Abstract
Post-traumatic stress disorder (PTSD) is characterized by the development of paradoxical memory disturbances including intrusive memories and amnesia for specific details of the traumatic experience. Despite evidence that women are at higher risk to develop PTSD, most animal research has focused on the processes by which male rodents develop adaptive fear memory. As such, the mechanisms contributing to sex differences in the development of PTSD-like memory disturbances are poorly understood. In this investigation, we exposed adult male and female Wistar rats to the synthetic alarm odor 2,4,5-trimethylthiazole (TMT) to assess development of generalized fear behavior and rapid modulation of glutamate uptake and signaling cascades associated with hippocampus-dependent long-term memory. We report that female Wistar rats exposed to alarm odor exhibit context discrimination impairments relative to TMT-exposed male rats, suggesting the intriguing possibility that females are at greater risk in developing generalized fear memories. Mechanistically, alarm odor exposure rapidly modulated signaling cascades consistent with activation of the CREB shut-off cascade in the male, but not the female hippocampus. Moreover, TMT exposure dampened glutamate uptake and affected expression of the glutamate transporter, GLT-1 in the hippocampus. Taken together, these results provide evidence for rapid sex-dependent modulation of CREB signaling in the hippocampus by alarm odor exposure which may contribute to the development of generalized fear.
Introduction
Post-traumatic stress disorder (PTSD) is characterized by both intrusive memories of the traumatic experience and amnesia for specific details associated with the trauma (Desmedt, Marighetto, & Piazza, Citation2015; Pitman et al., Citation2012). Recent theories postulate that the formation of inappropriate connections between traumatic memories and unrelated environmental cues underlies the paradoxical memory disturbances associated with PTSD (Besnard & Sahay, Citation2016; Desmedt et al., Citation2015). Although women exhibit greater risk of developing PTSD following a traumatic experience (Breslau, Citation2002; Kessler, Sonnega, Bromet, Hughes, & Nelson, Citation1995; Tolin & Foa, Citation2006), the mechanisms by which the female brain exhibits increased susceptibility to trauma remain poorly understood.
The hippocampus is critical for proper encoding and retrieval of contextual information (Squire, Citation1992). Given clinical observations of reduced hippocampal volume and impaired spatial memory in patients suffering from PTSD (Bremner, Citation2005, Citation2006; Bremner & Narayan, Citation1998; Tempesta, Mazza, Iaria, De Gennaro, & Ferrara, Citation2012), impaired hippocampus function may contribute to fear generalization, a cardinal symptom of PTSD (Acheson, Gresack, & Risbrough, Citation2012; Jovanovic, Kazama, Bachevalier, & Davis, Citation2012; Lopresto, Schipper, & Homberg, Citation2016). Deficits in context discrimination, a hippocampus-dependent process of distinguishing between similar environmental cues, may underlie fear generalization by facilitating fear responses to irrelevant spatial cues (Besnard & Sahay, Citation2016; Desmedt et al., Citation2015).
Previously, we and others have reported that alarm odor exposure supports conditioned avoidance and hyperactivity (Endres & Fendt, Citation2007, Citation2009; Homiack et al., Citation2017; Rosen et al., Citation2008). However, it remains unknown whether alarm odor supports the development of conditioned and generalized avoidant and hyperactive responses. Furthermore, the molecular changes in the hippocampus induced by alarm odor exposure remain poorly understood, particularly with respect to the female hippocampus.
The transcription factor cyclic-AMP response element binding protein (CREB), when phosphorylated on serine residue 133 (pCREB), transcribes immediate early genes which are critical for memory consolidation (Bourtchouladze et al., Citation2003; Pittenger & Kandel, Citation1998; Sekeres, Neve, Frankland, & Josselyn, Citation2010). During normal neurotransmission conditions, depolarization and activation of N-methyl d-aspartate acid receptors (NMDARs) by glutamate is a key event upstream of CREB phosphorylation and activation (Greenberg, Thompson, & Sheng, Citation1992; Impey et al., Citation1998). This pathway represents the canonical pathway responsible for synaptic plasticity and learning and memory. However, hyperactivation of NMDARs can also initiate a separate, cell death-associated cascade capable of rapidly reducing CREB phosphorylation (Hardingham & Bading, Citation2002; Hardingham, Fukunaga, & Bading, Citation2002; Kaufman et al., Citation2012). Previously, acute stress has been shown to elevate extracellular glutamate levels in the male hippocampus (Lowy, Wittenberg, & Yamamoto, Citation1995; Popoli, Yan, McEwen, & Sanacora, Citation2011; Venero & Borrell, Citation1999), which may increase NMDAR activity and potentially trigger the CREB shut-off pathway. Activation of the CREB shut-off pathway is characterized by reduced extracellular signal-regulated kinase 1/2 (ERK/MAPK) activity which drives nuclear translocation of the synaptonuclear messenger protein Jacob which reduces CREB phosphorylation through an undetermined mechanism (Dieterich et al., Citation2008; Hardingham & Bading, Citation2002; Hardingham et al., Citation2002). Importantly, prolonged reductions of CREB activation in the hippocampus have been reported following predator threat exposure, suggesting that disruptions of the pCREB pathway by predator stress may contribute to changes in behavioral responses to stress (Cohen, Kozlovsky, Matar, Zohar, & Kaplan, Citation2014). Our lab has demonstrated that alarm odor exposure rapidly dampens phosphorylation and subsequent activation of CREB in the throughout the male hippocampus in a manner consistent with the CREB shut-off cascade (Homiack et al., Citation2017), a finding which has also been observed 24 hours following learned helplessness training (Lin et al., Citation2009). Notably, acute stress does not appear to affect CREB phosphorylation in the female hippocampus (Homiack et al., Citation2017), suggesting that activation of the CREB shut-off cascade in the hippocampus represents a molecular locus by which the male and female hippocampus respond differently to acute stress.
Therefore, the goal of this investigation was to assess whether exposure to alarm odor causes sex-dependent context discrimination impairment and modulates glutamate uptake and the CREB shut-off pathway in the male and female hippocampus. Here we report that exposure to the synthetic alarm odor 2,4,5-trimethylthiazole (TMT), a sex-independent stressor, impaired context discrimination ability in females relative to TMT-exposed male rats. We observed molecular changes consistent with activation of the CREB shutoff pathway in the hippocampus of male, but not female rodents exposed to TMT. We also report that TMT exposure reduced expression of the primary EAAT, GLT-1, in homogenate prepared from the male, but not the female hippocampus. Furthermore, TMT exposure dampened glutamate reuptake in purified hippocampal synaptosomes to a larger degree in male than female rodents. Taken together, these findings suggest that female biological sex prevents the rapid molecular changes induced by TMT in the male hippocampus, which may contribute to the increased expression of generalized fear in female rodents.
Methods
Animals
Adult (56 days old on arrival) male and female Wistar rats (N = 88 total) from ENVIGO were habituated for one week prior to manipulation. Animals were pair-housed with phytoestrogen free chow and water available ad libitum on a 12:12 light/dark schedule. Due to space constraints, male and female rats were housed in the same room within the animal facility. No additional environmental enrichment was provided. To reduce stress associated with handling, female rats were not monitored for estrous status. All procedures were conducted based on protocols approved by the Tulane University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
TMT exposure
Animals were randomly assigned into two groups: control or TMT. On the day of exposure, animals were transported from the animal care facility to the experimental area and allowed to habituate for two hours prior to manipulation. Upon arrival in the experimental area, a petri dish to hold the stimulus was affixed to the grating of the home cage (dimensions: 28 cm height * 21.5 cm width * 44.5 cm length), above the area where animals could move freely. Animals then habituated to the environment for 120 minutes prior to handling and exposure. During the exposure, the home cage was placed inside a fume hood and 150 μL of either TMT or water was placed in the petri dish. TMT exposure always followed water exposure to prevent possible odor contamination. Both control and TMT groups were exposed each day during the experiment to control for the possibility that TMT lingered in the fume hood. Exposures were recorded and manually scored for time on the stimulus side (based on centroid position), midline crosses, vertical rearing, and immobility as previously described (Homiack et al., Citation2017).
