Abstract
Acute stressor experiences may influence cognition, possibly through actions of cognitive flexibility, which comprises the ability to modify cognitive and behavioral strategies in response to changing environmental demands. In the present investigation, we examined the effects of an acute psychosocial stressor (the Trier Social Stress Test) on a specific form of cognitive flexibility, namely that of set-shifting, which was assessed by the Berg’s Card Sorting Task (BCST). Among undergraduate students, the stressor promoted better performance on the BSCT relative to that evident among nonstressed individuals, including a reduction of perseverative (an index of enhanced set-shifting) and non-perseverative errors. They also required fewer trials to learn the first sorting category, reflecting augmented acquisition of an attentional set, but did not differ in the ability to maintain a set. Moreover, increased cortisol levels specifically mediated the enhancing effects of the acute stressor on set-shifting, but not the ability to acquire and maintain an attentional set. However, this enhancing effect was minimized among individuals who appraised the stressor as being uncontrollable. These data indicate that an acute, social-evaluative stressor can facilitate certain forms of cognitive flexibility, such as set-shifting. The present investigation also highlights the value of focusing on psychological and physiological mediators in determining the impact of stressful experiences on cognitive functioning.
A brief social stressor (public speaking) can have an enhancing effect on mental flexibility, and this seems to be related to the stress hormone, cortisol. This cognitive enhancing effect, however, might be minimized if a stressful situation is perceived as beyond a person’s control.
Lay summary
Introduction
Stressful experiences are believed to favor a shift from “top-down” cognitive control to “bottom-up” processing, resulting in the endorsement of more reflexive and well-learned (habitual) behaviors at the expense of carefully considered (slow) and flexible responses (Arnsten, Citation2015). Accordingly, it would be expected that cognitive flexibility, the ability to adjust cognitive (and behavioral) strategies in response to continuously changing environments (Izquierdo, Brigman, Radke, Rudebeck, & Holmes, Citation2017), would be impaired following an acute stressor. Indeed, in humans, acute stressor exposure was accompanied by a moderate reduction of cognitive flexibility across several paradigms (Shields, Sazma, & Yonelinas, Citation2016a). However, studies involving rodents suggest more complex interactions, indicating that acute stressors can have augmenting, impairing, or no effects on cognitive flexibility (Butts, Floresco, & Phillips, Citation2013; Thai, Zhang, & Howland, Citation2013; George et al., Citation2015), depending on the characteristics of the stressor and the task used (Hurtubise & Howland, Citation2017). Thus, the purpose of the present investigation was to examine the effects of an acute social stressor, the Trier Social Stress Test (TSST), on cognitive flexibility as expressed through set-shifting ability on an open source version of the Wisconsin Card Sorting Task (Berg’s Card Sorting Task; BCST).
The effects of acute stressors on cognitive flexibility might be partly related to the actions of glucocorticoids, such as cortisol, on neuronal circuits guiding this ability (Hurtubise & Howland, Citation2017). Cortisol elevations can promote advantageous cognitive and behavioral outcomes (Sapolsky, Romero, & Munck, Citation2000), being dependent on the magnitude and duration of this stress hormone’s release as well as the specific aspect of cognition required in a given situation (Lupien, Maheu, Tu, Fiocco, & Schramek, Citation2007; Wirth, Citation2015). In the context of cognitive flexibility, heightened levels of cortisol might engender different effects across specific processes underlying this ability. For instance, following an acute stressor, elevated cortisol levels were linked to enhanced “updating flexibility”, but reduced ability to switch between tasks (Goldfarb, Froböse, Cools & Phelps, Citation2017). Thus, in the present investigation, it was hypothesized that moderate elevations of cortisol would mediate the acute stressor effect on set-shifting performance.
Stressor appraisal processes play a central role in determining an individual’s response to a stressful situation (Folkman, Lazarus, Dunkel-Schetter, DeLongis, & Gruen, Citation1986), and might also be involved in predicting differences of cognitive flexibility (Crum, Akinola, Martin, & Fath, Citation2017). For instance, when faced with a stressful event, perceptions of threat might serve to mobilize necessary cognitive resources required to contend with the challenge, although behavioral responses might vary with perceived stressor controllability (Anisman & Matheson, Citation2005). Consistent with this view, individuals who learned to control a noise stressor exhibited increased cognitive control relative to those exposed to uncontrollable noise, and this was especially evident among those who also reported moderate levels of subjective stress (Henderson, Snyder, Gupta, & Banich, Citation2012). Given these findings, it was hypothesized that greater perceived threat would be accompanied by augmented cognitive performance, whereas greater perceived uncontrollability with be linked to reduced set-shifting ability.
