Abstract
Oxidative stress is one of the key mechanisms of sepsis related organ dysfunction including stress hyperglycemia. Silent mating type information regulation 2 homolog 1 (SIRT1) could regulate glucose metabolism through its deacetylase activity. In this study, we aimed to investigate the role of SIRT1/forkhead box protein 1 (FoxO1) pathway on lipopolysaccharide (LPS) induced INS-1 cells dysfunction from aspects of oxidative stress and apoptosis. After being treated with 1 mg/L LPS together with or without SIRT1 activator resveratrol (RSV) or SIRT1 inhibitor EX527, cell viability, ROS generation, malondialdehyde (MDA), superoxide, insulin secretion, and activity of superoxide dismutase (SOD) in INS-1 cells were measured by specific assays. Protein expression of SIRT1, FoxO1, toll-like receptor 4 (TLR4), and acetylated FoxO1 (ac-FoxO1) were detected by western blot analysis. Nuclear and cytoplasmic protein was extracted respectively to analyze SIRT1 and FoxO1 redistribution. Mitochondrial potentials and apoptosis were detected by flow cytometry or observed under fluorescence microscope. Results showed that LPS decreased cell viability and insulin secretion, increased ROS, MDA, and superoxide generation, whereas inhibited SOD activity and FoxO1 nuclear transportation. Activation of SIRT1 by RSV down-regulated TLR4 expression, SIRT1 and FoxO1 nuclear protein expression increased after RSV pretreatment. Additionally, LPS induced decreased mitochondrial membrane potentials and structural abnormalities, which could be partially reversed by RSV. SIRT1/FoxO1 may be one of potential targets which could resist against LPS-induced INS-1 cells from oxidative stress damage and mitochondrial dysfunction.
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (Shankar-Hari et al., Citation2016). The key mechanism of sepsis is oxidative stress, which is characterized by redundant reactive oxygen species (ROS) without scavenging in time (Mantzarlis, Tsolaki, & Zakynthinos, Citation2017). Oxidative stress could result in hyperglycemia by stimulating a large number of inflammatory cytokines secretion from pancreatic β cells, which further aggravates islet dysfunction and insulin resistance (Tao et al., Citation2017). It was recently reported that stress hyperglycemia contributed to mortality increase in septic patients (Nugent, Edriss, & Selvan, Citation2016). However, the molecular mechanisms between sepsis and stress hyperglycemia have not been fully understood.
Sirtuin 1 (SIRT1), a NAD+-dependent deacetylase, plays a role in a wide variety of cellular processes and has been demonstrated to protect against oxidative stress in several diseases (Wang, Tang, et al., Citation2017). It could regulate glucose and lipid metabolism through its deacetylase activity (Cao et al., Citation2016). Activation of SIRT1 could suppress neutrophil accumulation and alleviate TNF-α level in sepsis-induced myocardial injury in rats (An et al., Citation2016). Additionally, SIRT1 may participate in septic mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress (Zhao et al., Citation2015). SIRT1 activation inhibits hyperglycemia-induced apoptosis by reducing oxidative stress and mitochondrial dysfunction in human endothelial cells (Wang, Wang, Zhao, & Li, Citation2017). Furthermore, SIRT1 reduced mitochondrial damage by mediating oxidative stress-induced autophagy (Ou, Lee, Huang, Messina-Graham, & Broxmeyer, Citation2014). Transcription factor FoxO1, one of the family members in forkhead box protein (FoxO), is an important substrate of SIRT1, which plays a crucial role in multiple biological processes, including oxidative stress, apoptosis and cell cycle arrest (Kim et al., Citation2017). SIRT1 deacetylated FoxO1 to attenuate sepsis-induced injury (Lee, Jeong, Kim, Lee, & Bae, Citation2017). Several studies demonstrated that SIRT1 might regulate mammalian FoxO1 transcription factors through direct binding and/or deacetylation (Li et al., Citation2016). PI3K/pAkt/FoxO1 may be involved in islet cell proliferation and glycogen contents under hyperglycemia (Kim et al., Citation2017). Multiple effects depended on the changeable transcriptional activity of FoxO1 after posttranslational modifications (Fritz & Radziwill, Citation2011). Recently, drugs also have been used to identify potential Foxo1-dependent regulation in Sepsis (Ghosh et al., Citation2015). FoxO1 is specifically expressed on pancreatic β-cells. But the role of FoxO1 in maintenance of the β cells function remains controversial. Kobayashi et al. (Citation2012) showed that FoxO1 inhibited β-cell growth through a phosphoinositide-dependent protein kinase 1 (Pdk-1)-mediated mechanism, but is required for the maintenance of insulin secretion under metabolic stress.