The animals used for biochemistry and glutamate uptake experiments were sacrificed by rapid decapitation without anesthesia immediately following the conclusion of the 30-minute exposure to TMT or water. Trunk blood and hippocampi were collected as previously described (Ferland & Schrader, Citation2011a, Citation2011b; Homiack et al., Citation2017).
Trunk blood collection and corticosterone measurement
Trunk blood was collected into conical tubes. After collection, trunk blood incubated at room temperature for 90 minutes to clot. Following the incubation, the clot was disrupted, and tubes were centrifuged at 1,500 * g for 15 minutes at room temperature. The serum was collected and stored at −20 °C. Serum samples were shipped on dry ice to the ligand analysis core facility at the University of Virginia Center for Research in Reproduction. Samples were blindly measured in duplicate by radioimmunoassay for home cage exposures and by ELISA for context discrimination cohorts. One control male animal was excluded from the analysis of the context discrimination due to undetectable levels of corticosterone.
Predator odor context discrimination
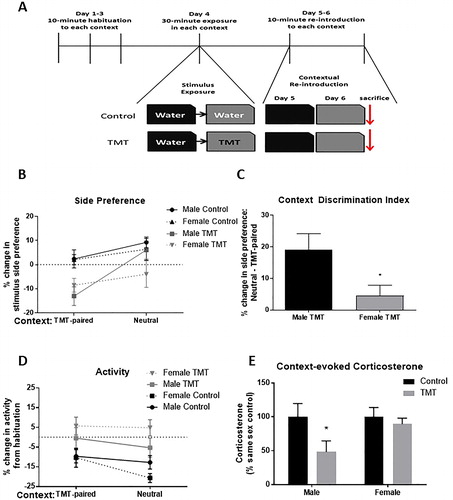
The design of the context discrimination is described schematically in . To control for potential olfactory confounds associated with male and female rodents being used at the same time (Hurst, Citation2009; Mak et al., Citation2007), male (n = 16) and female (n = 16) animals were run in separate cohorts. Prior to scent exposure, animals were brought to the room in which testing would take place two hours prior to habituation sessions for three days prior to manipulation. During habituation sessions, animals were placed in each context, an empty cage (dimensions: 28 cm height * 21.5 cm width * 44.5 cm length) with no bedding individually for 10 minutes. In order to create distinct, yet similar contexts spatial cues were placed around the separate fume hoods and masking tape was placed along the outside of the context in the shape of a plus, or an “X” in the same position of the arena. The order of the context exposure during habituation sessions was counterbalanced between TMT-exposed and control animals to eliminate order effects. On the fourth day of the experiment, all animals were exposed to water in the neutral context in pairs for 30 minutes. Thirty minutes following exposure to water in the neutral context, animals were exposed in pairs to either water (environmental control) (n = 8 male/female) or 150 μL TMT (n = 8 male/female) in the paired context for 30 minutes. Control exposures were conducted before TMT exposures to prevent unintentional scent exposure. Due to the strong TMT odor and in order to avoid any olfactory stimulation of control animals from lingering TMT odor, control animals were returned to the animal facility prior to TMT exposure sessions, and TMT-exposed animals were housed in a separate room in the animal facility. On the fifth day, all animals were returned to the neutral context for 10 minutes. On the sixth and final day, all animals were re-exposed to the context in which TMT had been presented for 10 minutes and sacrificed immediately afterward by rapid decapitation for trunk blood collection. All behavioral sessions were recorded and scored for side preference, activity, and immobility as previously described (Homiack et al., Citation2017). Analysis compared the average of all habituation sessions against re-introduction sessions as a two-way repeated-measures ANOVA.
Figure 1. Predator odor context discrimination. (A) Diagram depicting experimental design and timeline. (B) Animals exposed to TMT, regardless of sex, exhibited reduced preference for the stimulus-paired side of the TMT-paired, but not the neutral context. (C) Female animals exposed to TMT displayed an impaired ability, relative to TMT-exposed male rats, to limit avoidant behavior to the TMT-paired context. (D) Animals exposed to TMT exhibited an attenuated decrease in activity relative to control animals, regardless of sex and context. (E) When controlling for basal sex differences in corticosterone, TMT-exposed animals exhibited a trend toward reduced corticosterone levels. Post hoc analysis detected a significant difference between TMT-exposed and control males, but not females (*p < .05).
Tissue preparation
Brains were rapidly removed and placed in aCSF containing (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 25 d-glucose as previously described (Ferland & Schrader, Citation2011b; Homiack et al., Citation2017). The hippocampi from one cohort of animals (n = 24, 6 control M/F, 6 TMT M/F) was prepared for Western blot based on a commercially available protocol (Abcam, Cambridge, United Kingdom). Briefly, dissected whole hippocampi were homogenized in isotonic sucrose buffer containing (in mM): 250 sucrose, 20 HEPES pH = 7.9, 10 KCl, 1.5 MgCl2, 1 EDTA, 1 EGTA, and 0.1 DTT with protease (Calbiochem, San Diego, CA) and phosphatase (Sigma-Aldrich, St. Louis, MO) inhibitors and a portion was stored at −20 °C for molecular analysis. The remaining sample was centrifuged at 1,000 * g, and the pellet was washed twice and resuspended in physiological buffer containing (in mM): 120 NaCl, 4.7 KCl, 2.2 CaCl2, 1.2 MgCl2, 25 HEPES, 1.2 MgSO4, 1.2 KH2PO4, and 10 glucose, pH = 7.4.
Differential centrifugation for glutamate reuptake
The hippocampi from a separate cohort of animals (N = 32, 8 control M/F, 8 TMT M/F) were prepared for differential centrifugation as previously described (Carney et al., Citation2014; Dunkley, Jarvie, & Robinson, Citation2008; Stigliani et al., Citation2006). Percoll gradients were prepared the night prior to use by sequentially layering 2 mL each of 20%, 10%, 6%, and 2% Percoll in homogenization buffer containing: 0.32 M sucrose, 1 mM EDTA, 0.25 mM DTT, and 5 mM Tris, pH = 7.4. The day of the experiment, bilaterally dissected hippocampi were homogenized in homogenization buffer with added protease and phosphatase inhibitors. For molecular analysis, a portion of the homogenate was collected and stored at −20 °C prior to additional processing of the tissue.
Purified gliosomal and synaptosomal fractions were generated as previously described (Carney et al., Citation2014; Dunkley et al., Citation2008; Stigliani et al., Citation2006). Briefly, homogenized tissue was centrifuged at 1,000 * g and the supernatants were layered onto prepared Percoll gradients. Percoll gradients with sample were centrifuged at 33,500 * g for six minutes in a Sorvall SS34 rotor. The layers between the 2% and 6% fractions (gliosome), and the 10% and 20% fractions (synaptosome), were collected separately with a Pasteur pipette, washed with physiological buffer containing (in mM): 120 NaCl, 4.7 KCl, 2.2 CaCl2, 1.2 MgCl2, 25 HEPES pH = 7.4, 1.2 MgSO4, 1.2 KH2PO4, and 10 glucose and centrifuged at 1,000 * g to collect the final pellet. Pellets were resuspended in physiological buffer and stored on ice during same day measurement of glutamate uptake.