Methods
Participants
Initially, 80 participants were recruited from an online computerized recruitment system used by the university. From this initial sample, 16 individuals were excluded due to the use of antidepressants and/or psychostimulants (e.g. methylphenidate), presence of a neurological disorder, or learning disability. The final participant sample comprised 64 (female: n = 44, male: n = 20) undergraduate university students. Participant age ranged from 17 to 25 (M = 19.26, SD = 1.91) and reported ethnicities included White (52.3%, n = 34), Black (23.1%, n = 15), Arab/West Asian (9.2%, n = 6), South Asian (4.6%, n = 3), Latin American/Hispanic (3.1%, n = 2), Asian (1.5%, n = 1), and other (6.2%, n = 4).
Procedure
All procedures were approved by the Carleton University Ethics Committee for Psychological Research. Laboratory sessions were conducted between 1300 and 1730 hr, and participants were asked not to eat, drink (with the exception of water) or smoke for at least an hour before arriving to the session. Once informed consent was signed, participants completed several questionnaires assessing demographic information (e.g. age and gender), general health (e.g. neurological disorders) and medication history (e.g. psychostimulants), and depressive symptomatology. This allowed participants 30 minutes to habituate to the laboratory environment. Following the habituation period, participants were randomly assigned to either the stressor or control condition.
The trier social stress test (TSST)
The stressor condition comprised the TSST, which is a commonly used laboratory task designed to elicit psychological and physiological stress responses. Participants were told that they would engage in a public speaking task (about applying for a research assistantship), and given 5 minutes to prepare, after which they made their presentation in front of a panel of graduate student judges. Thereafter, participants engaged in an arithmetic task for 5 minutes. This consisted of participants being asked to subtract by 17, beginning with the number 1762. Participants were also told they were being videotaped during the TSST. Participants in the control condition were given 15 minutes to complete an employment task, which comprised writing about their strengths and past work/volunteer experience that would be relevant to a research assistant position.
Following the TSST or control task, participants were given 5 minutes to complete two questionnaires concerning measures of stressor appraisal and affect scores. At 5 minutes, participants completed the BCST. During the ensuing 20 minutes, participants completed several questionnaires in order to keep them occupied, after which they were fully debriefed concerning the purpose of the study.
Cognitive flexibility: Set-shifting
Cognitive flexibility, assessed through attentional set-shifting was determined using an open source computerized version of the Wisconsin Card Sorting Task, referred to as Berg’s Card Sorting Task (BCST), provided by the Psychology Experiment Building Language (PEBL) version 0.14 (Mueller & Piper, Citation2014). The BCST consists of a 128-card deck with each card containing a different combination of one of four shapes, colors, and quantities. Four key cards are displayed at the top of the screen as a guide to help determine to which of the four stacks the deck’s up-card is sorted. The deck is revealed one card at a time, and the visible card is matched to key cards depending on the particular rule (unknown to the participant) for a given set. After 10 cards have been successfully matched, the set is completed and the sorting rule changes (also unknown to the participant). The new rule must be discovered using trial and error via feedback received after each card is sorted. After a card is sorted, the participant is provided with feedback regarding whether the response was correct (i.e. according to the current rule). This process continues until the participant either completes all 128 cards, or until the nine sets/categories were successfully completed (see Fox et al., Citation2013). The BCST takes approximately 10 minutes to complete.
The main outcome measures of the BCST are the type of errors the individual makes. Perseverative errors occur when the individual continues to sort cards according to a previously, but no longer, relevant or correct sorting rule. In the present study, perseverative errors were computed according to procedures described by Heaton et al., (Citation1993). Perseverative errors are the primary measure of impaired cognitive flexibility (set-shifting) on this task, and thus were the main focus in the current experiment. Non-perseverative errors, by contrast, refer to all other errors. Among the non-perseverative errors are failures to maintain set, which refers to selecting an incorrect card once a sorting rule has been learned (i.e. switching after the fifth correctly sorted card). Failures to maintain a set are thought to represent distractibility or difficulties maintaining information online in working memory (Barcelo & Knight, Citation2002). Finally, the BCST also assesses trials to first category, which refers to how quickly an individual acquires an attentional set and, in the current study, was used as an index of learning efficiency. Scores pertaining to failures to maintain set and trials to first category were Log10 transformed as they were substantially positively skewed.
Salivary cortisol
Saliva samples were collected in Salivette tubes, (Sarstedt, Germany), 30 mins after participant’s arrival to the laboratory (baseline; 1 min prior to the commencement of the actual study) as well as 5, 15, 30, and 45 mins following the TSST or control condition. Immediately following the experimental session, saliva samples were frozen at −80 °C. Following the manufacturer’s protocol, a competitive radioimmunoassay, 125I kit (ICN Biomedicals Inc., Irvine, CA), was used to determine, in duplicate, salivary cortisol levels. The intra- and inter-assay variability was <10%. The minimum detectable level of cortisol was 0.02 µg/dl and the specificity was 100%. One participant did not have five valid cortisol measures and thus was removed from the repeated measures and AUCi analysis.
