, even though the general behavioral and neurobiological outcome is similar, different aspects and nuances are addressed in these three models and the final choice of model is determined by the research question.
Importantly, the three models share the ability to induce impaired social behaviors. This is highly relevant, as impaired social interaction, social avoidance up to social fear is likely to result in a feed-forward process of even more social stress and negative consequences. Using the three models side by side enables us to identify common neurobiological substrates to understand causes and consequences of social stress. From the physiological point of view, exposure to one of these three psychosocial stress models ischaracterized by alterations in the activity of the HPA-axis, and/or the brain OXT and AVP systems. Impairments in the OXT and AVP systems may contribute to the highly negative behavioral outcomes of social stress (). However, the complexity and flexibility of crosstalk between social interactions, components of the HPA axis as well as neuropeptide systems is still a challenge that needs further investigation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed, S.H., Stinus, L., Lemoal, M., & Cador, M. (1995). Social deprivation enhances the vulnerability of male wistar rats to stressor and amphetamine induced behavioral sensitization. Psychopharmacology, 117, 116–124. doi: 10.1007/BF02245106
- Albers, H.E. (2012). The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Hormones and Behavior, 61, 283–292. doi: 10.1016/j.yhbeh.2011.10.007
- Albert, D.J., Dyson, E.M., & Walsh, M.L. (1988). Cohabitation with a female activates testosterone-dependent social aggression in male rats independently of changes in serum testosterone concentration. Physiology & Behavior, 44, 735–740. doi: 10.1016/0031-9384(88)90054-6
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing Inc.
- Anisman, H., Hayley, S., Kelly, O., Borowski, T., & Merali, Z. (2001). Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: Mouse strain-dependent outcomes. Behavioral Neuroscience, 115, 443–454. doi: 10.1037/0735-7044.115.2.443
- Anisman, H., Lacosta, S., Kent, P., Mcintyre, D.C., & Merali, Z. (1998). Stressor-induced corticotropin-releasing hormone, bombesin, ACTH and corticosterone variations in strains of mice differentially responsive to stressors. Stress, 2, 209–220. doi: 10.3109/10253899809167284
- Apfelbach, R., Blanchard, C.D., Blanchard, R.J., Hayes, R.A., & McGregor, I.S. (2005). The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neuroscience and Biobehavioral Reviews, 29, 1123–1144. doi: 10.1016/j.neubiorev.2005.05.005
- Armario, A., Gavaldà, A., & Martí, J. (1995). Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology, 20, 879–890. doi: 10.1016/0306-4530(95)00018-6
- Arseneault, L. (2017). The long-term impact of bullying victimization on mental health. World Psychiatry, 16, 27–28. doi: 10.1002/wps.20399
- Bains, J.S., Cusulin, J.I.W., & Inoue, W. (2015). Stress: Stress-related synaptic plasticity in the hypothalamus. Nature Reviews Neuroscience, 16, 377–388. doi: 10.1038/nrn3881
- Baribeau, D.A., & Anagnostou, E. (2015). Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Frontiers in Neuroscience, 9, 335. doi: 10.3389/fnins.2015.00335
- Bartolomucci, A. (2007). Social stress, immune functions and disease in rodents. Frontiers in Neuroendocrinology, 28, 28–49. doi: 10.1016/j.yfrne.2007.02.001
- Beiderbeck, D.I., Neumann, I.D., & Veenema, A.H. (2007). Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. European Journal of Neuroscience, 26, 3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x
- Beiderbeck, D.I., Reber, S.O., Havasi, A., Bredewold, R., Veenema, A.H., & Neumann, I.D. (2012). High and abnormal forms of aggression in rats with extremes in trait anxiety – Involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology, 37, 1969–1980. doi: 10.1016/j.psyneuen.2012.04.011
- Berton, O., Aguerre, S., Sarrieau, A., Mormede, P., & Chaouloff, F. (1998). Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience, 82, 147–159. doi: 10.1016/S0306-4522(97)00282-0
- Berton, O., Covington, H.E., Ebner, K., Tsankova, N.M., Carle, T.L., Ulery, P., … Nestler, E.J. (2007). Induction of ΔFosB in the Periaqueductal Gray by stress promotes active coping responses. Neuron, 55, 289–300. doi: 10.1016/j.neuron.2007.06.033
- Berton, O., McClung, C.A., Dileone, R.J., Krishnan, V., Renthal, W., Russo, S.J., … Nestler, E.J. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science, 311, 864–868. doi: 10.1126/science.1120972
- Bhatnagar, S., Huber, R., Nowak, N., & Trotter, P. (2002). Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroendocrinology, 14(3), 403–410. doi: 10.1046/j.0007-1331.2002.00792.x
- Biro, L., Toth, M., Sipos, E., Bruzsik, B., Tulogdi, A., Bendahan, S., … Haller, J. (2016). Structural and functional alterations in the prefrontal cortex after post-weaning social isolation: Relationship with species-typical and deviant aggression. Brain Structure and Function, 222, 1861–1875. doi: 10.1007/s00429-016-1312-z
- Björkqvist, K. (2001). Social defeat as a stressor in humans. Physiology &Amp; Behavior, 73, 435–442. doi: 10.1016/S0031-9384(01)00490-5
- Blanchard, R.J., McKittrick, C.R., & Blanchard, D.C. (2001). Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiology & Behavior, 73, 261–271. doi: 10.1016/S0031-9384(01)00449-8
- Bosch, O.J., & Neumann, I.D. (2012). Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior, 61, 293–303. doi: 10.1016/j.yhbeh.2011.11.002
- Bowlby, J. (1951). Maternal care and mental health. Bulletin of the World Health Organization, 3, 355–533.