Glutamate uptake
Glutamate uptake was measured essentially as previously described elsewhere (Huang, Chen, Yeh, & Hsu, Citation2012; Ullensvang, Lehre, Storm-Mathisen, & Danbolt, Citation1997). Briefly, the protein concentrations of gliosomal-enriched and synaptosomal-enriched layers were measured using the Bio-Rad DC protein assay. In a final volume of 500 µL of physiological buffer, 75 μg of protein from each sample was incubated for five minutes in the presence of 10 nM L-[3,4-3H]-glutamate (Perkin-Elmer, Waltham, MA, 49.1 Ci/mmol) and 30 μM unlabeled glutamate (Santa Cruz Biotechnology, Dallas, TX) at 37 °C. The incubation was terminated by filtration of samples onto glass fiber filters using a cell harvester under vacuum. Glass filters were then washed four times with physiological buffer. Filters were placed in liquid scintillation fluid (Perkin-Elmer) overnight and [3H]-radiation was detected the following day by a scintillation counter. Samples were run in triplicate. Remaining sample was stored at −20 °C for molecular analysis.
Western blotting
Protein concentration was determined using the Bio-Rad DC protein assay. The 4x sample buffer containing: 0.3 M Tris pH = 6.8, 40% glycerol, 8% SDS, 200 mM DTT, and 0.01% bromophenol blue was added and samples were stored at −20 °C until use. As previously described (Homiack et al., Citation2017), 25–50 μg of protein was separated on 10% polyacrylamide gels and transferred to nitrocellulose membranes for Western blotting. Membranes were blocked with Odyssey® Tris-buffered saline (TBS) blocking buffer (LI-COR® Lincoln, NE) and incubated overnight at 4° C in Odyssey® blocking buffer with rabbit-anti-pS133-CREB (1:1000, Cell Signaling Technology, Danvers, MA), rabbit-anti-CREB (1:1000, Cell Signaling), rabbit-anti-pERK (1:1000, Cell Signaling), rabbit anti-t-ERK (1:1000, Cell Signaling), rabbit-anti-β-tubulin (1:1000, Cell Signaling), rabbit-anti-ezrin (1:1000, Cell Signaling), rabbit-anti-GFAP (1:1000, PhosphoSolutions, Aurora, CO), mouse-anti-GAPDH (1:10,000, Calbiochem), rabbit-anti-GLT-1 (1:1,000, Boster Biochem), rabbit-anti-GLAST (1:1,000, Boster Biochem, Pleasonton, CA). Rabbit-anti-pan-Jacob antibody was previously described in Dieterich et al. (Citation2008) and Karpova et al. (Citation2013). Following primary incubation, membranes were washed 4x in TBS containing 0.1% Tween-20 and exposed to goat-anti-rabbit (1:10,000) and goat-anti-mouse (1:10,000) antibodies conjugated to near infrared dyes from LI-COR®. Immunofluorescence was detected using the LI-COR Odyssey® system and quantified using LI-COR Image Studio Lite® 3.1 analytical software. Data presented represent combined results from hippocampal homogenate from each cohort (N = 56, n = 14 each for male/female control and TMT-exposed), unless otherwise specified. Data from both cohorts were normalized to samples run on the same gel and combined for a single analysis as results did not differ between cohorts. Western blot and glutamate uptake data were analyzed using a two-way ANOVA comparison across sex and TMT exposure. Based on previous reports indicating sex differences in the response to acute stress between male and female rodents (Homiack et al., Citation2017; Huang et al., Citation2012; Lin et al., Citation2009), a priori Sidak’s multiple comparisons were used to compare the effects of TMT on animals of the same sex.
Results
TMT-evoked behaviors
Male and female animals exposed to TMT in the home cage exhibited increased immobility (F(TMT)(1,52) = 18.98, p < .001, ) and defensive digging behavior (F(TMT)(1,52) = 5.64, p < .05, ). Additionally, TMT-exposed animals avoided the side of the arena in which the stimulus was presented (F(TMT)(1,52) = 64.86, p < .001, ) and exhibited reduced activity (F(TMT)(1,52) = 22.50, p < .001, ), measured as both horizontal crossings (F(TMT)(1,52) = 18.41, p < .001, ) and rearing behavior (F(TMT)(1,52) = 14.80, p < .001, ). There was a main effect of sex for rearing behavior (F(sex)(1,52) = 6.34, p < .05, ), as both TMT and control females exhibited increased rearing relative to male Wistar rats. These results indicate that TMT exposure elicits similar behavioral responses in male and female rodents.
Table 1. Behavioral and physiological outcomes associated with TMT exposure.
Effect of TMT exposure on circulating corticosterone
Animals exposed to TMT exhibited higher levels of corticosterone in trunk blood collected immediately following a 30-minute exposure (F(TMT)(1,52) = 17.40, p < .001, ). Samples from female rodents exhibited higher levels of corticosterone regardless of treatment condition (F(sex)(1,52) = 9.70, p < .01). These data suggest that acute TMT exposure activates the HPA axis, with female Wistar rats having higher levels of circulating corticosterone. Taken together, these data indicate that TMT evokes biological stress responses and species-specific defensive and fear behaviors in both male and female Wistar rats.
Effect of biological sex on predator odor contextual discrimination
Previously, we and others have shown that TMT-exposed male rats exhibit long-term avoidance, but not freezing, when re-introduced to a context in which TMT had previously been presented (Endres & Fendt, Citation2009; Homiack et al., Citation2017; Rosen, Pagani, Rolla, & Davis, Citation2008). However, it remains unknown if TMT-exposed animals can distinguish between a threat-associated context and a separate, but similar context. Furthermore, it is also unclear whether male and female rodents exhibit differing abilities to discriminate between safe and threat-associated contexts, as recently reported with a traditional fear conditioning paradigm (Keiser et al., Citation2017). Therefore, we designed a novel predator odor context discrimination paradigm to test whether male and female rodents exposed to TMT exhibited maladaptive, generalized avoidance, and hyperactive responses (). Consistent with our previous reports, TMT-exposed animals avoided the side of the arena in which TMT had previously been presented relative to a baseline determined as the average of each individual habituation across all habituation sessions (F(trial*TMT)(1,28) = 16.35 p < .001, left). Animals exposed to TMT, when re-introduced to the neutral context, did not exhibit avoidant behavior (F(trial*TMT)(1,28) = 2.60, p = .12 right). However, we observed a significant interaction between TMT-exposed male and females with respect to the level of avoidance in each context (F(trial*sex*context)(1,14) = 5.88, p < .05, ), suggesting that female rats exposed to TMT do not discriminate between neutral and threat-associated contexts as well as male rodents as measured by avoidance.
With respect to hyperactivity, TMT-exposed animals exhibited elevated total activity, relative to habituation averages, in both the TMT-paired (F(trial*TMT)(1,28) = 12.13 p < .01, left) and neutral contexts (F(trial*TMT)(1,28) = 9.28 p < .01). Consistent with previous reports (Blanchard, Griebel, & Blanchard, Citation2003; Endres & Fendt, Citation2009; Homiack et al., Citation2017; McGregor, Schrama, Ambermoon, & Dielenberg, Citation2002; Wallace & Rosen, Citation2000), we observed negligible freezing behavior when animals were re-introduced to either the TMT-paired (F(trial*TMT)(1,28) = 0.05, p = .82) or neutral contexts (F(TMT*trial)(1,28) = 0.44, p = .51; Supplemental Figure 1(A)).