Our previous observations have demonstrated that LPS, a main component of outer membrane of Gram-negative bacteria, increased TLR4 expression in INS-1 cells (Ge, Du, Bian, Lin, & Su, Citation2011). INS-1 cells derived from transplanted rat insulinoma which are insulin positive and can synthesize proinsulin I and II, which can be used for pancreatic β-cell function investigation. Lee et al. (Citation2014) found that acetylated FoxO1 might take part in the regulation of hepatic gluconeogenesis-related genes in diabetic db/db mice. The acetylation and phosphorylation of FoxO1 markedly increased and changeable SIRT1 content after oleanolic acid treatment in type 2 diabetes mice (Zhou et al., Citation2014). High glucose could reduce SIRT1 protein expression, modulate acetylation level of FoxO1 and p53. Herein, SIRT1/FoxO1 might play an important role in the regulation of glucose metabolism. Moreover, TLR4 is involved in the regulation of LPS inflammatory signals pathway, activation of downstream factors, finally leading to inflammatory damage. Inhibition of TLR4 may prevent rat islet β cells dysfunction from oxidative stress (Wang, Ge, Bian, Dong, & Huang, Citation2017).
Therefore, we try to detect the oxidative damage extent of LPS in INS-1 cells and further make a more comprehensive understanding of the mechanism that SIRT1 might be one of the potential targets in delineating sepsis and stress hyperglycemia.
Materials and methods
Reagents
LPS, resveratrol (RSV), EX527, and DHE were purchased from Sigma (The United States). RPMI-1640 medium, fetal bovine serum, and other cell culture reagents were obtained from Gibco (The United States). SIRT1 and FoxO1 antibodies were purchased from Cell Signaling Technology (The United States). TLR4 and acylated FoxO1 (FKHR) antibody were obtained from Santa Cruz (The United States). Actin antibody, histone H3 mouse monoclonal antibody and HRP-conjugated goat anti-mouse or anti-rabbit antibodies were purchased from Beyotime company (China). Rat insulin Elisa kit, BCA protein assay kit, mitochondrial membrane potential detection kit (JC-1), SOD activity, and malondialdehyde (MDA) test kit were purchased from Beyotime Biotechnology company (China). Apoptosis detect kit was purchased from BD Company (The United States). Nuclear and Cytoplasmic Extraction Reagents were purchased from Thermo company (The United States).
Cell culture and treatment
INS-1 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 10 mmol/L glutamine, 10 mmol/L HEPES, 50 µmol/L β-mercaptoethanol, 100 U/L penicillin, and 100 mg/L streptomycin. Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
INS-1 cells were grouped as follows: (1) CON group (normal control group), (2) LPS group (treated with 1 mg/L LPS), (3) LPS + RSV (pretreated with 10 µmol/L RSV, then treated with 1 mg/L LPS), (4) LPS + EX527 group (pretreated with 20 µmol/L EX527, then treated with 1 mg/L LPS).
MTT assay for INS-1 cell viability
MTT reduction assay was used as a qualitative index of cell viability. The logarithmic growth cells were seeded in 96-well dish. Incubated with MTT solution for 2 h at 37 °C. Subsequently, removed the supernatant and measured at 540 nm.
Western blotting
INS-1 cells lysates were gently mixed and then centrifuged at 12,000g for 15 min at 4 °C. Detected protein concentration to prepare for electrophoresis, then transferred gel to PVDF membranes. Primary antibodies SIRT1 (1:1000), FKHR (1:200), FoxO1 (1:1000), TLR4 (1:2000) were covered on the membrane overnight at 4 °C, followed by incubation with the HRP-conjugated goat anti-mouse or anti-rabbit antibody (1:1000) for 1 h at room temperature. Protein bands were analyzed by Image Lab software and normalized against the intensity of β-actin.
Detection of intracellular ROS by fluorescent microscope and flow cytometry
The oxidative fluorescent dye DHE (10 µmol/L) was used to evaluate the formation of ROS. Red fluorescence intensity signifies ROS production observed under fluorescent microscopy. After DHE incubation, INS-1 cells were trypsinized, harvested, washed twice with PBS, and directly collected before an immediate detection of mean fluorescence intensity (MFI) of DHE by flow cytometry.