Measures
Stressor appraisals
The Stress Appraisal Measure (SAM; Peacock & Wong, Citation1990) was used to assess stressor appraisals immediately following the TSST (or control task). The SAM is a 28-item self-report measure that assesses six relatively independent dimensions of stressor appraisal, threat, challenge, centrality, control-by-self, control-by-others, uncontrollable-by anyone. The items are measured on a five-point scale ranging from 1 (not at all) to 5 (extremely), and scores for each appraisal dimension are computed by taking the mean across all relevant items. The stressfulness component of the SAM has been previously used to assess the effects of an acute stressor on cognitive flexibility (Alexander et al., Citation2007). In the present study, it was of particular interest to determine whether perceptions of threat and control over the stressor would be related the cognitive flexibility and thus only items comprising these stressor appraisals were included. This shortened version of the SAM included 8 items concerning perceptions of threat (“How threatening was this situation?”) and uncontrollability (“Was this a totally hopeless situation?” “Was the outcome of this situation uncontrollable by anyone?”), with higher scores indicating higher levels of perceived threat (α = .88) and uncontrollability (α = .70). As threat and uncontrollability scores were moderately positively skewed, they were square root transformed.
Affective state
The 20-item Positive and Negative Affect Schedule (PANAS; Watson et al., Citation1988) was used to assess positive and negative affect poststressor. Responses ranged on a six-point scale from 1 (very slightly or not at all) to 5 (extremely). Positive (α = .85) and negative (α = .90) affect scores were computed by summing across all 10 relevant items for each subscale. Negative affect scores were substantially positively skewed and thus Log10 transformed.
Statistical analyses
Statistical analyses were performed using SPSS for Windows 20.0 (SPSS Science, Chicago, IL). Multivariate analyses of variance (MANOVAs) were used to examine group differences (stressor vs control) in BCST performance indices as well as stressor appraisals and affective state. Cortisol changes over time as a function of stressor condition were analyzed using a 2 (Stressor Condition) × 2 (Sex) × 5 (Time: 5 time-points) mixed measures ANOVA with Time serving as the within-group factor and Stressor Condition and Sex comprising the between-group factors. Follow-up comparisons comprised t-tests with a Bonferroni correction to maintain the alpha level at 0.05. In addition, Area Under the Curve (AUCi) was computed in order to examine overall changes, indexed by a single value, of cortisol in relation to the BCST performance (Pruessner et al., Citation2003). Moderation and mediation analyses were conducted using the PROCESS v3.0 add-on to SPSS provided by Hayes (Citation2017), using unstandardized predictor, mediator/moderator, and outcome variables, 95% Confidence Intervals (C.I.), and 5000 bootstrap samples.
Results
Acute stressor effects on BCST performance
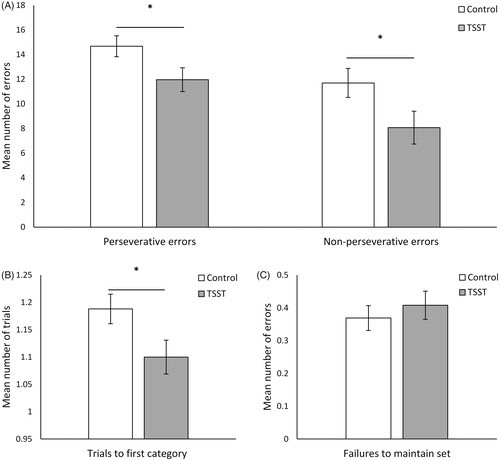
A MANOVA indicated that individuals who completed the TSST made significantly fewer perseverative errors, F(1, 62) = 4.42, p = .040, ω2partial = .051, and non-perseverative errors, F(1, 62) = 4.15, p = .046, ω2partial = .047 on the BCST relative to those in the control condition who were writing about their strengths and previous volunteer experiences (). These individuals also learned the first sorting category more readily, F(1, 62) = 4.65, p = .035, ω2partial = .054 (). However, the groups did not differ in the frequency of failures to maintain a set, F(1, 62) = .48, p = .492, ω2partial = .000 (). A subsequent MANOVA revealed no significant Stressor Condition × Sex interaction on the frequency of perseverative, F(1, 60) = .15, p = .697, ω2partial = .000, and non-perseverative errors, F(1, 60) = .09, p = .770, ω2partial = .000, as well as trials to first sorting category, F(1, 60) = 1.03, p = .314, ω2partial = .000, or failures to maintain set, F(1, 60) = .343, p = .560, ω2partial = .000.