Previous work suggests that heightened feedback inhibition of the HPA axis may be associated with PTSD (Delahanty, Raimonde, & Spoonster, Citation2000; Galatzer-Levy, Karstoft, Statnikov, & Shalev, Citation2014; Resnick, Yehuda, Pitman, & Foy, Citation1995; Yehuda, Citation1998; Yehuda, Giller, Southwick, Lowy, & Mason, Citation1991; Yehuda, Teicher, Trestman, Levengood, & Siever, Citation1996; Yehuda, Yang, Buchsbaum, & Golier, Citation2006). Therefore, we assessed circulating corticosterone immediately following re-introduction to the TMT-paired context. Female rodents exhibited higher corticosterone, regardless of treatment condition when re-exposed to the context (F(sex)(1,27) = 11.36, p < .01; Supplemental Figure 1(B)). To assess the effect of TMT exposure on contextually evoked HPA axis responses independent of regulation by sex, values were normalized to those of same sex controls. TMT-exposed animals exhibited significantly reduced corticosterone (F(TMT)(1,27) = 4.45, p < .05, ) with no interaction between sex and TMT (F(sex*TMT)(1,27) = 1.92, p = .18). This result suggests that prior TMT exposure can dampen HPA axis activation during subsequent context re-exposure.
Effect of TMT exposure on activation of the CREB shut-off cascade
TMT exposure reduced phosphorylated CREB relative to total CREB in the hippocampus (F(TMT)(1,52) = 4.74, p < .05, ) with no main effect of sex (F(sex)(1,52) = 1.22, p = .27), though a significant interaction between sex and TMT exposure was detected (F(TMT*sex)(1,52) = 4.40, p < .05). Sidak’s multiple comparisons post hoc test detected a significant decrease in pCREB expression in homogenate from the hippocampus of TMT-exposed males (t(52) = 3.02, p < .01), but not females (t(52) = 0.06, p > .05) relative to same-sex controls. This result suggests that TMT exposure rapidly reduces CREB phosphorylation in the male, but not the female hippocampus. Since reduced ERK activation is implicated in the CREB shutoff pathway, the expression of phosphorylated ERK relative to total ERK in homogenate was investigated. TMT exposure affected pERK (F(TMT)(1,52) = 5.22, p < .05, ), with no main effect of sex (F(sex)(1,52) = 3.29, p = .08), and no interaction between sex and TMT exposure (F(TMT*sex)(1,52) = 1.50, p = .23). This result suggests that TMT exposure rapidly decreases ERK phosphorylation in the hippocampus.
Figure 2. Effect of TMT exposure on markers associated with CREB shut-off cascade. Left: Representative Western blot images from whole hippocampal homogenate prepared from male and female control and TMT-exposed animals. (A) Male, but not female rats exposed to TMT exhibited lower levels of pCREB relative to CREB. (B) Phosphorylation of ERK was reduced in the male, but not the female hippocampus following TMT exposure. (C) Following TMT exposure, accumulation of pan-Jacob was observed in crude nuclear preparations from the male, but not the female hippocampus. *p < .05 and **p < .01 denote post hoc difference (Sidak’s multiple comparisons test) relative to same sex control animals.
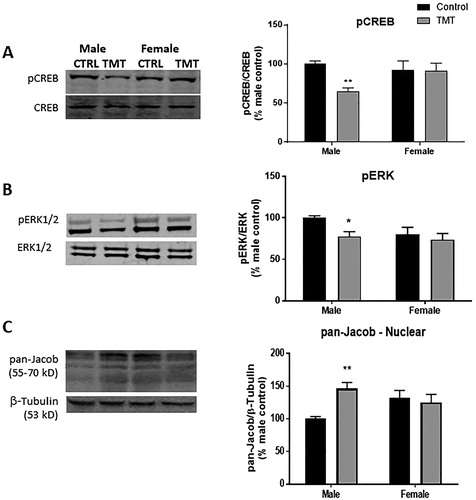
We also observed a significant main effect of TMT exposure (F(TMT)(1,52) = 4.03 p < .05), but not sex (F(sex)(1,52) = 0.29, p = .59) on nuclear expression of pan-Jacob. Furthermore, nuclear pan-Jacob expression was affected by the interaction between TMT exposure and sex (F(TMT*sex)(1,52) = 7.68 p < .01, ). Post hoc analysis revealed that TMT exposure significantly increased expression of Jacob in the crude nuclear fraction of the male (t(52) = 3.38, p < .01), but not the female (t(52) = 0.54, p > .05) hippocampus. Taken together, these data suggest that TMT exposure activates the CREB shut-off cascade in the male, but not the female hippocampus.
Effect of TMT exposure on glutamate reuptake
Previous reports suggest that acute stress can reduce glutamate reuptake in the male but not the female hippocampus (Huang et al., Citation2012). However, these studies assessed glutamate reuptake using crude synaptosomes, which may overrepresent neuronal glutamate reuptake (Petr et al., Citation2015). In consideration of this recent finding, we generated gliosome- and synaptosome-enriched fractions () by differential centrifugation using Percoll® gradient fractionation as previously described (Acheson et al., Citation2012; Carney et al., Citation2014; Dunkley et al., Citation2008; Stigliani et al., Citation2006) to assess glutamate reuptake in both fractions. Gliosomes were enriched with ezrin, a protein associated with fine astroglial processes (Carney et al., Citation2014; Derouiche & Frotscher, Citation2001; Lavialle et al., Citation2011), and glial fibrillary acidic protein (GFAP). Expression of GFAP and ezrin was essentially absent in synaptosomes. However, PSD95 was expressed at high levels in both gliosomal and synaptosomal fractions (). This suggests that differential Percoll centrifugation yields relatively pure synaptosomal fractions whereas gliosomal fractions also contain postsynaptic elements (), consistent with another report (Carney et al., Citation2014).
Figure 3. Effect of TMT on glutamate uptake. (A) Diagram of differential centrifugation. (B) Fraction integrity – expression of ezrin and GFAP, proteins associated with distal and primary astroglial processes were essentially absent from synaptosomal fractions, but PSD95 a postsynaptic marker was enriched in the synaptosome fraction, but also expressed in the gliosomal fraction. (C) Glutamate uptake was dampened in synaptosomes prepared from TMT-exposed animals, an effect which was driven by a larger effect in the male hippocampus. (D) Exposure to TMT also dampened glutamate reuptake in gliosome-enriched fractions, though no post hoc differences were detected. **p < .01 denotes post hoc difference (Sidak’s multiple comparisons test) relative to control animals of the same sex.
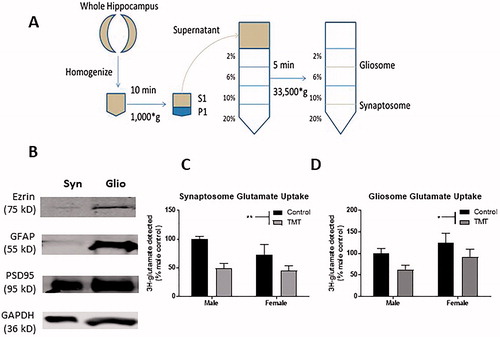
TMT exposure decreased the amount of [3H]-glutamate detected (F(TMT)(1,28) = 12.40, p < .01, ) in the synaptosome-enriched fraction. We did not observe a main effect of sex (F(sex)(1,28) = 1.96, p = .17) or an interaction between TMT exposure and sex (F(TMT*sex)(1,28) = 1.07, p = .31) with respect to [3H]-glutamate detected in synaptosome-enriched fractions. With respect to the gliosome-enriched fraction, similar to the synaptosomal fraction, we observed a significant main effect of TMT (F(TMT)(1,28) = 4.63, p < .05, ), but we did not observe a significant main effect of sex (F(sex)(1,28) = 2.86, p = .10) or interaction between TMT exposure and sex (F(TMT*sex)(1,28) = 0.02, p = .89) on [3H]-glutamate uptake. It is important to note that gliosome and synaptosome fractions exhibited similar levels of [3H]-glutamate uptake (t(62) = 0.18, p = .85), indicating that glial and synaptic transporters exhibit similar amounts of glutamate reuptake. Taken together, these results suggest that TMT exposure dampens glutamate reuptake in the hippocampus.