MitoSOXTM red method to detect superoxide levels
INS-1 cells were seeded in 6-well dish at a density of 2 × 106/well, grouped as mentioned above. Added 1 mL MitoSOX™ working solution and incubated the INS-1 cells for 10 min at 37 °C in dark. Washed gently with warm buffer then stained cells with DAPI. Finally, fluorescent microscopy was used to observe red fluorescence intensity and flow cytometry was used to detect the superoxide levels.
Cytoplasmic and nuclear protein extraction
INS-1 cells were harvested with trypsin-EDTA and centrifugated at 2000 rpm for 5 min. Added ice-cold 200 µL CER I to fully suspend the cell pellet. Added ice-cold 11 μL CER II and vortexed for 5 s. Centrifuged the tube for 5 min at 1,6000g. Immediately collected supernatant, added ice-cold 100 µL NER and centrifuged the tube at maximum speed for 10 min. Immediately collected the supernatant (nuclear extract).
SOD and MDA levels detection
Collected cell lysate and prepared WST-8/enzyme working solution. Added 160 μL WST-8/enzyme working solution per reaction system, the solution should be stored at 4 °C. Prepared the reaction starting solution and measured at the absorbance of 450 nm.
Made TBA storage solution and MDA testing solution. Put samples in boiling water for 15 min and centrifuged the tube at 1000g, 10 min, and 4 °C. Measured at the absorbance of 532 nm by using a microplate reader.
Glucose-stimulated insulin secretion
INS-1 cells were balanced in KRB (115 mmol/L NaCl; 4.7 mmol/L KCl; 2.6 mmol/L CaCl2; 1.2 mmol/L KH2PO4; 1.2 mmol/L MgSO4; 10 mmol/L NaHCO3; 10 mmol/L Hepes; 0.1% BSA, pH 7.4) containing 3.3 mmol/L D-glucose for 30 min. Then INS-1 cells were incubated in KRB containing 3.3 mmol/L D-glucose for another 1 h, or in KRB containing 16.7 mmol/L D-glucose for 1 h. Buffer was collected from the last 3.3 and 16.7 mmol/L incubations and stored at −80 °C until analysis. Insulin concentration in the medium was measured by Rat Insulin Elisa Kit.
Determination of mitochondrial membrane potential
INS-1 cells seeded in a 6-well dish were treated with 1 mg/L LPS at a density of 2 × 106/well for 24 h. The medium was removed 24 h later and the cells were incubated with 1 mL JC-1 staining buffer for another 30 min in the dark at 37 °C. Then the cells were collected and washed once with PBS. Data were obtained and analyzed using a flow cytometer.
Detect changes in apoptosis
INS-1 cells were cultured in 6-well plates at a density of 2 × 106 cells/well. Trypsinized and collected INS-1 cells. Binding buffer (300 µL) containing 5 µL Annexin V-PE, 5 µL 7-AAD solution were added and cells were incubated at room temperature in the dark for 15 min. The stained cells were measured and analyzed by BD FACS Calibur™.
Statistical analysis
Data were collected from at least three times repeated experiments and presented as means ± standard deviation. Differences between two groups were analyzed by Student’s t-test. One-way analysis of variance after Least Significant Difference analysis and Student–Newman–Keuls test were used for comparison between groups. Statistical significance was considered as p < .05.
Results
INS-1 cell viability in different groups
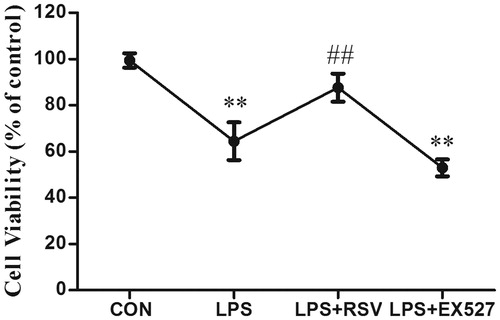
Cultured INS-1 cells with above-mentioned treatments for 24 h, INS-1 cells viability significantly decreased after 1 mg/L LPS stimulation, which was about 0.65-fold to that of control groups (64.46 ± 8.2, p < .01). Pretreated with RSV could reverse the inhibitory effect of LPS (87.63 ± 6.05, p < .01), while pretreated with SIRT1 inhibitor EX527, the viability of INS-1 cells was about 0.52-fold to that of control groups (52.97 ± 3.65, p < .01) ().