- Bredewold, R., Smith, C.J., Dumais, K.M., & Veenema, A.H. (2014). Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Frontiers in Behavioral Neuroscience, 8, 216. doi: 10.3389/fnbeh.2014.00216
- Brown, K.J., & Grunberg, N.E. (1995). Effects of housing on male and female rats: Crowding stresses male but calm females. Physiology & Behavior, 58, 1085–1089. doi: 10.1016/0031-9384(95)02043-8
- Buckley, T., Bartrop, R., Mckinley, S., Ward, C., Bramwell, M., Roche, D., … Tofler, G. (2009). Prospective study of early bereavement on psychological and behavioural cardiac risk factors. Internal Medicine Journal, 39, 370–378. doi: 10.1111/j.1445-5994.2008.01879.x
- Buske-Kirschbaum, A., Geiben, A., & Hellhammer, D. (2001). Psychobiological aspects of atopic dermatitis: An overview. Psychotherapy and Psychosomatics, 70, 6–16. doi: 10.1159/000056219
- Cacioppo, J.T., Cacioppo, S., Capitanio, J.P., & Cole, S.W. (2015). The neuroendocrinology of social isolation. Annual Review of Psychology, 66, 733–767. doi: 10.1146/annurev-psych-010814-015240
- Calcagnoli, F., De Boer, S.F., Althaus, M., De Boer, J.A., & Koolhaas, J.M. (2013). Antiaggressive activity of central oxytocin in male rats. Psychopharmacology, 229, 639–651. doi: 10.1007/s00213-013-3124-7
- Calcagnoli, F., de Boer, S.F., Beiderbeck, D.I., Althaus, M., Koolhaas, J.M., & Neumann, I.D. (2014). Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behavioural Brain Research, 261, 315–322. doi: 10.1016/j.bbr.2013.12.050
- Caldwell, H.K. (2017). Oxytocin and Vasopressin: Powerful regulators of social behavior. The Neuroscientist, 23, 517–528. doi: 10.1177/1073858417708284
- Campbell, J.C. (2002). Health consequences of intimate partner violence. Lancet (London, England), 359, 1331–1336. doi: 10.1016/S0140-6736(02)08336-8
- Cannon, W.B. (1929). Organization for physiological homeostasis. Physiological Reviews, 9, 399–431. doi: 10.1152/physrev.1929.9.3.399
- Caramaschi, D., de Boer, S.F., & Koolhaas, J.M. (2008). Is hyper-aggressiveness associated with physiological hypoarousal? A comparative study on mouse lines selected for high and low aggressiveness. Physiology & Behavior, 95, 591–598. doi: 10.1016/j.physbeh.2008.08.019
- Carpenter, L., Shattuck, T., Tyrka, A., Geracioti, T., & Price, L.H. (2013). Effect of childhood physical abuse on cortisol stress response. Psychopharmacology, 214, 367–375. doi: 10.1007/s00213-010-2007-4
- Caspi, A., Mcclay, J., Moffitt, T.E., Mill, J., Martin, J., Craig, I.W., … Poulton, R. (2002). Role of genotype in the cycle of violence in maltreated children. Science (New York, N.Y.), 297, 851–854. doi: 10.1126/science.1072290
- Champagne, D., Beaulieu, J., & Drolet, G. (1998). CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: A tract-tracing study. Journal of Neuroendocrinology, 10, 119–131. doi: 10.1046/j.1365-2826.1998.00179.x
- Choi, J., Park, M., Kim, C., Lee, Y., & Choi, E. (2017). Long-term consumption of sugar- sweetened beverage during the growth period promotes social aggression in adult mice with proinflammatory responses in the brain. Scientific Reports, 7, 45693. doi: 10.1038/srep45693
- Cohen, S., Janicki-Deverts, D., & Miller, G.E. (2007). Psychological stress and disease. Journal of American Medical Association, 298, 1685–1687. doi: 10.1001/jama.298.14.1685
- Coker, A.L., Davis, K.E., Arias, I., Desai, S., Sanderson, M., Brandt, H.M., & Smith, P.H. (2002). Physical and mental health effects of intimate partner violence for men and women. American Journal of Preventive Medicine, 23, 260–268. doi: 10.1016/S0749-3797(02)00514-7
- Cordero, M.I., Ansermet, F., & Sandi, C. (2013). Long-term programming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology, 38, 2758–2769. doi: 10.1016/j.psyneuen.2013.07.005
- Dackis, M.N., Rogosch, F.A., & Cicchetti, D. (2015). Child maltreatment, callous – unemotional traits, and defensive responding in high-risk children: An investigation of emotion-modulated startle response. Development and Psychopathology, 27, 1527–1545. doi: 10.1017/S0954579415000929
- Davidson, R.J., & McEwen, B.S. (2012). Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience, 15, 689–695. doi: 10.1038/nn.3093
- de Jong, T.R., & Neumann, I.D. (2017). Oxytocin and Aggression. In: Current Topics in Behavioral Neurosciences. Heidelberg, Berlin: Springer. doi: 10.1007/7854_2017_13
- de Jong, T.R., Beiderbeck, D.I., & Neumann, I.D. (2014). Measuring virgin female aggression in the Female Intruder Test (FIT): Effects of oxytocin, estrous cycle, and anxiety. PLoS One, 9, e91701. doi: 10.1371/journal.pone.0091701
- de Kloet, E.R., Joëls, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6, 463–475. doi: 10.1038/nrn1683
- DeVries, A.C., Glasper, E.R., & Detillion, C.E. (2003). Social modulation of stress responses. Physiology & Behavior, 79, 399–407. doi: 10.1016/S0031-9384(03)00152-5 doi: 10.1016/S0031-9384(03)00152-5