TMT exposure and biological sex affect EAAT expression
We also assessed expression of the primary EAATs in the hippocampus of control and TMT-exposed male and female Wistar rats. With respect to GLT-1, the primary EAAT responsible for ∼90% of glutamate reuptake in the rodent hippocampus (Danbolt, Citation1994; Holmseth et al., Citation2009), we observed a significant main effect of sex (F(sex)(1,52) = 4.28, p < .05, ), and no main effect of TMT (F(TMT)(1,52) = 3.24, p = .078) or interaction between TMT and sex (F(TMT*sex)(1,52) = 3.36, p = .073). Sidak’s multiple comparisons post hoc test detected a significant decrease in GLT-1 expression in hippocampus of TMT-exposed males (t(52) = 2.6, p = .03), but not females (t(52) = 0.02, p > .05) relative to same-sex controls.
Figure 4. Effect of TMT exposure on EAAT expression. (A) Exposure to TMT selectively reduced expression of GLT-1 in the male hippocampus. (B) Expression of GLAST was higher in the female hippocampus and unaffected by TMT exposure. p < .05, **p < .01, and ***p < .001. Brackets denote main effect of sex. Symbols above TMT data points denote post hoc (Sidak’s multiple comparisons test) differences between control and TMT-exposed animals of the same sex.
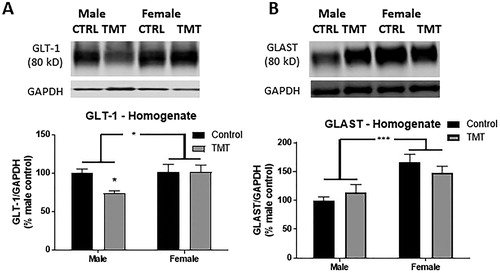
We also assessed expression of GLAST, or EAAT1, which also contributes to glutamate reuptake in the hippocampus. We observed no main effect of TMT exposure (F(TMT)(1,52) = 0.05, p = .83), or interaction between TMT and sex (F(TMT*sex)(1,52) = 1.92, p = .17) with respect to GLAST expression. However, we observed a significant main effect of sex (F(sex)(1,52) = 18.24, p < .001, ) as females, regardless of treatment condition expressed GLAST at higher levels. Taken together, these results suggest that GLAST is expressed at higher levels in the female hippocampus but is unaffected by TMT exposure.
Discussion
Our results demonstrate that alarm odor exposure induces greater generalized avoidance behavior in female rats, yet rapidly reduces CREB phosphorylation in the male, but not the female hippocampus. Taken together with other reports, our findings suggest that severe acute stress differentially affects CREB signaling in the hippocampus in a sex-dependent manner, which may contribute to sex differences in the development of generalized fear (Day, Reed, & Stevenson, Citation2016; Keiser et al., Citation2017; Lynch, Winiecki, Vanderhoof, Riccio, & Jasnow, Citation2016).
In response to TMT exposure, Wistar rats exhibited avoidance, defensive behaviors, immobility, and HPA axis activation. Although 2,4,5-trimethylthiazole is a synthetic compound which is not secreted by a predator, it is commonly used as a stressor (Bronson & Bale, Citation2014; Galliot, Laurent, Hacquemand, Pourie, & Millot, Citation2012; Pérez-Gomez et al., Citation2015) and activates cells in the Grueneberg ganglion of the rostral nasal septum which detect alarm pheromones (Brechbuhl, Klaey, & Broillet, Citation2008; Brechbuhl et al., Citation2014; Brechbuhl et al., Citation2013). Furthermore, TMT activates the HPA axis and increased circulating corticosterone levels and evokes behavioral responses characteristic of alarm odors secreted by predators (Brechbuhl et al., Citation2008; Brechbuhl et al., Citation2014; Brechbuhl et al., Citation2013; Homiack et al., Citation2017; Vernet-Maury, Polak, & Demael, Citation1984; Wallace & Rosen, Citation2000). Although previous studies have shown that butyric acid, an acrid odor, evokes separate behaviors than TMT and is processed by a distinct neural circuit (Day, Masini, & Campeau, Citation2004), we acknowledge the possibility that rodents may perceive TMT as both an alarm odor and a noxious stimulus (Brechbuhl et al., Citation2008; Brechbuhl et al., Citation2014; Brechbuhl et al., Citation2013; Endres & Fendt, Citation2009; Homiack et al., Citation2017; Rosen et al., Citation2008; Vernet-Maury et al., Citation1984; Wallace & Rosen, Citation2000). Nonetheless, our findings indicate that TMT reliably activates biological stress responses and species-specific fear and defensive behaviors in both male and female Wistar rats. Furthermore, consistent with previous reports (Brechbuhl et al., Citation2008; Brechbuhl et al., Citation2014; Brechbuhl et al., Citation2013; Endres & Fendt, Citation2009; Homiack et al., Citation2017; Rosen et al., Citation2008; Vernet-Maury et al., Citation1984; Wallace & Rosen, Citation2000), we observed that TMT supports conditioned avoidance and hyperactivity responses, but not freezing, in both male and female Wistar rats. As such, we conclude that TMT, regardless of whether it is perceived primarily as a predator odor or a noxious stimulus, represents a severe stressor which evokes similar physiological and behavioral responses in male and female Wistar rats. To further characterize the behavioral response to TMT exposure, we investigated whether TMT exposure could elicit generalized avoidance and hyperactivity behaviors as well as HPA axis disturbances in male and female Wistar rats.
Male and female rats exposed to TMT exhibited comparable avoidance and hyperactivity when re-introduced to the TMT-paired context, suggesting similar levels of memory formation. However, female rats exhibited greater avoidance behavior when re-introduced to the neutral context, suggesting greater context generalization, consistent with previous studies (Keiser et al., Citation2017; Lynch, Cullen, Jasnow, & Riccio, Citation2013). Interestingly, TMT-exposed males exhibited reduced circulating corticosterone following re-introduction to the TMT-associated context, indicating altered context-specific HPA axis responsivity. Mechanistically, it remains poorly understood how context discrimination impairments and HPA axis disturbances develop following severe stress exposure. An inability to distinguish between neutral and threat-associated contextual information could be the result of incomplete acquisition during the stressor, or inappropriate retrieval of a fear memory in a neutral context. One possible explanation for such a phenomenon is that the mechanisms by which associative contextual information is encoded following alarm odor exposure differ between male and female rodents.