The effects of TLR4 expression by activation or inhibition of SIRT1
INS-1 cells were pretreated with SIRT1 agonist RSV or inhibitor EX527. After RSV pretreatment alone on INS-1 cells, SIRT1 expression was up-regulated compared with control group (p < .01). Additionally, RSV pretreatment could reverse LPS-induced decreased SIRT1 protein expression (p < .01). EX527 significantly inhibited the SIRT1 expression, meanwhile its pretreatment aggravated the inhibitory effect of LPS on SIRT1 expression (p < .01). Therefore, RSV or EX527 pretreatment alone did have effects on SIRT1 expression in the absence of LPS.
Figure 2. (A) Effects of RSV or EX527 pretreatment alone on SIRT1 protein expression. (B) Effects of changeable SIRT1 activity on the expression of TLR4 protein. Pretreated INS-1 cells with RSV or EX527 to detect the expression of TLR4 and investigate the relationship between TLR4 and SIRT1. More than three individual experiments were implemented. * p < .05, ** p < .01, vs. control. # p < .05 vs. LPS group.
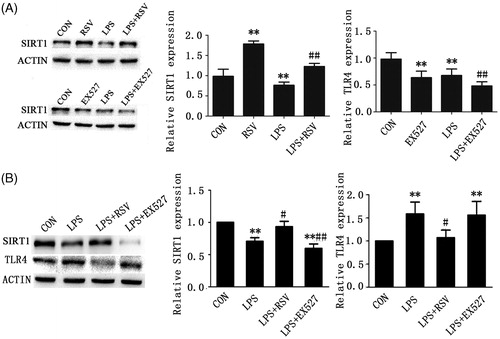
Our previous study (Wang, Tang, et al., Citation2017) demonstrated that TLR4 was partly involved in LPS-induced INS-1 cells dysfunction. To explore the role of SIRT1 in this process, we pretreated INS-1 cells with RSV or EX527 and detected the protein expression of TLR4. One-way ANOVA revealed statistically significant differences among groups in the protein expression of SIRT1 and TLR4 respectively (n = 3, F = 9.577, p = .005; n = 3, F = 6.471, p = .016). LPS stimulation induced downregulation of SIRT1 compared with control group (p < .01), which could be reversed by RSV. Additionally, pretreated with RSV could reverse LPS-induced increased TLR4 protein expression (p < .05). Conversely, pretreated INS-1 cells with EX527 increased the protein expression of TLR4 compared with control (p < .01), whereas did not aggravate LPS induced TLR4 expression (p > .05) ().
Activation of SIRT1 reduced ROS production induced by LPS
DHE is one of the most commonly used detection probes of superoxide anion. It penetrates freely into living cell and can be oxidized by intracellular ROS to form ethidium oxide, which can combine with RNA or DNA to generate red fluorescence.
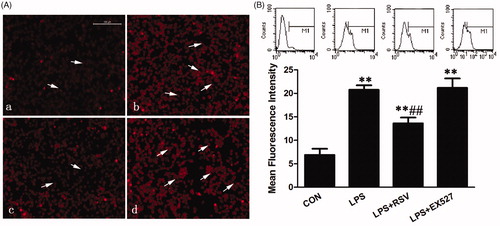
As shown, red fluorescence was relatively weak in normal group which means little ROS production (white arrows pointed area). Exposure of the cells to 1 mg/L LPS for 24 h increased ROS production significantly compared with control (p < .01) (). Pretreated INS-1 cells with RSV and EX527, respectively, we found that the mean fluorescence intensity (MFI) decreased significantly in RSV pretreatment group to that of LPS group (13.61 ± 1.23 vs. 20.75 ± 0.97, p < .01), which explained why RSV pretreatment reversed LPS-induced increased ROS generation. Conversely, the MFI of EX527 group increased compared with control group (21.17 ± 2.02 vs. 6.89 ± 1.32, p < .01), but had not significantly increased ROS generation compared with LPS group (p > .05) ().