Figure 1. Performance on the BCST, including the frequency of (A) perseverative and non-perseverative errors, (B) trials to first category, and (C) failures in maintaining a set, between individuals who experienced the TSST and control conditions. Error bars represent ± S.E. *p <.05. Note: Figures 1B and 1C display Log10 transformed scores.
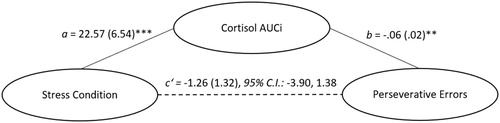
Acute stressor effects on set-shifting: mediating role of cortisol
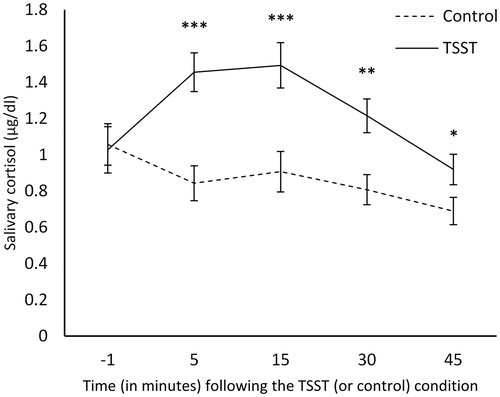
A mixed-measures ANOVA revealed a significant main effect of Time, F(4, 56) = 10.25, p < .001, ω2partial = .378, and Stressor Condition × Time interaction, F(4, 56) = 9.81, p < .001, ω2partial = .367, but no Stressor Condition x Time x Sex interaction, F(4, 56) = 1.08, p = .375, ω2partial = .005, on cortisol over the course of the experiment. As illustrated in , relative to those who were in the control condition, individuals who experienced the TSST exhibited elevated cortisol levels at 5, 15, 30, and 45 minutes following the challenge. A univariate ANOVA revealed a significant main effect of Stressor Condition F(1, 59) = 14.70, p < .001, ω2partial = .183, but no Stressor Condition x Sex interaction F(1, 59) = 1.83, p = .181, ω2partial = .013, on cortisol AUCi. Specifically, relative to the control condition (M = -10.93, S.D. = 29.05), a larger cortisol was apparent among individuals in the TSST condition (M = 14.25, S.D. = 26.48). Additional analyses among females revealed no significant interactions comprising Stressor Condition x Menstrual Cycle, F(4, 36) = 1.51, p = .221, ω2partial = .000, or Stressor Condition x Oral Contraceptive use, F(4, 36) = 2.18, p = .091, ω2partial = .000. However, the lack of statistical significance in this respect might be due to the lack of power in detecting these effects.
Figure 2. Salivary cortisol levels (±S.E.) before and after the TSST and control conditions. **p <.01, ***p <.001.
Greater cortisol AUCi was associated with fewer perseverative (r = −.39, p = .002) and, to a lesser extent, non-perseverative errors (r = −.28, p = .025). Furthermore, increased cortisol AUCi mediated the effects of stressor condition on perseverative errors (ab = −1.40, S.E. = .64, 95% C.I.: −2.72, −.28). As depicted in , the TSST was accompanied by elevated cortisol AUCi and, this in turn, was linked to fewer perseverative errors. By contrast, cortisol AUCi did not mediate the acute stressor effects on non-perseverative errors (ab = −1.24, S.E. = 1.07, 95% C.I.: −3.67, .57). Moreover, cortisol AUCi was unrelated to the number of trials required to learn the first category (r = −.22, p = .092), and failures in maintaining a set (r = −.06, p = .649).
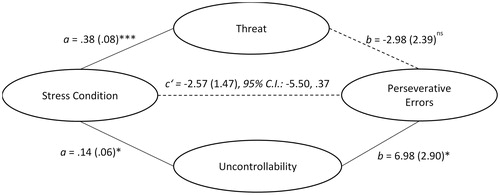
Acute stressor effects on set-shifting: mediating role of stressor appraisals
A MANOVA indicated that relative to the control condition, the TSST was appraised as more threatening, F(1, 62) = 23.46, p < .001, η2partial = .274, and to some extent, more uncontrollable, F(1, 62) = 4.84, p = .031, η2partial = .072. A multiple mediation analysis indicated that the effects of the TSST on perseverative errors were mediated by perceptions of uncontrollability (ab = .98, S.E. = .58, 95% C.I.: .03, 2.29), but not threat (ab = −1.12, S.E. = .77, 95% C.I.: −2.67, .37). As shown in , the TSST was appraised as more uncontrollable, which in turn, was linked to more frequent perseverative errors. These mediating effects were unique to perseverative errors as threat (ab = −.10, S.E. = 1.00, 95% C.I.: −1.92, 2.15) or uncontrollability (ab = .32, S.E. = .61, 95% C.I.: −.90, 1.67) appraisals did not mediated the effects of the TSST on non-perseverative errors, or on trials required to learn first sorting category or failures in maintaining a set (p’s > .05).