Sex differences have been reported in acquisition and retention of associative learning paradigms in rodent studies, and in general, females acquire and retain stable fear memories more effectively compared to males (Dalla, Papachristos, Whetstone, & Shors, Citation2009). Previous studies show that female rodents exhibit enhanced hippocampus-dependent conditioned fear memory following stress exposure as well as an impaired ability to discriminate between threat-associated and neutral contexts relative to male rodents following classical fear conditioning (Day et al., Citation2016; Keiser et al., Citation2017). Our present observation that females exhibited reduced ability to discriminate alarm odor and neutral contexts is consistent with these results. However, we did not observe sex differences in memory acquisition as male and female rodents exhibited comparable amounts of avoidance upon re-exposure to the stress context, indicating that observed deficits in context discrimination were not secondary to differences in long-term memory. Consistent with these rodent studies, clinical studies have shown that women demonstrate impaired ability to discriminate contexts in a contextual fear paradigm (Lonsdorf et al., Citation2015). These findings suggest the intriguing possibility that the female hippocampus encodes fear memory representations which are stronger, yet also less specific than the male hippocampus.
In the present investigation, we extended previous observations that acute stress induces signaling changes consistent with the CREB shutoff pathway in the male to directly compare the effects of TMT and biological sex on the phosphorylation state of CREB and ERK as well as the subcellular expression of Jacob. Phosphorylation of CREB was rapidly reduced by TMT exposure in the male, but not the female hippocampus. Furthermore, TMT exposure increased expression of the synaptonuclear messenger protein Jacob in crude nuclear preparations in the male, but not the female hippocampus. Together, these results indicate that TMT rapidly activates the CREB shut-off pathway in the male but not the female hippocampus (Homiack et al., Citation2017). However, we cannot rule out the possibility that modulation of glutamatergic signaling is temporally shifted in the female hippocampus as we only investigated molecular changes immediately following TMT exposure. Future physiological investigations will further address the mechanistic determinants of CREB phosphorylation in female hippocampus.
Activation of the CREB shut-off cascade is thought to be mediated by extrasynaptic NMDA receptors (Dieterich et al., Citation2008; Hardingham & Bading, Citation2002; Hardingham et al., Citation2002). Extrasynaptic NMDA receptors are paired with signaling cascades which dampen and oppose phosphorylation of ERK downstream of synaptic NMDA receptor activity (Ivanov et al., Citation2006; Karpova et al., Citation2013). One possibility is that alarm odor exposure affects a mechanism which preferentially activates extrasynaptic NMDA receptors in the male hippocampus. Observations that acute stress facilitates long-term depression and increases ambient glutamate by increasing glutamate release probability and dampening glutamate reuptake through EAATs in the male hippocampus provide indirect support for this possibility (Karst & Joels, Citation2005; Lowy et al., Citation1995).
To test this hypothesis, we generated purified synaptosome- as well as a gliosome-enriched fraction by differential centrifugation (Carney et al., Citation2014; Dunkley et al., Citation2008) to measure glutamate uptake in the male and female hippocampus following acute TMT exposure. TMT exposure dampened glutamate uptake in both purified synaptosome and gliosome-enriched fractions. Given that TMT exposure dampened glutamate uptake we assessed the expression of the EAATs, GLT-1 and GLAST, which mediate glutamate uptake in the rodent hippocampus. TMT exposure rapidly reduced expression of GLT-1, which mediates approximately 90% of glutamate reuptake in the rodent hippocampus. Interestingly, we observed higher expression of GLAST in homogenate prepared from the female hippocampus, indicating that GLAST expression is affected by biological sex, a finding consistent with previous studies (Mitrovic, Maddison, & Johnston, Citation1999; Sajjad, Felice, Golubeva, Cryan, & O'Mahony, Citation2016). Furthermore, we observed greater variability in glutamate reuptake in both preparations from the female hippocampus (Supplemental Figure 2). Although this observation could be due to many different causes, one possible explanation is that glutamate uptake may fluctuate across the estrous cycle, as has previously been reported in the cerebral cortex (Mitrovic et al., Citation1999). However, in this study, we did not assess the possible contribution of the estrous cycle or ovarian hormones on fear generalization, glutamate reuptake, or activation of the CREB shut-off cascade.
Taken together, the data presented in this report indicate that alarm odor exposure supports sex differences in the development of generalized avoidance behavior in Wistar rats. Furthermore, alarm odor exposure induces rapid signaling changes and altered expression of EAATs in the male, but not the female hippocampus. However, it remains unknown whether sex differences in the development of generalized fear and HPA axis disruption are dependent on modulation of hippocampal glutamatergic signaling. Future studies will assess whether in vivo disruption of glutamate communication during alarm odor exposure contributes to the development of generalized fear and altered HPA axis activation.
Supplemental Figure 2
Download JPEG Image (35 KB)Supplemental Figure 1
Download JPEG Image (43.5 KB)Disclosure statement
DH, EOC, SH, LS, and GD reported no biomedical financial interests or potential conflicts of interest.
Additional information
Funding
References
- Acheson, D.T., Gresack, J.E., & Risbrough, V.B. (2012). Hippocampal dysfunction effects on context memory: Possible etiology for posttraumatic stress disorder. Neuropharmacology, 62, 674–685. doi:10.1016/j.neuropharm.2011.04.029
- Besnard, A., & Sahay, A. (2016). Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology, 41, 24–44. doi:10.1038/npp.2015.167
- Blanchard, D.C., Griebel, G., & Blanchard, R.J. (2003). Conditioning and residual emotionality effects of predator stimuli: Some reflections on stress and emotion. Progress in Neuropsychopharmacology and Biological Psychiatry, 27, 1177–1185. doi:10.1016/j.pnpbp.2003.09.012
- Bourtchouladze, R., Lidge, R., Catapano, R., Stanley, J., Gossweiler, S., Romashko, D., … Tully, T. (2003). A mouse model of Rubinstein-Taybi syndrome: Defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proceedings of the National Academy of Sciences of the United States of America, 100, 10518–10522. doi:10.1073/pnas.1834280100
- Brechbuhl, J., Klaey, M., & Broillet, M.C. (2008). Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science, 321, 1092–1095. doi:10.1126/science.1160770
- Brechbuhl, J., Klaey, M., Moine, F., Bovay, E., Hurni, N., Nenniger-Tosato, M., & Broillet, M.-C. (2014). Morphological and physiological species-dependent characteristics of the rodent Grueneberg ganglion. Frontiers in Neuroanatomy, 8, 87. doi:10.3389/fnana.2014.00087