Figure 3. Activation of SIRT1 could reverse LPS-induced ROS generation. A. ROS production was observed under fluorescence microscope (×200). Intensity of the fluorescence where white arrows point representing ROS production. (a) CON group, (b) LPS group (treated with 1 mg/L LPS), (c) LPS + RSV (pretreated with 10 μmol/L RSV, then treated with 1 mg/L LPS), (d) LPS + EX527 group (pretreated with 20 μmol/L EX527, then treated with 1 mg/L LPS). B. ROS production was detected by flow cytometry. Values are means ± SD of more than three individual experiments. ** p < .01 vs. control. ## p < .01 vs. LPS group.
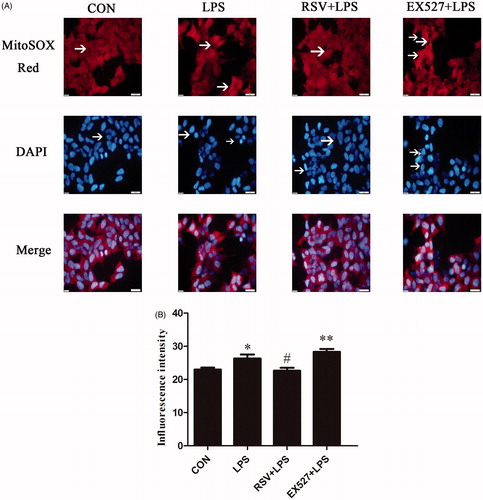
Changes of mitochondrial superoxide levels
MitoSOXTM Red, a novel mitochondrial fluorescent probe, specifically targets mitochondria and selectively detects superoxide originate from mitochondria. The probe passes through the living cell membrane and selectively enters the mitochondria. Once enter into mitochondria, the probe can be oxidized by the superoxide to emit red fluorescence. Red fluorescence represents the site of superoxide origin, whereas the blue represents nuclear location. On the other hand, the reduced cell number represents the effect of drugs on cell damage.
INS-1 cells were seeded in 6-well dish at a density of 2 × 106/well, but LPS and EX527 induced reduced cell numbers. One-way ANOVA revealed statistically significant differences among groups in superoxide generation (na = 3, F = 8.922, p = .006). Compared with the control group, red fluorescence intensity of INS-1 cells in LPS group was enhanced, whereas some nucleuses were pale and densely stained (p < .05). Pretreated with RSV could reduce red influorescence intensity, decrease the superoxide production, and alleviate nuclear damage compared with LPS group (22.67 ± 1.53 vs. 26.33 ± 2.08, p < .05). Pretreated with EX527 had no significant changes on superoxide generation compared with LPS group (p > .05) (28.33 ± 1.53 vs. 26.33 ± 2.08), but have statistical significance compared with control group (p < .01) ().
Figure 4. SIRT1 inhibited superoxide production in LPS-stimulated INS-1 cells. Fluorescence intensity by MitoSOX Red where white arrows point represents the amount of superoxide production, DAPI stained region which white arrows point represents damaged nucleus. A. Effects of SIRT1 activity on superoxide production in INS-1 cells by fluorescence microscopy (×400). B. Fluorescence intensity analysis in INS-1 cells by flow cytometry. Vertical coordinate represents the red MFI of superoxide. * p < .05 vs. control. # p < .05 vs. LPS group.
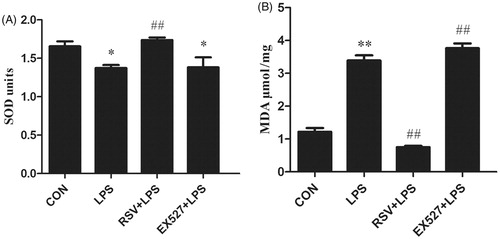
Changes of SOD and MDA levels by activation or inhibition of SIRT1
SOD is an important antioxidant enzyme, which can catalyze the disproportionation of superoxide anion to produce H2O2 and O2. MDA is a natural product of lipid oxidation of organisms. When oxidative stress occurs, lipid oxidation and MDA content will increase. Both indexes were widely used as indicators of oxidative stress. One-way ANOVA revealed statistically significant differences among groups in SOD activity and MDA content (n = 3, F = 5.923, p = .020; n = 3, F = 149.470, p = .000). Compared with control group (SOD: 1.66 ± 0.11, MDA: 1.22 ± 0.20), SOD activity decreased (1.37 ± 0.07, p < .05) and MDA production increased (3.39 ± 0.28, p < .01) in LPS group. Conversely, SOD activity in EX527 group had no significant difference with LPS group (1.38 ± 0.22, p > .05), whereas MDA production increased (3.77 ± 0.25, p < .01). Otherwise, we found increased SOD activity (1.74 ± 0.66, p < .01) and decreased MDA production in RSV group (0.75 ± 0.77, p < .01) ().