Affective state in relation to BCST performance
Individuals in the TSST condition reported greater negative affect, F(1, 62) = 46.17, p < .001, η2partial = .427, following the challenge. However, elevated negative affect did not mediate the acute stressor effects on perseverative (ab = −.57, S.E. = .89, 95% C.I.: −2.26, 1.29) and non-perseverative (ab = −1.22, S.E. = 1.23, 95% C.I.: −3.90, 1.04) errors, as well as trials required to learn first sorting category (ab = −.02, S.E. = .03, 95% C.I.: −.08, .04) or failures in maintaining a set (ab = .09, S.E. = .05, 95% C.I.: −.00, .18).
Discussion
Exposure to an acute social stressor was accompanied by enhanced cognitive flexibility, reflected through more efficient set-shifting performance. The augmenting effects of this stressor were not limited to set-shifting as individuals who were exposed to the TSST also made fewer non-perseverative errors and required less trials to learn the first sorting category. The only parameter of the BCST that was unaffected was the ability to maintain an attentional set, which may have been due to a ceiling effect in both the stressor and control conditions. Thus, exposure to an acute social stressor can facilitate certain forms of cognitive flexibility, including the ability to effectively disengage from no longer relevant information and toward that which is newly relevant.
Importantly, the present data should not be interpreted to suggest that acute stressors have an enhancing effect on all types of cognitive flexibility. Cognitive flexibility is a multifaceted construct that can be manifested in a variety of ways, depending on context or task demands (Goldfarb et al., Citation2017). As such, it is possible that, through effects on different cognitive processes and neural systems, distinct features comprising this ability might be differentially affected by stressors (Hurtubise & Howland, Citation2017). Indeed, whereas we found that the TSST facilitated set-shifting, the same psychosocial stressor impaired another form of cognitive flexibility, that of multi-tasking (Plessow, Fischer, Kirschbaum, & Goschke, Citation2011; Plessow, Schade, Kirschbaum, & Fischer, Citation2012).
As cognitive flexibility appears to be manifested through multiple behaviors, which seem to be governed by different cognitive processes and neuronal circuits (Bissonette & Roesch, Citation2017; Izquierdo et al., Citation2017; Klanker, Feenstra, & Denys, Citation2013), it might be counterproductive to directly compare stressor effects across different tasks. Instead, a more productive comparison might be made by examining the specific cognitive processes required for each aspect of this ability. In this respect, set-shifting is dependent on multiple distinct, but overlapping, processes, including performance and error monitoring, certain aspects of working memory, response inhibition, and to a significant extent, stimulus-response (trial-and-error) learning.
Acute stressors have frequently been shown to impair working memory, although these effects were dependent on the component of this ability being investigated (Luethi, Meier, & Sandi, Citation2008; Oei, Everaerd, Elzinga, van Wall, & Bermond, Citation2006; Schoofs, Preusz, & Wolf, Citation2008; Schoofs, Wolf, & Smeets, Citation2009). Generally, following a mild to moderate stressor, working memory tasks that involve greater cognitive load (effort) were more readily impaired than those requiring less effort (Sandi, Citation2013). For instance, acute stressor exposure specifically impaired the manipulation component of working memory, and to a lesser extent, if at all, the temporary storage of information (Schoofs et al., Citation2009). In line with these findings, we observed that the stressor employed in the present study did not affect the ability to maintain an attentional set, which likely reflects the temporary storage aspect of working memory.
Differential stressor effects have also been observed for inhibitory control processes. Acute stressor exposure was generally accompanied by diminished cognitive inhibition (i.e., selectively attending to or ignoring information), but enhanced response inhibition (i.e., the suppression of a preponent response) (Shields et al., Citation2016a). There has, however, been some contention as to which cognitive tasks reflect cognitive versus response inhibition (Dang, Citation2017; Shields, Citation2017). In accordance with a meta-analysis reporting stress-enhancing effects on response inhibition (Shields et al., Citation2016a), the effects on set-shifting observed in the present study might have partly occurred through an increased ability to suppress a preponent response.
Mild to moderate acute stressors typically facilitate encoding and consolidation processes, but impair information retrieval (Schwabe, Joëls, Roozendaal, Wolf, & Oitzl, Citation2012). Particularly relevant to the present study, stimulus-response learning was shown to be augmented following an acute stressor challenge in humans and animals (Roebuck, Liu, Lins, Scott, & Howland, Citation2018; Vogel & Schwabe, Citation2018). In line with these findings, we observed that the TSST facilitated the acquisition of an attentional set within BCST performance, a process that is heavily dependent on trial-and-error learning. Indeed, enhanced stimulus-response learning may have contributed to the overall better performance on the BCST that was observed among individuals in the stressor condition.