- Brechbuhl, J., Moine, F., Klaey, M., Nenniger-Tosato, M., Hurni, N., Sporkert, F., … Broillet, M.-C. (2013). Mouse alarm pheromone shares structural similarity with predator scents. Proceedings of the National Academy of Sciences of the United States of America, 110, 4762–4767. doi:10.1073/pnas.1214249110
- Bremner, J.D. (2005). Effects of traumatic stress on brain structure and function: Relevance to early responses to trauma. Journal of Trauma and Dissociation, 6, 51–68. doi:10.1300/J229v06n02_06
- Bremner, J.D. (2006). The relationship between cognitive and brain changes in posttraumatic stress disorder. Annals of the New York Academy of Sciences, 1071, 80–86. doi:10.1196/annals.1364.008
- Bremner, J.D., & Narayan, M. (1998). The effects of stress on memory and the hippocampus throughout the life cycle: Implications for childhood development and aging. Development and Psychopathology, 10, 871–885. doi:10.1017/S0954579498001916
- Breslau, N. (2002). Gender differences in trauma and posttraumatic stress disorder. The Journal of Gender-Specific Medicine : JGSM : The Official Journal of the Partnership for Women's Health at Columbia, 5, 34–40. https://www.ncbi.nlm.nih.gov/pubmed/11859685
- Bronson, S.L., & Bale, T.L. (2014). Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology, 155, 2635–2646. doi:10.1210/en.2014-1040
- Carney, K.E., Milanese, M., van Nierop, P., Li, K.W., Oliet, S.H.R., Smit, A.B., … Verheijen, M.H.G. (2014). Proteomic analysis of gliosomes from mouse brain: Identification and investigation of glial membrane proteins. Journal of Proteome Research, 13, 5918–5927. doi:10.1021/pr500829z
- Cohen, H., Kozlovsky, N., Matar, M.A., Zohar, J., & Kaplan, Z. (2014). Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. European Neuropsychopharmacology, 24, 1925–1944. doi:10.1016/j.euroneuro.2014.09.009
- Dalla, C., Papachristos, E.B., Whetstone, A.S., & Shors, T.J. (2009). Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proceedings of the National Academy of Sciences, 106, 2927–2932. doi:10.1073/pnas.0809650106
- Danbolt, N.C. (1994). The high affinity uptake system for excitatory amino acids in the brain. Progress in Neurobiology, 44, 377–396. doi:10.1016/0301-0082(94)90033-7
- Day, H.E., Masini, C.V., & Campeau, S. (2004). The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Research, 1025, 139–151. doi:10.1016/j.brainres.2004.07.079
- Day, H.L.L., Reed, M.M., & Stevenson, C.W. (2016). Sex differences in discriminating between cues predicting threat and safety. Neurobiology of Learning and Memory, 133, 196–203. doi:10.1016/j.nlm.2016.07.014
- Delahanty, D.L., Raimonde, A.J., & Spoonster, E. (2000). Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological Psychiatry, 48, 940–947. doi:10.1016/S0006-3223(00)00896-9
- Derouiche, A., & Frotscher, M. (2001). Peripheral astrocyte processes: Monitoring by selective immunostaining for the actin-binding ERM proteins. Glia, 36, 330–341. doi:10.1002/glia.1120
- Desmedt, A., Marighetto, A., & Piazza, P.V. (2015). Abnormal fear memory as a model for posttraumatic stress disorder. Biological Psychiatry, 78, 290–297. doi:10.1016/j.biopsych.2015.06.017
- Dieterich, D.C., Karpova, A., Mikhaylova, M., Zdobnova, I., König, I., Landwehr, M., … Kreutz, M.R. (2008). Caldendrin-Jacob: A protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biology, 6, e34. doi:10.1371/journal.pbio.0060034
- Dunkley, P.R., Jarvie, P.E., & Robinson, P.J. (2008). A rapid Percoll gradient procedure for preparation of synaptosomes. Nature Protocols, 3, 1718–1728. doi:10.1038/nprot.2008.171
- Endres, T., & Fendt, M. (2007). Conditioned behavioral responses to a context paired with the predator odor trimethylthiazoline. Behavioral Neuroscience, 121, 594–601. doi:10.1037/0735-7044.121.3.594
- Endres, T., & Fendt, M. (2009). Aversion- vs fear-inducing properties of 2,4,5-trimethyl-3-thiazoline, a component of fox odor, in comparison with those of butyric acid. Journal of Experimental Biology, 212, 2324–2327. doi:10.1242/jeb.028498
- Ferland, C.L., & Schrader, L.A. (2011a). Cage mate separation in pair-housed male rats evokes an acute stress corticosterone response. Neuroscience Letters, 489, 154–158. doi:10.1016/j.neulet.2010.12.006
- Ferland, C.L., & Schrader, L.A. (2011b). Regulation of histone acetylation in the hippocampus of chronically stressed rats: A potential role of sirtuins. Neuroscience, 174, 104–114. doi:10.1016/j.neuroscience.2010.10.077
- Galatzer-Levy, I.R., Karstoft, K.I., Statnikov, A., & Shalev, A.Y. (2014). Quantitative forecasting of PTSD from early trauma responses: A Machine Learning application. Journal of Psychiatric Research, 59, 68–76. doi:10.1016/j.jpsychires.2014.08.017
- Galliot, E., Laurent, L., Hacquemand, R., Pourie, G., & Millot, J.L. (2012). Fear-like behavioral responses in mice in different odorant environments: Trigeminal versus olfactory mediation under low doses. Behavioural Processes, 90, 161–166. doi:10.1016/j.beproc.2012.01.002
- Greenberg, M.E., Thompson, M.A., & Sheng, M. (1992). Calcium regulation of immediate early gene transcription. Journal of Physiology-Paris, 86, 99–108. doi:10.1016/S0928-4257(05)80013-0
- Hardingham, G.E., & Bading, H. (2002). Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochimica et Biophysica Acta, 1600, 148–153. doi:10.1016/S1570-9639(02)00455-7
- Hardingham, G.E., Fukunaga, Y., & Bading, H. (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neuroscience, 5, 405–414. doi:10.1038/nn835
- Holmseth, S., Scott, H.A., Real, K., Lehre, K.P., Leergaard, T.B., Bjaalie, J.G., & Danbolt, N.C. (2009). The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience, 162, 1055–1071. doi:10.1016/j.neuroscience.2009.03.048
- Homiack, D., O'Cinneide, E., Hajmurad, S., Barrileaux, B., Stanley, M., Kreutz, M.R., & Schrader, L.A. (2017). Predator odor evokes sex-independent stress responses in male and female Wistar rats and reduces phosphorylation of cyclic-adenosine monophosphate response element binding protein in the male, but not the female hippocampus. Hippocampus, 27, 1016–1029. doi:10.1002/hipo.22749
- Huang, C.C., Chen, J.P., Yeh, C.M., & Hsu, K.S. (2012). Sex difference in stress-induced enhancement of hippocampal CA1 long-term depression during puberty. Hippocampus, 22, 1622–1634. doi:10.1002/hipo.21003
- Hurst, J.L. (2009). Female recognition and assessment of males through scent. Behavioural Brain Research, 200, 295–303. doi:10.1016/j.bbr.2008.12.020
- Impey, S., Smith, D.M., Obrietan, K., Donahue, R., Wade, C., & Storm, D.R. (1998). Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nature Neuroscience, 1, 595–601. doi:10.1038/2830
- Ivanov, A., Pellegrino, C., Rama, S., Dumalska, I., Salyha, Y., Ben-Ari, Y., & Medina, I. (2006). Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. The Journal of Physiology, 572, 789–798. doi:10.1113/jphysiol.2006.105510
- Jovanovic, T., Kazama, A., Bachevalier, J., & Davis, M. (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmaco-logy, 62, 695–704. doi:10.1016/j.neuropharm.2011.02.023
- Karpova, A., Mikhaylova, M., Bera, S., Bär, J., Reddy, P.P., Behnisch, T., … Kreutz, M.R. (2013). Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell, 152, 1119–1133. doi:10.1016/j.cell.2013.02.002
- Karst, H., & Joels, M. (2005). Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. Journal of Neurophysiology, 94, 3479–3486. doi:10.1152/jn.00143.2005