- Dubois-Dauphin, M., Pevet, P., Tribollet, E., & Dreifuss, J.J. (1990). Vasopressin in the brain of the golden hamster: The distribution of vasopressin binding sites and of immunoreactivitz to the vasopressin-related glycopeptide. The Journal of Comparative Neurology, 300, 535–548. doi: 10.1002/cne.903000408
- Dumais, K.M., & Veenema, A.H. (2016). Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Frontiers in Neuroendocrinology, 40, 1–23. doi: 10.1016/j.yfrne.2015.04.003
- Dunbar, R.I., & Shultz, S. (2007). Evolution in the social brain. Science, 317, 1344–1347. doi: 10.1126/science.1145463
- Ebner, K., Wotjak, C.T., Landgraf, R., & Engelmann, M. (2000). A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Research, 872, 87–92. doi: 10.1016/S0006-8993(00)02464-1
- Ebner, K., Wotjak, C.T., Landgraf, R., & Engelmann, M. (2005). Neuroendocrine and behavioral response to social confrontation: Residents versus intruders, active versus passive coping styles. Hormones and Behavior, 47, 14–21. doi: 10.1016/j.yhbeh.2004.08.002
- Eliava, M., Melchior, M., Knobloch-Bollmann, H.S., Wahis, J., da Silva Gouveia, M., Tang, Y., … Grinevich, V. (2016). A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron, 89, 1291–1304. doi: 10.1016/j.neuron.2016.01.041
- Elzinga, B.M., Spinhoven, P., Berretty, E., de Jong, P., & Roelofs, K. (2010). The role of childhood abuse in HPA-axis reactivity in social anxiety disorder: A pilot study. Biological Psychology, 83, 1–6. doi: 10.1016/j.biopsycho.2009.09.006
- Engelmann, M., Ebner, K., Landgraf, R., Holsboer, F., & Wotjak, C.T. (1999). Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. Journal of Neuroendocrinology, 11, 867–872. doi: 10.1046/j.1365-2826.1999.00403.x
- Engelmann, M., Landgraf, R., & Wotjak, C. (2004). The hypothalamic – neurohypophysial system regulates the hypothalamic – pituitary – adrenal axis under stress: An old concept revisited. Frontiers in Neuroendocrinology, 25, 132–149. doi: 10.1016/j.yfrne.2004.09.001
- Faucher, J., Koszycki, D., Bradwejn, J., Merali, Z., & Bielajew, C. (2016). Effects of CBT versus MBSR treatment on social stress reactions in social anxiety disorder. Mindfulness, 7, 514–526. doi: 10.1007/s12671-015-0486-4
- Foertsch, S., Füchsl, A.M., Faller, S.D., Hölzer, H., Langgartner, D., Messmann, J., … Reber, S.O. (2017). Splenic glucocorticoid resistance following psychosocial stress requires physical injury. Scientific Reports, 7, 1–12. doi: 10.1038/s41598-017-15897-2
- Franklin, T.B., Linder, N., Russig, H., Thony, B., & Mansuy, I.M. (2011). Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One, 6, e21842. doi: 10.1371/journal.pone.0021842
- Füchsl, A.M., Langgartner, D., & Reber, S. (2013). Mechanisms underlying the increased plasma ACTH levels in chronic psychosocially stressed male mice. PLoS One, 8, e84161. doi: 10.1371/journal.pone.0084161
- Füchsl, A.M., Neumann, I.D., & Reber, S.O. (2014). Stress resilience: A low-anxiety genotype protects male mice from the consequences of chronic psychosocial stress. Endocrinology, 155, 117–126. doi: 10.1210/en.2013-1742
- Füchsl, A.M., Uschold-Schmidt, N., & Reber, S.O. (2013). Chronic psychosocial stress in male mice causes an up-regulation of scavenger receptor class B type 1 protein in the adrenal glands. Stress, 16, 461–468. doi: 10.3109/10253890.2013.793303
- Gilbert, P. (2001). Evolution and social anxiety: The role of attraction, social competition, and social hierarchies. The Psychiatric Clinics of North America, 24, 723–751. doi: 10.1016/S0193-953X(05)70260-4
- Golden, S.A., Covington, H.E., Berton, O., & Russo, S.J. (2011). A standardized protocol for repeated social defeat stress in mice. Nature Protocols, 6, 1183–1191. doi: 10.1038/nprot.2011.361
- Goldin, P.R., Manber, T., Hakimi, S., Canli, T., & Gross, J.J. (2009). Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66, 170–180. doi: 10.1001/archgenpsychiatry.2008.525
- Gruver, A.L., & Sempowski, G.D. (2008). Cytokines, leptin, and stress-induced thymic atrophy. Journal of Leukocyte Biology, 84, 915–923. doi: 10.1189/jlb.0108025
- Gunnar, M.R., & Hostinar, C.E. (2015). The social buffering of the hypothalamic – pituitary – adrenocortical axis in humans: Developmental and experiential determinants. Social Neuroscience, 10, 479–488. doi: 10.1080/17470919.2015.1070747
- Guzmán, Y.F., Tronson, N.C., Jovasevic, V., Sato, K., Guedea, A.L., Mizukami, H., … Radulovic, J. (2013). Fear-enhancing effects of septal oxytocin receptors. Nature Neuroscience, 16, 1185–1187. doi: 10.1038/nn.3465
- Halász, J., Tóth, M., Kalló, I., Liposits, Z., & Haller, J. (2006). The activation of prefrontal cortical neurons in aggression – A double labeling study. Behavioural Brain Research, 175, 166–175. doi: 10.1016/j.bbr.2006.08.019
- Haller, J. (2013). The neurobiology of abnormal manifestations of aggression – A review of hypothalamic mechanisms in cats, rodents, and humans. Brain Research Bulletin, 93, 97–109. doi: 10.1016/j.brainresbull.2012.10.003
- Haller, J. (2016). Preclinical models of conduct disorder − principles and pharmacologic perspectives. Neuroscience and Biobehavioral Reviews, 7634, 30005–30007. doi: 10.1016/j.neubiorev.2016.05.032.