Assessing the effects of acute stressors on cognitive functioning across studies has been made difficult partly due to discrepancies in stressor protocols (e.g. the cold pressor test versus the TSST) (Roos, Knight, Beauchamp, Giuliano, Fisher, & Berkman, Citation2017). As such, focusing on how different stressors influence subjective (e.g., stressor appraisals, emotions) and objective (e.g. sympathetic arousal, cortisol) stressor response markers might be one solution to this issue. In the present investigation, the TSST elicited a moderate increase of cortisol levels (approximately 40 to 50% increase), and heightened cortisol mediated the stressor effects on set-shifting performance (i.e. fewer perseverative errors). Elevated cortisol levels were also linked non-perseverative errors. Similar to these findings, stressor-provoked elevations of cortisol were accompanied by enhanced stimulus-response learning on an explicit rule knowledge test (Vogel, Klumpers, Schroder, Oplaat, Krugers, Oitzl et al, Citation2017; Vogel & Schwabe, Citation2018) and updating flexibility on a task-switching paradigm (Goldfarb et al., Citation2017). Moreover, in a meta-analysis, it was reported that cortisol administration enhanced response inhibition (Shields et al., Citation2015), a process that is fundamental to efficient set-shifting performance.
The impact of a stressful situation on subsequent cognitive functioning might be dependent on the characteristics of the stressor (Arsten, 2015; Anisman & Matheson, Citation2005). At the same time, individuals vary considerably in their response to challenging situations, and it has long been suggested that the stressor appraisal process might play a key role in this respect (Folkman et al, Citation1986). Among individuals who received positive feedback during a job interview stressor (which was aimed to elicit perceptions of challenge instead of threat), a “stress-is-enhancing” mindset was accompanied by enhanced cognitive flexibility, whereas a “stress-is-debilitating” mindset was linked to reduced flexibility (Crum et al., Citation2017). Additionally, among individuals who reported moderate levels of stress following a noise stressor, those who were capable of exerting control over the stressor displayed greater cognitive (interference) control relative to those who were exposed to an uncontrollable noise stressor (Henderson et al., Citation2012).
Similar to these findings, we found that the effects on set-shifting performance was mediated by stressor appraisals, although not quite as expected. Relative to the control condition, the TSST was appraised as more threatening and uncontrollable, and greater uncontrollability mediated the effects of the stressor on decreased set-shifting performance. At first glance, this mediating effect might seem counterintuitive given that, as previously indicated, the TSST was accompanied by augmented BCST performance. In the current study, although greater perceived uncontrollability was strongly related to more frequent perseverative errors on the BCST, the TSST was not appraised as being exceptionally uncontrollable. Thus, it seems that individuals who appraised their previously-experienced situation (i.e. TSST or control condition) as being uncontrollable were more likely to display reduced set-shifting performance. From this perspective, the present data suggest that the potential enhancing effects of a stressful event on cognitive performance might be diminished if the individual interprets their current situation as being uncontrollable.
Exposure to a group version of the TSST was associated with a reduction of set-shifting, but only among men (Shields, Trainor, Lam, & Yonelinas, Citation2016b). Although it is difficult to compare these findings to the present data as a different TSST protocol was used, we tested whether the stressor effects on BCST performance where dependent on sex. In contrast to Shields et al., (Citation2016b), we did not find a significant Stressor × Sex effect on set-shifting, but the meaningfulness of this null finding might be precluded by the relatively small sample size in the current study. In fact, most of the participants in the present study were females which, together with the findings reported by Shields et al., (Citation2016b), raises the possibility that an acute, social stressor might have opposing effects on set-shifting for males and females.
There are several limitations attributed to the present study that should be considered when interpreting the results. Most notably, given that the present study was limited by sample size, we were unable to sufficiently test potential moderating effects, such as sex and age, and thus, the generalizability in this regard should be considered. This notwithstanding, the current data might provide important insight into the processes, including stressor appraisals and cortisol, through which an acute stressor might affect set-shifting ability. It is unclear, however, whether similar effects might be apparent across different types of stressors and other aspects of cognitive flexibility, such as task switching. The present investigation also highlights the value of focusing on potential mediators in determining whether and how cognitive functioning might be affected following a stressor, which frequently are not considered in linking stressful events to cognitive performance.