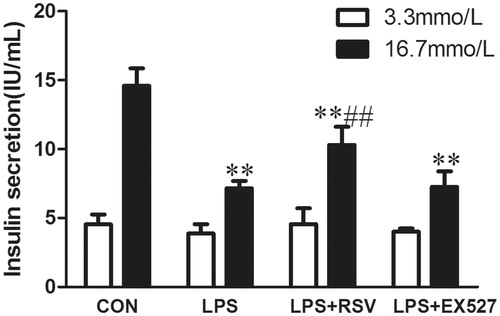
Activation of SIRT1 protected insulin secretion function
To evaluate the role of SIRT1 in LPS-induced pancreatic β-cell dysfunction, we conducted glucose stimulated insulin secretion (GSIS) assays. When cells were stimulated with 3.3 mmol/L basal glucose, there was no difference among groups (n = 3, F = 0.663, p = .598). After stimulating INS-1 cells with 16.7 mmol/L glucose (n = 3, F = 29.835, p = .000). Insulin secretion was measured as basal insulin secretion (BIS) or GSIS. After treating INS-1 cells with LPS for 24 h, there was no insulin secretion peak and insulin secretion was significantly lower than that of control group (p < .01). Pretreated INS-1 cells with RSV increased insulin secretion compared with LPS group (10.31 ± 1.31vs. 7.49 ± 1.11, p < .01), while there was no significant difference between EX527 intervention group and LPS group (p > .05) ().
Figure 6. Activation of SIRT1 inhibited LPS induced INS-1 cells insulin secretion. Incubated INS-1 cells with 1 mg/L LPS for 24 h induced a significant decrease in GSIS. Pretreated INS-1 cells with 10 μmol/L RSV reversed insulin secretion reduction induced by LPS. White column represents BIS induced by 3.3 mmol/L glucose. Black column represents GSIS induced by 16.7 mmol/L glucose. Values are means ± SD of more than three individual experiments. * p < .05, ** p < .01 vs. control, # p < .05 vs. LPS group.
The acetylation of FoxO1 and nuclear localization of SIRT1/FoxO1
Data revealed that the expressions of FoxO1 and ac-FoxO1 were significantly up-regulated in INS-1 cells exposed to 1 mg/L LPS for 24 h. The ratio of ac-FoxO1/FoxO1 increased about 1.7 folds to that of control group (p < .01). Pretreatment INS-1 cells with RSV decreased the expression of FoxO1, ratio of ac-FoxO1/FoxO1 decreased about 3.4 folds to that of LPS group (p < .01) ().
Figure 7. Acetylation of FoxO1 and nucleus-cytoplasm distribution of SIRT1/FoxO1. A. Protein expression of acetylated FoxO1 in INS-1 cells by LPS stimulation with or without RSV for 24 h. B. Cytoplasmic protein expression of SIRT1 and FoxO1. C. Nuclear protein expression of SIRT1 and FoxO1. Values are means ± SD of more than three individual experiments. * p < .05, ** p < .01 vs. control. ## p < .01, # p < .05 vs. LPS group.
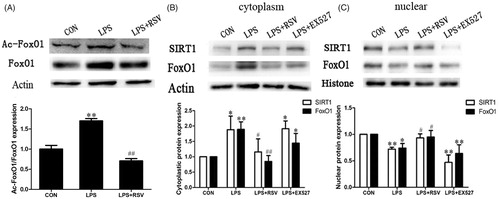
The nuclear expressions of SIRT1 and FoxO1 were higher than the cytoplasmic expression on normal occasion. One-way ANOVA revealed statistically significant differences among groups in SIRT1 and FoxO1 redistribution (nuclear SIRT1 translocation: n = 3, F = 25.486, p = .000; nuclear FoxO1 translocation: n = 3, F = 7.096, p = .012; cytoplasm SIRT1 translocation: n = 3, F = 6.191, p = .018; cytoplasm FoxO1 translocation: n = 3, F = 13.486, p = .002). After LPS treatment for 24 h, cytoplasmic protein expression of SIRT1 and FoxO1 were both up-regulated compared with that of control (p < .05, p < .01). LPS stimulation limited SIRT1 and FoxO1 in INS-1 cytoplasm. Activating SIRT1 by RSV resulted in SIRT1/FoxO1 nuclear redistribution and increased nuclear protein expression of SIRT1/FoxO1 (p < .05), while EX527 has the opposite effect as decreasing FoxO1 nuclear protein expression (p < .01) ().