- Haller, J., & Bakos, N. (2002). Stress-induced social avoidance: A new model of stress-induced anxiety? Physiology and Behavior, 77, 327–332. doi: 10.1016/S0031-9384(02)00860-0
- Haller, J., Halász, J., Mikics, É., & Kruk, M.R. (2004). Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. Journal of Neuroendocrinology, 16, 550–557. doi: 10.1111/j.1365-2826.2004.01201.x
- Haller, J., Harold, G., Sandi, C., & Neumann, I.D. (2014). Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. Journal of Neuroendocrinology, 26, 724–738. doi: 10.1111/jne.12182
- Hansen, A.M., Hogh, A., Persson, R., Karlson, B., Garde, A.H., & Ørbaek, P. (2006). Bullying at work, health outcomes, and physiological stress response. Journal of Psychosomatic Research, 60, 63–72. doi: 10.1016/j.jpsychores.2005.06.078
- Heinrichs, S.C., Pich, E.M., Miczek, K.A., Britton, K.T., & Koob, G.F. (1992). Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Research, 581, 190–197. doi: 10.1016/0006-8993(92)90708-H
- Herman, J.P., Mcklveen, J.M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., … Myers, B. (2016). Regulation of the hypothalamic-pituitary- adrenocortical stress response. Comprehensive Physiology, 6, 603–621. doi: 10.1002/cphy.c150015
- Hernández, V.S., Hernández, O.R., Perez de la Mora, M., Gómora, M.J., Fuxe, K., Eiden, L.E., & Zhang, L. (2016). Hypothalamic vasopressinergic projections innervate central amygdala GABAergic neurons: Implications for anxiety and ctress coping. Frontiers in Neural Circuits, 10, 92. doi: 10.3389/fncir.2016.00092
- Hinduja, S., & Patchin, J. (2010). Bullying, cyberbullying, and suicide. Archives of Suicide Research, 14, 206–221. doi: 10.1080/13811118.2010.494133
- Hollis, F., Wang, H., Dietz, D., Gunjan, A., & Kabbaj, M. (2010). The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology, 211, 69–77. doi: 10.1007/s00213-010-1869-9
- Huhman, K.L., Bunnell, B.N., Mougey, E.H., & Meyerhoff, J.L. (1990). Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiology & Behavior, 47, 949–956. doi: 10.1016/0031-9384(90)90023-W
- Ieraci, A., Mallei, A., & Popoli, M. (2016). Social isolation stress induces anxious-depressive-like behavior and alterations of Neuroplasticity-related genes in adult male mice. Neural Plasticity, 2016, 13. doi: 10.1155/2016/6212983
- Ingram, C.D., & Moos, F. (1992). Oxytocin-containing pathway to the bed nuclei of the stria terminalis of the lactating rat brain: Immunocytochemical and in vitro electrophysiological evidence. Neuroscience, 47, 439–452. doi: 10.1016/0306-4522(92)90258-4
- Johnson, Z.V., & Young, L.J. (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neuroscience and Biobehavioral Reviews, 76, 87–98. doi: 10.1016/j.neubiorev.2017.01.034
- Jurek, B., & Neumann, I.D. (2018). The oxytocin receptor: From intracellular signaling to behavior. Physiological Reviews (in press).
- Kamakura, R., Kovalainen, M., Leppäluoto, J., Herzig, K.H., & Mäkelä, K.A. (2016). The effects of group and single housing and automated animal monitoring on urinary corticosterone levels in male C57BL/6 mice. Physiological Reports, 4, e12703. doi: 10.14814/phy2.12703
- Karandrea, D., Kittas, C., & Kitraki, E. (2002). Forced swimming differentially affects male and female brain corticosteroid receptors. Neuroendocrinology, 75, 217–226. doi: 10.1159/000054713
- Keeney, A., Jessop, D.A., Harbuz, M.S., Marsden, C.A., Hogg, S., & Blackburn-Munro, R.E. (2006). Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. Journal of Neuroendocrinology, 18, 330–338. doi: 10.1111/j.1365-2826.2006.01422.x
- Keeney, A.J., & Hogg, S. (1999). Behavioural consequences of repeated social defeat in the mouse, preliminary evalution of a potential animal model of depression. Behavioural Pharmacology, 10, 753–764. doi: 10.1097/00008877-199912000-00007
- Knobloch, H.S., Charlet, A., Hoffmann, L.C., Eliava, M., Khrulev, S., Cetin, A.H., … Grinevich, V. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron, 73, 553–566. doi: 10.1016/j.neuron.2011.11.030
- Knobloch, H.S., Grinevich, V., & Dabrowska, J. (2014). Evolution of oxytocin pathways in the brain of vertebrates. Frontiers in Behavioral Neuroscience, 8, 1–13. doi: 10.3389/fnbeh.2014.00031
- Kohl, C., Wang, X.D., Grosse, J., Fournier, C., Harbich, D., Westerholz, S., … Schmidt, M.V. (2015). Hippocampal neuroligin-2 links early-life stress with impaired social recognition and increased aggression in adult mice. Psychoneuroendocrinology, 55, 128–143. doi: 10.1016/j.psyneuen.2015.02.016
- Koolhaas, J.M., Bartolomucci, A., Buwalda, B., de Boer, S.F., Flügge, G., Korte, S.M., … Fuchs, E. (2011). Stress revisited: A critical evaluation of the stress concept. Neuroscience and Biobehavioral Reviews, 35, 1291–1301. doi: 10.1016/j.neubiorev.2011.02.003
- Koolhaas, J.M., Coppens, C.M., de Boer, S.F., Buwalda, B., Meerlo, P., & Timmermans, P.J. (2013). The resident-intruder paradigm: A standardized test for aggression, violence and social stress. Journal of Visualized Experiments, 77, 1–7.doi: 10.3791/4367.
- Koolhaas, J.M., DeBoer, S.F., Buwalda, B., & Meerlo, P. (2017). Social stress models in rodents: Towards enhanced validity. Neurobiology of Stress, 6, 104–112. doi: 10.1016/j.ynstr.2016.09.003
- Kowalski, R.M., Giumetti, G.W., Schroeder, A.N., & Lattanner, M.R. (2014). Bullying in the digital age: A critical review and meta-analysis of cyberbullying research among youth. Psychological Bulletin, 140, 1073–1137. doi: 10.1037/a0035618
- Krishnan, V., Han, M.H., Graham, D.L., Berton, O., Renthal, W., Russo, S.J., … Nestler, E.J. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell, 131, 391–404. doi: 10.1016/j.cell.2007.09.018
- Laman-Maharg, A., & Trainor, B.C. (2017). Stress, sex, and motivated behaviors. Journal of Neuroscience Research, 95, 83–92. doi: 10.1002/jnr.23815
- Landgraf, R., Häcker, R., & Buhl, R. (1982). Plasma vasopressin and oxytocin in response to exercise and during a day-night cycle in man. Endokrinologie, 79, 281–291.
- Langgartner, D., Füchsl, A.M., Uschold-Schmidt, N., Slattery, D.A., & Reber, S.O. (2015). Chronic subordinate colony housing paradigm: A mouse model to characterize the consequences of insufficient glucocorticoid signaling. Frontiers in Psychiatry, 6, 18. doi: 10.3389/fpsyt.2015.00018
- Larrieu, T., Cherix, A., Lei, H., & Gruetter, R. (2017). Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress. Current Biology, 27, 2202–2210. doi: 10.1016/j.cub.2017.06.027
- Litvin, Y., Murakami, G., & Pfaff, D.W. (2011). Effects of chronic social defeat on behavioral and neural correlates of sociality: Vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiology and Behavior, 103, 393–403. doi: 10.1016/j.physbeh.2011.03.007
- Lukas, M., & de Jong, T.R. (2017). Conspecific interactions in adult laboratory rodents: Friends or foes?. Currents Topics in Behavioral Neuroscience, 30, 3–24. doi: 10.1007/7854_2015_428.