Acknowledgements
We would like to thank Shane Mueller for his work on PEBL and for scoring the BCST. We would also like to thank the reviewers as their suggestions significantly improved this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alexander, J.K., Hillier, A., Smith, R.M., Tivarus, M.E., & Beversdorf, D.Q. (2007). Beta-adrenergic modulation of cognitive flexibility during stress. Journal of Cognitive Neuroscience, 19, 468–478. doi: 10.1162/jocn.2007.19.3.468
- Anisman, H., & Matheson, K. (2005). Stress, depression, and anhedonia: caveats concerning animal models. Neuroscience & Biobehavioral Reviews, 29, 525–546. doi:10.1016/j.neubiorev.2005.03.007
- Arnsten, A.F. (2015). Stress weakens prefrontal networks: molecular insults to higher cognition. Nature Neuroscience, 18, 1376. doi:10.1038/nn.4087
- Barceló, F., & Knight, R.T. (2002). Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia, 40, 349–356. doi:10.1016/S0028-3932(01)00110-5
- Bissonette, G.B., & Roesch, M.R. (2017). Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience, 345, 64–76. doi:10.1016/j.neuroscience.2016.01.062
- Butts, K.A., Floresco, S.B., & Phillips, A.G. (2013). Acute stress impairs set-shifting but not reversal learning. Behavioural Brain Research, 252, 222–229. doi:10.1016/j.bbr.2013.06.007
- Crum, A.J., Akinola, M., Martin, A., & Fath, S. (2017). The role of stress mindset in shaping cognitive, emotional, and physiological responses to challenging and threatening stress. Anxiety, Stress, and Coping, 30, 379–395. doi:10.1080/10615806.2016.1275585
- Dang, J. (2017). Commentary: the effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Frontiers in Psychology, 8, 1711. doi:10.3389/fpsyg.2017.01711
- Folkman, S., Lazarus, R.S., Dunkel-Schetter, C., DeLongis, A., & Gruen, R.J. (1986). Dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. Journal of Personality and Social Psychology, 50, 992. doi:10.1037/0022-3514.50.5.992
- Fox, C.J., Mueller, S.T., Gray, H.M., Raber, J., & Piper, B.J. (2013). Evaluation of a short-form of the Berg Card Sorting Test. PloS one, 8, e63885. doi:10.1371/journal.poe.0063885
- George, S.A., Rodriguez-Santiago, M., Riley, J., Abelson, J.L., Floresco, S.B., & Liberzon, I. (2015). Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behavioural Brain Research, 286, 256–264. doi:10.1016/j.bbr.2015.02.051
- Goldfarb, E.V., Froböse, M.I., Cools, R., & Phelps, E.A. (2017). Stress and cognitive flexibility: cortisol increases are associated with enhanced updating but impaired switching. Journal of Cognitive Neuroscience, 29, 14–24. doi:10.1162/jocn_a_01029
- Hayes, A.F. (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. NY:Guilford Publications.
- Heaton, R.K., Chelune, G.J., Talley, J.L., Kay, G.G., & Curtiss, G. (1993). Wisconsin Card Sorting Test (WCST): Manual: Revised and Expanded. Odessa, FL:Psychological Assessment Resources (PAR).
- Henderson, R.K., Snyder, H.R., Gupta, T., & Banich, M.T. (2012). When does stress help or harm? The effects of stress controllability and subjective stress response on stroop performance. Frontiers in Psychology, 3, 179. doi:10.3389/fpsyg.2012.00179
- Hurtubise, J.L., & Howland, J.G. (2017). Effects of stress on behavioral flexibility in rodents. Neuroscience, 345, 176–192. doi:10.1016/j.neuroscience.2016.04.007
- Izquierdo, A., Brigman, J.L., Radke, A.K., Rudebeck, P.H., & Holmes, A. (2017). The neural basis of reversal learning: an updated perspective. Neuroscience, 345, 12–26. doi:10.1016/j.neuroscience.2016.03.021
- Klanker, M., Feenstra, M., & Denys, D. (2013). Dopaminergic control of cognitive flexibility in humans and animals. Frontiers in Neuroscience, 7, 201. doi:10.3389/fnins.2013.00201
- Luethi, M., Meier, B., & Sandi, C. (2008). Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Frontiers in Behavioral Neuroscience, 2, 5. doi:10.3389/neuro.08.005.2008
- Lupien, S.J., Maheu, F., Tu, M., Fiocco, A., & Schramek, T.E. (2007). The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition, 65, 209–237. doi:10.1016/j.bandc.2007.02.007
- Mueller, S.T., & Piper, B.J. (2014). The psychology experiment building language (PEBL) and PEBL test battery. Journal of Neuroscience Methods, 222, 250–259. doi:10.1016/j.jneumeth.2013.10.024