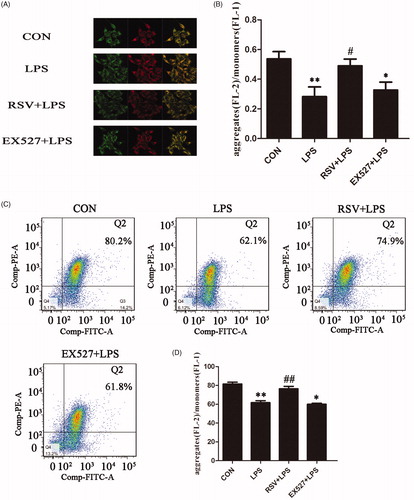
Effects of SIRT1 activation on LPS-induced mitochondrial membrane potential in INS-1 cells
Mitochondrial membrane potential decline is an early landmark event of apoptosis. When the mitochondrial membrane potential is high, JC-1 is shown as the form of polymer aggregation in the matrix of mitochondria and emitted red fluorescence; when the mitochondrial membrane potential is low, JC-1 cannot aggregate in the matrix of mitochondria, where JC-1 emitted green fluorescence as a monomer. As shown, red and green fluorescence intensities of INS-1 cells were measured and pictures were merged. The intensities of red and green color represented the change of mitochondrial membrane potential and it could be used as an early indicator of apoptosis (). One-way ANOVA revealed statistically significant differences among groups in mitochondrial membrane potential (fluorescence intensity: n = 3, F = 5.252, p = .027; cytometry: n = 3, F = 29.070, p = .000). Compared with control group (0.54 ± 0.09), there was lower fluorescence intensity ratio in LPS group (0.28 ± 0.11, p < .01). Compared with LPS group, the fluorescence intensity ratio of RSV group could reverse LPS effect (0.52 ± 0.11, p < .05), but there was no statistically significant changes on EX527 plus LPS group (0.33 ± 0.09, p > .05) (). The mitochondrial potential changes induced by SIRT1 activator or inhibitor were illustrated under flow cytometry (). Data on the right showed the ratios of JC-1 polymer/monomer, which represented mitochondrial potential changes. The ratio of each group showed as follows: control group (81.57 ± 3.27), LPS group (61.73 ± 3.56), RSV pretreatment group (76.43 ± 4.48), EX527 pretreatment group (60.03 ± 1.91). RSV, the activator of SIRT1 could alleviate LPS induced decreased mitochondrial potential ().
Figure 8. Activation of SIRT1 alleviated LPS-induced decreased mitochondrial membrane potential in INS-1 cells. A. Fluorescence intensity pictures were collected from laser confocal microscopy, 6 pictures gotten from in each group. B. Fluorescence intensity was measured by ZEN 2009 Light Edition software. C. Effects of different treatments on mitochondrial membrane potential in INS-1 cells by flow cytometry. Abscissa represents the form of JC-1 monomer while ordinate represents the form of JC-1 polymer, value of quadrant Q2 represents the red/green fluorescence ratio at the early stage of cellular apoptosis. D. Right histogram was based on the statistical results from flow cytometry. Values are means ± SD of more than three individual experiments. ** p < .01 vs. control. # p < .05, ## p < .01 vs. LPS group.
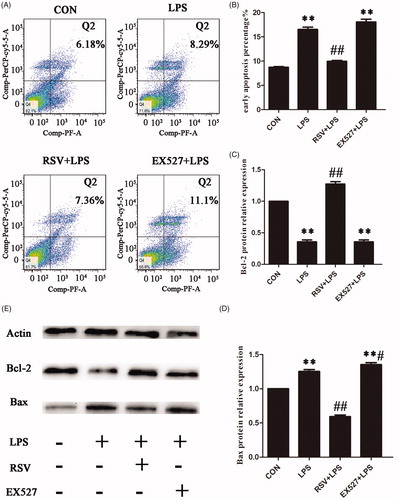
Activation of SIRT1 reduced apoptosis in INS-1 cells
In order to maintain the stability of the internal environment, cells usually react against the impact from the external environment by apoptosis, a self-help orderly death way. One-way ANOVA revealed statistically significant differences among groups in apoptosis and apoptotic proteins (apoptosis: n = 3, F = 139.344, p = .000; Bcl-2: n = 3, F = 274.790, p = .000; Bax: n = 3, F = 213.888, p = .000). As shown, compared with the control group (8.72 ± 0.27), the apoptotic rate of LPS group was significantly higher (16.53 ± 0.83, p < .01) with decreased protein expression of Bcl-2 and increased pro-apoptotic protein Bax. But the apoptotic rate in RSV group was significantly lower than that of LPS group (10.49 ± 0.73, p < .01) with decreased protein expression of Bax. Otherwise EX527 didn’t statistically significant increase apoptosis induced by LPS ().