- Lukas, M., & Neumann, I.D. (2013). Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behavioural Brain Research, 251, 85–94. doi: 10.1016/j.bbr.2012.08.011
- Lukas, M., & Neumann, I.D. (2014). Social preference and maternal defeat-induced social avoidance in virgin female rats: Sex differences in involvement of brain oxytocin and vasopressin. Journal of Neuroscience Methods, 234, 101–107. doi: 10.1016/j.jneumeth.2014.03.013
- Lukas, M., Bredewold, R., Landgraf, R., Neumann, I.D., & Veenema, A.H. (2011). Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology, 36, 843–853. doi: 10.1016/j.psyneuen.2010.11.007
- Lukas, M., Toth, I., Reber, S.O., Slattery, D.A., Veenema, A.H., & Neumann, I.D. (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology, 36, 2159–2168. doi: 10.1038/npp.2011.95
- MacDonald, K., & Feifel, D. (2014). Oxytocin's role in anxiety: a critical appraisal. Brain Research, 1580, 22–56. doi: 10.1016/j.brainres.2014.01.025
- Marquez, C., Poirier, G.L., Cordero, M.I., Larsen, M.H., Groner, A., Marquis, J., … Sandi, C. (2013). Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Translational Psychiatry, 3, e216. doi: 10.1038/tp.2012.144
- Martinez, M., Phillips, P.J., & Herbert, J. (1998). Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. The European Journal of Neuroscience, 10, 20–33. doi: 10.1046/j.1460-9568.1998.00011.x.
- McEwen, B.S., Bowles, N.P., Gray, J.D., Hill, M.N., Hunter, R.G., Karatsoreos, I.N., & Nasca, C. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18, 1353–1363. doi: 10.1038/nn.4086
- Menon, R., Grund, T., Zoicas, I., Althammer, F., Fiedler, D., Biermeier, V., … Neumann, I.D. (2018). Oxytocin signalling in the lateral septum prevents social fear during lactation. Current Biology, 28, 1–13. doi: 10.1016/j.cub.2018.02.044
- Miczek, K.A., Maxson, S.C., Fish, E.W., & Faccidomo, S. (2001). Aggressive behavioral phenotypes in mice. Behavioural Brain Research, 125, 167–181. doi: 10.1016/S0166-4328(01)00298-4
- Mitre, M., Marlin, B.J., Schiavo, J.K., Morina, E., Norden, S.E., Hackett, T., … Froemke, R.C. (2016). A distributed network for social cognition enriched for oxytocin receptors. The Journal of Neuroscience, 36, 2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016
- Modecki, K.L., Minchin, J., Harbaugh, A.G., Guerra, N.G., & Runions, K.C. (2014). Bullying prevalence across contexts: A meta-analysis measuring cyber and traditional bullying. Journal of Adolescent Health, 55, 602–611. doi: 10.1016/j.jadohealth.2014.06.007
- Morimoto, M., Morita, N., Ozawa, H., Yokoyama, K., & Kawata, M. (1996). Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistocheminal and in situ hybridization study. Neuroscience Research, 26, 235–269. doi: 10.1016/S0168-0102(96)01105-4
- Nagaraja, H.S., & Jeganathan, P.S. (1999). Forced swimming stress-induced changes in the physiological and biochemical parameters in albino rats. Indian Journal of Physiology and Pharmacology, 43, 53–59.
- Nelson, R.J., & Trainor, B.C. (2007). Neural mechanisms of aggression. Nature Reviews Neuroscience, 8, 536–546. doi: 10.1038/nrn2174
- Nelson, C.A., Zeanah, C.H., Fox, N.A., Marshall, P.J., Smyke, A.T., & Guthrie, D. (2007). Cognitive recovery in socially deprived young children: The Bucharest early intervention project. Science, 318, 1937–1940. doi: 10.1126/science.1143921
- Nemeroff, C.B. (2016). Paradise lost: The neurobiological and clinical consequences of child abuse and neglect. Neuron, 89, 892–909. doi: 10.1016/j.neuron.2016.01.019
- Neumann, I.D. (2007). Stimuli and consequences of dendritic release of oxytocin within the brain. Biochemical Society Transactions, 35, 1252–1257. doi: 10.1042/BST0351252
- Neumann, I.D. (2009). The advantage of social living: Brain neuropeptides mediate the beneficial consequences of sex and motherhood. Frontiers in Neuroendocrinology, 30, 483–496. doi: 10.1016/j.yfrne.2009.04.012
- Neumann, I.D., & Landgraf, R. (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35, 649–659. doi: 10.1016/j.tins.2012.08.004
- Neumann, I.D., & Slattery, D.A. (2016). Oxytocin in general anxiety and social fear: A translational approach. Biological Psychiatry, 79, 213–221. doi: 10.1016/j.biopsych.2015.06.004
- Neumann, I.D., Johnstone, H.A., Hatzinger, M., Liebsch, G., Shipston, M., Russell, J.A., … Douglas, A.J. (1998). Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. The Journal of Physiology, 508, 289–300. doi: 10.1111/j.1469-7793.1998.289br.x
- Neumann, I.D., Kromer, S.A., Toschi, N., & Ebner, K. (2000). Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regulatory Peptides, 96, 31–38. doi: 10.1016/S0167-0115(00)00197-X
- Neumann, I.D., Torner, L., & Wigger, A. (2000). Brain oxytocin: Differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience, 95, 567–575. doi: 10.1016/S0306-4522(99)00433-9
- Neumann, I.D., Veenema, A.H., & Beiderbeck, D.I. (2010). Aggression and anxiety: Social context and neurobiological links. Frontiers in Behavioral Neuroscience, 4, 12. doi: 10.3389/fnbeh.2010.00012
- Newman, S.W. (1999). The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Annals of the New York Academy of Sciences, 877, 242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x
- Nyuyki, K.D., Beiderbeck, D.I., Lukas, M., Neumann, I.D., & Reber, S.O. (2012). Chronic subordinate colony housing (CSC) as a model of chronic psychosocial stress in male rats. PLoS One, 7, e52371.doi: 10.1371/journal.pone.0052371
- O’Connell, L.A., & Hofmann, H.A. (2011). The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology, 519, 3599–3639. doi: 10.1002/cne.22735