- Kaufman, A.M., Milnerwood, A.J., Sepers, M.D., Coquinco, A., She, K., Wang, L., … Raymond, L.A. (2012). Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. Journal of Neuroscience, 32, 3992–4003. doi:10.1523/JNEUROSCI.4129-11.2012
- Keiser, A.A., Turnbull, L.M., Darian, M.A., Feldman, D.E., Song, I., & Tronson, N.C. (2017). Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology, 42, 397–407. doi:10.1038/npp.2016.174
- Kessler, R.C., Sonnega, A., Bromet, E., Hughes, M., & Nelson, C.B. (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52, 1048–1060. doi:10.1001/archpsyc.1995.03950240066012
- Lavialle, M., Aumann, G., Anlauf, E., Prols, F., Arpin, M., & Derouiche, A. (2011). Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America, 108, 12915–12919. doi:10.1073/pnas.1100957108
- Lin, Y., Ter Horst, G.J., Wichmann, R., Bakker, P., Liu, A., Li, X., & Westenbroek, C. (2009). Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cerebral Cortex, 19, 1978–1989. doi:10.1093/cercor/bhn225
- Lonsdorf, T.B., Haaker, J., Schümann, D., Sommer, T., Bayer, J., Brassen, S., … Kalisc, R. (2015). Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: The role of biological sex, contraceptives and menstrual cycle phases. Journal of Psychiatry & Neuroscience, 40, 368–375. doi:10.1503/jpn.140336
- Lopresto, D., Schipper, P., & Homberg, J.R. (2016). Neural circuits and mechanisms involved in fear generalization: Implications for the pathophysiology and treatment of posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews, 60, 31–42. doi:10.1016/j.neubiorev.2015.10.009
- Lowy, M.T., Wittenberg, L., & Yamamoto, B.K. (1995). Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. Journal of Neurochemistry, 65, 268–274. doi:10.1046/j.1471-4159.1995.65010268.x
- Lynch, J., 3rd, Cullen, P.K., Jasnow, A.M., & Riccio, D.C. (2013). Sex differences in the generalization of fear as a function of retention intervals. Learn & Memory, 20, 628–632. doi:10.1101/lm.032011.113
- Lynch, J.F., 3rd, Winiecki, P., Vanderhoof, T., Riccio, D.C., & Jasnow, A.M. (2016). Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiology of Learning and Memory, 130, 83–92. doi:10.1016/j.nlm.2016.01.010
- Mak, G.K., Enwere, E.K., Gregg, C., Pakarainen, T., Poutanen, M., Huhtaniemi, I., & Weiss, S. (2007). Male pheromone-stimulated neurogenesis in the adult female brain: Possible role in mating behavior. Nature Neuroscience, 10, 1003–1011. doi:10.1038/nn1928
- McGregor, I.S., Schrama, L., Ambermoon, P., & Dielenberg, R.A. (2002). Not all 'predator odours' are equal: Cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behavioural Brain Research, 129, 1–16. doi:10.1016/S0166-4328(01)00324-2
- Mitrovic, A.D., Maddison, J.E., & Johnston, G.A. (1999). Influence of the oestrous cycle on L-glutamate and L-aspartate transport in rat brain synaptosomes. Neurochemistry International, 34, 101–108. doi:10.1016/S0197-0186(98)00066-7
- Pérez-Gómez, A., Bleymehl, K., Stein, B., Pyrski, M., Birnbaumer, L., Munger, S.D., … Chamero, P. (2015). Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Current Biology, 25, 1340–1346. doi:10.1016/j.cub.2015.03.026
- Petr, G.T., Sun, Y., Frederick, N.M., Zhou, Y., Dhamne, S.C., Hameed, M.Q., … Rosenberg, P.A.. (2015). Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. Journal of Neuroscience, 35, 5187–5201. doi:10.1523/JNEUROSCI.4255-14.2015
- Pitman, R.K., Rasmusson, A.M., Koenen, K.C., Shin, L.M., Orr, S.P., Gilbertson, M.W., … Liberzon, I. (2012). Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience, 13, 769–787. doi:10.1038/nrn3339
- Pittenger, C., & Kandel, E. (1998). A genetic switch for long-term memory. Comptes Rendus De L'academie Des Sciences. Serie Iii, Sciences De La Vie, 321, 91–96. doi:10.1016/S0764-4469(97)89807-1
- Popoli, M., Yan, Z., McEwen, B.S., & Sanacora, G. (2011). The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience, 13, 22–37. doi:10.1038/nrn3138
- Resnick, H.S., Yehuda, R., Pitman, R.K., & Foy, D.W. (1995). Effect of previous trauma on acute plasma cortisol level following rape. American Journal of Psychiatry, 152, 1675–1677. doi:10.1176/ajp.152.11.1675
- Rosen, J.B., Donley, M.P., Gray, D., West, E.A., Morgan, M.A., & Schulkin, J. (2008). Chronic corticosterone administration does not potentiate unconditioned freezing to the predator odor, trimethylthiazoline. Behavioural Brain Research, 194, 32–38. doi:10.1016/j.bbr.2008.06.019
- Rosen, J.B., Pagani, J.H., Rolla, K.L., & Davis, C. (2008). Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: A model for animal phobias. Neuroscience & Biobehavioral Reviews, 32, 1267–1276. doi:10.1016/j.neubiorev.2008.05.006
- Sajjad, J., Felice, V.D., Golubeva, A.V., Cryan, J.F., & O’Mahony, S.M. (2016). Sex-dependent activity of the spinal excitatory amino acid transporter: Role of estrous cycle. Neuroscience, 333, 311–319. doi:10.1016/j.neuroscience.2016.07.036
- Sekeres, M.J., Neve, R.L., Frankland, P.W., & Josselyn, S.A. (2010). Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learning and Memory, 17, 280–283. doi:10.1101/lm.1785510
- Squire, L.R. (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review, 99, 195–231. doi:10.1037/0033-295X.99.2.195
- Stigliani, S., Zappettini, S., Raiteri, L., Passalacqua, M., Melloni, E., Venturi, C., … Bonanno, G. (2006). Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. Journal of Neurochemistry, 96, 656–668. doi:10.1111/j.1471-4159.2005.03631.x
- Tempesta, D., Mazza, M., Iaria, G., De Gennaro, L., & Ferrara, M. (2012). A specific deficit in spatial memory acquisition in post-traumatic stress disorder and the role of sleep in its consolidation. Hippocampus, 22, 1154–1163. doi:10.1002/hipo.20961
- Tolin, D.F., & Foa, E.B. (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132, 959–992. doi:10.1037/0033-2909.132.6.959
- Ullensvang, K., Lehre, K.P., Storm-Mathisen, J., & Danbolt, N.C. (1997). Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. European Journal of Neuroscience, 9, 1646–1655. doi:10.1111/j.1460-9568.1997.tb01522.x
- Venero, C., & Borrell, J. (1999). Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: A microdialysis study in freely moving rats. European Journal of Neuroscience, 11, 2465–2473. doi:10.1046/j.1460-9568.1999.00668.x
- Vernet-Maury, E., Polak, E.H., & Demael, A. (1984). Structure-activity relationship of stress-inducing odorants in the rat. Journal of Chemical Ecology, 10, 1007–1018. doi:10.1007/BF00987509
- Wallace, K.J., & Rosen, J.B. (2000). Predator odor as an unconditioned fear stimulus in rats: Elicitation of freezing by trimethylthiazoline, a component of fox feces. Behavioral Neuroscience, 114, 912–922. doi:10.1037/0735-7044.114.5.912
- Yehuda, R. (1998). Psychoneuroendocrinology of post-traumatic stress disorder. The Psychiatric Clinics of North America, 21, 359–379. doi:10.1016/S0193-953X(05)70010-1
- Yehuda, R., Giller, E.L., Southwick, S.M., Lowy, M.T., & Mason, J.W. (1991). Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biological Psychiatry, 30, 1031–1048. doi:10.1016/0006-3223(91)90123-4
- Yehuda, R., Teicher, M.H., Trestman, R.L., Levengood, R.A., & Siever, L.J. (1996). Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry, 40, 79–88. doi:10.1016/0006-3223(95)00451-3
- Yehuda, R., Yang, R.K., Buchsbaum, M.S., & Golier, J.A. (2006). Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology, 31, 447–451. doi:10.1016/j.psyneuen.2005.10.007