- Oei, N.Y., Everaerd, W.T., Elzinga, B.M., van Well, S., & Bermond, B. (2006). Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress, 9, 133–141. doi:10.1080/10253890600965773
- Peacock, E.J., & Wong, P.T. (1990). The stress appraisal measure (SAM): A multidimensional approach to cognitive appraisal. Stress Medicine, 6, 227–236. doi:10.1002/smi.2460060308
- Plessow, F., Fischer, R., Kirschbaum, C., & Goschke, T. (2011). Inflexibly focused under stress: acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. Journal of Cognitive Neuroscience, 23, 3218–3227. doi:10.1162/jocn_a_00024
- Plessow, F., Schade, S., Kirschbaum, C., & Fischer, R. (2012). Better not to deal with two tasks at the same time when stressed? Acute psychosocial stress reduces task shielding in dual-task performance. Cognitive, Affective, and Behavioral Neuroscience, 12, 557–570. doi:10.3758/s13415-012-0098-6
- Pruessner, J.C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D.H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. doi:10.1016/S0306-4530(02)00108-7
- Roebuck, A.J., Liu, M.C., Lins, B.R., Scott, G.A., & Howland, J.G. (2018). Acute stress, but not corticosterone, facilitates acquisition of paired associates learning in rats using touchscreen-equipped operant conditioning chambers. Behavioural Brain Research, 348, 139–149. doi:10.1016/j.bbr.2018.04.027
- Roos, L.E., Knight, E.L., Beauchamp, K.G., Giuliano, R.J., Fisher, P.A., & Berkman, E.T. (2017). Conceptual precision is key in acute stress research: a commentary on Shields, Sazma, & Yonelinas, 2016. Neuroscience & Biobehavioral Reviews, 83, 140–144. doi:10.1016/j.neubiorev.2017.10.005
- Sandi, C. (2013). Stress and cognition. Wiley Interdisciplinary Reviews: Cognitive Science, 4, 245–261. doi:10.1002/wcs.1222
- Sapolsky, R.M., Romero, L.M., & Munck, A.U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21, 55–89. doi:10.1210/edrv.21.1.0389
- Schoofs, D., Preusz, D., & Wolf, O.T. (2008). Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology, 33, 643–653. doi:10.1016/j.psyneuen.2008.02.004
- Schoofs, D., Wolf, O.T., & Smeets, T. (2009). Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behavioral Neuroscience, 123, 1066. doi:10.1037/a0016980
- Schwabe, L., Höffken, O., Tegenthoff, M., & Wolf, O.T. (2013). Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology, 38, 2319–2326. doi:10.1016/j.psyneuen.2013.05.001
- Schwabe, L., Joëls, M., Roozendaal, B., Wolf, O.T., & Oitzl, M.S. (2012). Stress effects on memory: an update and integration. Neuroscience and Biobehavioral Reviews, 36, 1740–1749. doi:10.1016/j.neubiorev.2011.07.002
- Shields, G.S., Bonner, J.C., & Moons, W.G. (2015). Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology, 58, 91–103. doi:10.1016/j.psyneuen.2015.04.017
- Shields, G.S., Sazma, M.A., & Yonelinas, A.P. (2016a). The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668. doi:10.1016/j.neubiorev.2016.06.038
- Shields, G.S., Trainor, B.C., Lam, J.C., & Yonelinas, A.P. (2016b). Acute stress impairs cognitive flexibility in men, not women. Stress (Amsterdam, Netherlands), 19, 542–546. doi:10.1080/10253890.2016.1192603
- Shields, G.S. (2017). Response: Commentary: The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Frontiers in Psychology, 8, 2090. doi:10.3389/fpsyg.2017.02090
- Thai, C.A., Zhang, Y., & Howland, J.G. (2013). Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cognitive, Affective, and Behavioral Neuroscience, 13, 164–173. doi:10.3758/s13415-012-0124-8
- Vogel, S., Klumpers, F., Schröder, T.N., Oplaat, K.T., Krugers, H.J., Oitzl, M.S., … Fernández, G. (2017). Stress induces a shift towards striatum-dependent stimulus-response learning via the mineralocorticoid receptor. Neuropsychopharmacology, 42, 1262. doi:10.1038/npp.2016.262
- Vogel, S., & Schwabe, L. (2018). tell me what to do: Stress facilitates stimulus-response learning by instruction. Neurobiology of Learning and Memory, 151, 43–52. doi:10.1016/j.nlm.2018.03.022
- Watson, D., Clark, L.A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54, 1063. doi:10.1037/0022-3514.54.6.1063
- Wirth, M.M. (2015). Hormones, stress, and cognition: the effects of glucocorticoids and oxytocin on memory. Adaptive Human Behavior and Physiology, 1, 177–201. doi:10.1007/s40750-014-0010-4