- Oliva, A.M., Salcedo, E., Hellier, J.L., Ly, X., Koka, K., Tollin, D.J., & Restrepo, D. (2010). Toward a mouse neuroethology in the laboratory environment. PLoS One, 5, e11359. doi: 10.1371/journal.pone.0011359
- Palanza, P., Gioiosa, L., & Parmigiani, S. (2001). Social stress in mice: Gender differences and effects of estrous cycle and social dominance. Physiology & Behavior, 73, 411–420. doi: 10.1016/S0031-9384(01)00494-2
- Paré, W.P., & Glavin, G.B. (1986). Restraint stress in biomedical research: A review. Neuroscience and Biobehavioral Reviews, 10, 339–370. doi: 10.1016/0149-7634(86)90017-5
- Peters, S., Slattery, D.A., Flor, P.J., Neumann, I.D., & Reber, S.O. (2013). Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addiction Biology, 18, 66–77. doi: 10.1111/adb.12001
- Peters, S., Slattery, D.A., Uschold-Schmidt, N., Reber, S.O., & Neumann, I.D. (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology, 42, 225–236. doi: 10.1016/j.psyneuen.2014.01.021
- Pohl, T.T., Young, L.J., & Bosch, O.J. (2018). Lost connections: Oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships. International Journal of Psychophysiology, in press. doi: 10.1016/j.ijpsycho.2017.12.011. [Epub ahead of print]
- Pryce, C.R. (2008). Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: Inter-species and intra-species differences. Brain Research Reviews, 57, 596–605. doi: 10.1016/j.brainresrev.2007.08.005
- Reber, S., Birkeneder, L., Veenema, A.H., Obermeier, F., Falk, W., Straub, R.H., & Neumann, I.D. (2007). Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: Implications and mechanisms. Endocrinology, 148, 670–682. doi: 10.1210/en.2006-0983
- Reber, S.O. (2012). Stress and animal models of inflammatory bowel disease-An update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology, 37, 1–19. doi: 10.1016/j.psyneuen.2011.05.014
- Reber, S.O., & Neumann, I.D. (2008). Defensive behavioral strategies and enhanced state anxiety during chronic subordinate colony housing are accompanied by reduced hypothalamic vasopressin, but not oxytocin, expression. Annals of the New York Academy of Sciences, 1148, 184–195. doi: 10.1196/annals.1410.003
- Reber, S.O., Langgartner, D., Foertsch, S., Postolache, T.T., Brenner, L.A., Guendel, H., & Lowry, C.A. (2016). Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment – 2016 Curt Richter Award paper. Psychoneuroendocrinology, 74, 221–230. doi: 10.1016/j.psyneuen.2016.08.031
- Reber, S.O., Obermeier, F., Straub, H.R., Falk, W., & Neumann, I.D. (2006). Chronic intermittent psychosocial stress (Social Defeat/Overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology, 147, 4968–4976. doi: 10.1210/en.2006-0347
- Reiche, E.M., Nunes, S.O., & Morimoto, H.K. (2004). Stress, depression, the immune system, and cancer. The Lancet. Oncology, 5, 617–625. doi: 10.1016/S1470-2045(04)01597-9
- Roelofs, K., van Peer, J., Berretty, E., Jong, P. D., Spinhoven, P., & Elzinga, B.M. (2009). Hypothalamus-pituitary-adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biological Psychiatry, 65, 336–343. doi: 10.1016/j.biopsych.2008.08.022
- Rotondo, F., Butz, H., Syro, L.V., Yousef, G.M., Di Ieva, A., Restrepo, L.M., … Kovacs, K. (2016). Arginine vasopressin (AVP): A review of its historical perspectives, current research and multifunctional role in the hypothalamo-hypophysial system. Pituitary, 19, 345–355. doi: 10.1007/s11102-015-0703-0
- Russo, S.J., Murrough, J.W., Han, M., Charney, D.S., & Nestler, E.J. (2012). Neurobiology of resilience. Nature Neuroscience, 15, 1475–1484. doi: 10.1038/nn.3234
- Sandi, C., & Haller, J. (2015). Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nature Reviews Neuroscience, 16, 290–304. doi: 10.1038/nrn3918
- Sántha, P., Veszelka, S., Hoyk, Z., Mészáros, M., Walter, F.R., Tóth, A.E., … Deli, M.A. (2016). Restraint stress-induced morphological changes at the blood-brain barrier in adult rats. Frontiers in Molecular Neuroscience, 8, 88. doi: 10.3389/fnmol.2015.00088
- Sapolsky, R.M. (2015). Stress and the brain: Individual variability and the inverted-U. Nature Neuroscience, 18, 1344–1346. doi: 10.1038/nn.4109
- Sarro, E.C., Sullivan, R.M., & Barr, G. (2014). Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience, 258, 147–161. doi: 10.1016/j.neuroscience.2013.10.064
- Schmidt, M.V., Scharf, S.H., Sterlemann, V., Ganea, K., Liebl, C., Holsboer, F., & Müller, M.B. (2010). High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology, 35, 635–643. doi: 10.1016/j.psyneuen.2009.10.002
- Schmidt, M.V., Sterlemann, V., & Müller, M.B. (2008). Chronic stress and individual vulnerability. Annals of the New York Academy of Sciences, 1148, 174–183. doi: 10.1196/annals.1410.017
- Schmidt, M.V., Sterlemann, V., Ganea, K., Liebl, C., Alam, S., Harbich, D., … Müller, M.B. (2007). Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology, 32, 417–429. doi: 10.1016/j.psyneuen.2007.02.011
- Selye, H. (1936). A syndrome produced by diverse nocuous agents. Nature, 138, 32. doi: 10.1038/138032a0
- Sial, O.K., Warren, B.L., Alcantara, L.F., Parise, E.M., & Bolaños-Guzman, C.A. (2017). Vicarious social defeat stress: Bridging the gap between physical and emotional stress Omar. Journal of Neuroscience Methods, 258, 94–103. doi: 10.1016/j.jneumeth.2015.10.012
- Singewald, G.M., Nguyen, N.K., Neumann, I.D., Singewald, N., & Reber, S. (2009). Effect of chronic psychosocial stress-induced by subordinate colony (CSC) housing on brain neuronal activity patterns in mice. Stress, 12, 58–69. doi: 10.1080/10253890802042082
- Singewald, N., Schmuckermair, C., Whittle, N., Holmes, A., & Ressler, K.J. (2015). Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacology & Therapeutics, 149, 150–190. doi: 10.1016/j.pharmthera.2014.12.004
- Slattery, D.A., & Neumann, I.D. (2008). No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. The Journal of Physiology, 586, 377–385. doi: 10.1113/jphysiol.2007.145896
- Slattery, D.A., Uschold, N., Magoni, M., Bär, J., Popoli, M., Neumann, I.D., & Reber, S.O. (2012). Behavioural consequences of two chronic psychosocial stress paradigms: Anxiety without depression. Psychoneuroendocrinology, 37, 702–714. doi: 10.1016/j.psyneuen.2011.09.002
- Smith, A.S., & Wang, Z. (2014). Hypothalamic oxytocin mediates social buffering of the stress response. Biological Psychiatry, 76, 281–288. doi: 10.1016/j.biopsych.2013.09.017
- Soravia, L.M., Heinrichs, M., Aerni, A., Maroni, C., Schelling, G., Ehlert, U., … der Quervain, D.J. (2006). Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences of the United States of America, 103, 5585–5590. doi: 10.1073/pnas.0509184103
- Stefanski, V., & Engler, H. (1999). Social stress, dominance and blood cellular immunity. Journal of Neuroimmunology, 94, 144–152. doi: 10.1016/S0165-5728(98)00242-2. doi: 10.1016/S0165-5728(98)00242-2
- Steinman, M.Q., Duque-Wilckens, N., Greenberg, G.D., Hao, R., Campi, K.L., Laredo, S.A., … Trainor, B.C. (2016). Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biological Psychiatry, 80, 406–414. doi: 10.1016/j.biopsych.2015.10.007
- Steinman, M.Q., Laredo, S.A., Lopez, E.M., Manning, C.E., Hao, R.C., Doig, I.E., … Trainor, B.C. (2015). Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology, 51, 122–134. doi: 10.1016/j.psyneuen.2014.09.009
- Takahashi, A., Chung, J., Zhang, S., Zhang, H., Grossman, Y., Flanigan, M., … Russo, S.J. (2017). Establishment of a repeated social defeat stress model in female mice. Scientific Reports, 7, 12838. doi: 10.1038/s41598-017-12811-8.
- Tamashiro, K.L., Nguyen, M.M., & Sakai, R.R. (2005). Social stress: From rodents to primates. Frontiers in Neuroendocrinology, 26, 27–40. doi: 10.1016/j.yfrne.2005.03.001
- Terranova, J.I., Song, Z., Larkin, T.E., Hardcastle, N., Norvelle, A., Riaz, A., & Albers, H.E. (2016). Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proceedings of the National Academy of Sciences of the United States of America, 113, 13233–13238. doi: 10.1073/pnas.1610446113
- Thorndike, B. (1933). A theory of the action of the after-effects of a connection upon it. Psychological Review, 40, 434–439. doi: 10.1037/h0071025
- Tobin, V.A., Hashimoto, H., Wacker, D.W., Takayanagi, Y., Langnaese, K., Caquineau, C., … Ludwig, M. (2010). An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature, 464, 413–417. doi: 10.1038/nature08826
- Tokunaga, R. (2010). Following you home from school: A critical review and synthesis of research on cyberbullying victimization. Computers in Human Behavior, 26, 277–287. doi: 10.1016/j.chb.2009.11.014
- Torner, L., Plotsky, P.M., Neumann, I.D., & de Jong, T.R. (2017). Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology, 77, 165–174. doi: 10.1016/j.psyneuen.2016.12.006
- Tost, H., Champagne, F.A., & Meyer-Lindenberg, A. (2015). Environmental influence in the brain, human welfare and mental health. Nature Neuroscience, 18, 4121–4131. doi: 10.1038/nn.4108
- Toth, I., Neumann, I.D., & Slattery, D.A. (2012). Social fear conditioning: A novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology, 37, 1433–1443. doi: 10.1038/npp.2011.329
- Toth, I., Neumann, I.D., & Slattery, D.A. (2013). Social fear conditioning as an animal model of social anxiety disorder. Current Protocols in Neuroscience, Chapter 9, Unit9.42. doi: 10.1002/0471142301.ns0942s63
- Touma, C., Bunck, M., Glasl, L., Nussbaumer, M., Palme, R., Stein, H., … Landgraf, R. (2008). Mice selected for high versus low stress reactivity: A new animal model for affective disorders. Psychoneuroendocrinology, 33, 839–862. doi: 10.1016/j.psyneuen.2008.03.013
- Tulogdi, Á., Tóth, M., Barsvári, B., Biró, L., Mikics, É., & Haller, J. (2014). Effects of resocialization on post-weaning social isolation-induced abnormal aggression and social deficits in rats. Developmental Psychobiology, 56, 49–57. doi: 10.1002/dev.21090
- Tzanoulinou, S., Riccio, O., de Boer, M.W., & Sandi, C. (2014). Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Translational Psychiatry, 4, e410. doi: 10.1038/tp.2014.54
- Ulrich-Lai, Y.M., & Herman, J.P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10, 397–409. doi: 10.1038/nrn2647
- Uschold-Schmidt, N., Nyuyki, K.D., Füchsl, A.M., Neumann, I.D., & Reber, S.O. (2012). Chronic psychosocial stress results in sensitization of the HPA axis to acute heterotypic stressors despite a reduction of adrenal in vitro ACTH responsiveness. Psychoneuroendocrinology, 37, 1676–1687. doi: 10.1016/j.psyneuen.2012.02.015
- Valzelli, L. (1973). The “isolation syndrome” in mice” . Psychopharmacologia, 31, 305–320. doi: 10.1007/BF00421275. doi: 10.1007/BF00421275
- van Eekelen, J.A.M., Bohn, M.C., & de Kloet, E.R. (1991). Postnatal ontogeny of mineralocorticoid and glucocorticoid receptor gene expression in regions of the rat tel- and diencephalon. Developmental Brain Research, 61, 33–43. doi: 10.1016/0165-3806(91)90111-U
- Veenema, A.H. (2012). Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Hormones and Behavior, 61, 304–312. doi: 10.1016/j.yhbeh.2011.12.002
- Veenema, A.H., Beiderbeck, D., Lukas, M., & Neumann, I.D. (2010). Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Hormones and Behavior, 58, 273–281. doi: 10.1016/j.yhbeh.2010.03.006
- Veenema, A.H., Blume, A., Niederle, D., Buwalda, B., & Neumann, I.D. (2006). Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. European Journal of Neuroscience, 24, 1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x
- Veenema, A.H., Bredewold, R., & De Vries, G.J. (2013). Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology, 38, 2554–2561. doi: 10.1016/j.psyneuen.2013.06.002
- Veenema, A.H., Reber, S.O., Selch, S., Obermeier, F., & Neumann, I.D. (2008). Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology, 149, 2727–2736. doi: 10.1210/en.2007-1469
- Veenema, A.H., Torner, L., Blume, A., Beiderbeck, D.I., & Neumann, I.D. (2007). Low inborn anxiety correlates with high intermale aggression: Link to ACTH response and neuronal activation of the hypothalamic paraventricular nucleus. Hormones and Behavior, 51, 11–19. doi: 10.1016/j.yhbeh.2006.07.004
- Veenit, V., Cordero, M.I., Tzanoulinou, S., & Sandi, C. (2013). Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Frontiers in Behavioral Neuroscience, 7, 26. doi: 10.3389/fnbeh.2013.00026
- Võikar, V., Polus, A., Vasar, E., & Rauvala, H. (2005). Long-term individual housing in C57BL/6J and DBA/2 mice: Assessment of behavioral consequences. Genes, Brain and Behavior, 4, 240–252. doi: 10.1111/j.1601-183X.2004.00106.x
- Waldherr, M., & Neumann, I.D. (2007). Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proceedings of the National Academy of Sciences of the United States of America, 104, 16681–16684. doi: 10.1073/pnas.0705860104
- Waldherr, M., Nyuyki, K., Maloumby, R., Bosch, O.J., & Neumann, I.D. (2010). Attenuation of the neuronal stress responsiveness and corticotrophin releasing hormone synthesis after sexual activity in male rats. Hormones and Behavior, 57, 222–229. doi: 10.1016/j.yhbeh.2009.11.006
- Walker, S.E., Sandi, C., Walker, S.E., & Sandi, C. (2018). Long-term programing of psychopathology- like behaviors in male rats by peripubertal stress depends on individual’s glucocorticoid responsiveness to stress to stress. Stress, 7, 1–10. doi: 10.1080/10253890.2018.1435639
- Walker, S.E., Zanoletti, O., Guillot de Suduiraut, I., & Sandi, C. (2017). Constitutive differences in glucocorticoid responsiveness to stress are related to variation in aggression and anxiety-related behaviors. Psychoneuroendocrinology, 84, 1–10. doi: 10.1016/j.psyneuen.2017.06.011
- Wallace, D.L., Han, M.H., Graham, D.L., Green, T.A., Vialou, V., Iñiguez, S.D., … Nestler, E.J. (2009). CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nature Neuroscience, 12, 200–209. doi: 10.1038/nn.2257
- Webster Marketon, J.I., & Glaser, R. (2008). Stress hormones and immune function. Cellular Immunology, 252, 16–26. doi: 10.1016/j.cellimm.2007.09.006
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology (Berlin), 134, 319–329. doi: 10.1007/s002130050456
- Windle, R.J., Kershaw, Y.M., Shanks, N., Wood, S.S., Lightman, S.L., & Ingram, C.D. (2004). Oxytocin attenuates stress-induced c- fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo - pituitary - adrenal activity. The Journal of Neuroscience, 24, 2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004
- Wommack, J.C., Salinas, A., & Delville, Y. (2005). Glucocorticoids and the development of agonistic behaviour during puberty in male golden hamsters. Journal of Neuroendocrinology, 17, 781–787. doi: 10.1111/j.1365-2826.2005.01369.x
- Wong, L.C., Wang, L., D’amour, J.A., Yumita, T., Chen, G., Yamaguchi, T., … Lin, D. (2016). Effective modulation of male aggression through Lateral septum to medial hypothalamus projection. Current Biology, 26, 593–604. doi: 10.1016/j.cub.2015.12.065
- Wood, S.K., & Bhatnagar, S. (2015). Resilience to the effects of social stress: Evidence from clinical and preclinical studies on the role of coping strategies. Neurobiology of Stress, 1, 164–173. doi: 10.1016/j.ynstr.2014.11.002
- Wotjak, C.T., Kubota, M., Liebsch, G., Montkowski, A., Holsboer, F., Neumann, I., & Landgraf, R. (1996). Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: A novel mechanism of regulating adrenocorticotropic hormone secretion?. The Journal of Neuroscience, 16, 7725–7732. doi: 10.1016/S0079-6123(08)61571-X. doi: 10.1523/JNEUROSCI.16-23-07725.1996
- Yan, H.C., Cao, X., Das, M., Zhu, X.H., & Gao, T.M. (2010). Behavioral animal models of depression. Neuroscience Bulletin, 26, 327–337. doi: 10.1007/s12264-010-0323-7
- Yang, T., Yang, C.F., Chizari, M.D., Bender, K.J., Ganguli, S., & Shah, N.M. (2017). Social control of Hypothalamus-mediated male aggression. Neuron, 95, 955–970.e4. doi: 10.1016/j.neuron.2017.06.046
- Yoshida, M., Takayanagi, Y., Inoue, K., Kimura, T., Young, L.J., Onaka, T., & Nishimori, K. (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. Journal of Neuroscience, 29, 2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009
- Ziegler, C., Dannlowski, U., Bräuer, D., Stevens, S., Laeger, I., Wittmann, H., … Domschke, K. (2015). Oxytocin receptor gene methylation: Converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology, 40, 1528–1538. doi: 10.1038/npp.2015.2
- Zoicas, I., & Neumann, I.D. (2016). Maternal separation facilitates extinction of social fear in adult male mice. Behavioural Brain Research, 297, 323–328. doi: 10.1016/j.bbr.2015.10.034
- Zoicas, I., Slattery, D.A., & Neumann, I.D. (2014). Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum. Neuropsychopharmacology, 39, 3027–3035. doi: 10.1038/npp.2014.156