Figure 9. Effects of SIRT1 on LPS-induced apoptosis in INS-1 Cells. Incubated INS-1 cells with 1 mg/L LPS for 24 h induced a significant increase in apoptosis. Pretreated INS-1 cells with 10 µmol/L RSV alleviated LPS-induced apoptosis. A. Effects of SIRT1 activation on LPS-induced apoptosis in INS-1 cells by flow cytometry. B. The histogram was based on the statistical results from flow cytometry. C–E. Detection of apoptotic protein expression by western blot. More than three individual experiments were implemented in each group. ** p < .01 vs. control. # p < .05, ## p < .01 vs. LPS group.
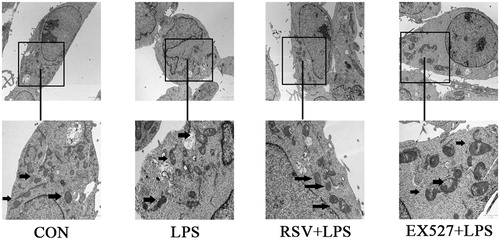
Effects of different treatment on mitochondrial structure in INS-1 cells
Normal mitochondria are elliptical with well-developed ridges, even the electron density groups can be seen in control. In our study, INS-1 cells were incubated with 1 mg/L LPS for 24 h which induced irregular, swelling mitochondria and disordered ridges in INS-1 cells. The mitochondrial structure damage was alleviated by RSV pretreatment. In EX527 group, mitochondrial structure was also irregular, swelling due to mitochondrial inner and outer membrane damage ().
Figure 10. Effects of different treatments on mitochondrial structure in INS-1 cells by transmission electron microscope (×2 μm), the second row pictures were adapted from upper pictures (×1 μm). Arrows refer to mitochondria in different groups. Mitochondria became irregular, swelling and showed more disordered ridges induced by LPS. RSV pretreatment alleviated these damages, but EX527 pretreatment further damaged mitochondrial structure, showing more obscuring or even fragmentary outer membrane.
Discussion
Sepsis is one of the most important causes of death in intensive care units. Oxidative stress, which is the imbalance of the oxidants and antioxidants, plays a key role in sepsis process (Mantzarlis et al., Citation2017). During this process, tremendous proinflammatory cytokines and oxidative products can continuously contribute to a wide range of pathologies. Inadequate antioxidant in pancreatic β cells makes the cells more vulnerable to oxidative stress (Lenzen, Drinkgern, & Tiedge, Citation1996).
Our study showed that 1 mg/L LPS incubation for 24 h decreased INS-1 cells viability which was 0.65-fold lower than control group. LPS increased ROS production and induced apoptosis in INS-1 cells which were reversed by RSV. This was consistent with our previous results that ROS may participate in LPS induced apoptosis (Du, Ge, Lin, Dong, & Su, Citation2012). In this study, we found decreased activity of SOD and increased content of MDA in LPS stimulated group. Arruda et al. (Citation2017) also showed that LPS induced oxidative stress by activating superoxide dismutase and glutathione peroxidase. We speculated that 1 mg/L LPS could induce excessive oxidative side products generation and decrease antioxidase activity. It might determine cellular fate by activating certain signal pathways.
In clinic, stress hyperglycemic is part of adaptive metabolic response to sepsis, leading to the occurrence of insulin resistance (Balloni, Lari, & Giostra, Citation2017), which in turn aggravate islet dysfunction. SIRT1 acting as an antioxidant takes part in protecting against oxidative stress and hyperglycemia, decreasing the production of inflammatory factors (Rada et al., Citation2017). SIRT1 has been demonstrated to improve GSIS by β cell-specific SIRT1 overexpression transgenic mice (Ramsey, Mills, Satoh, & Imai, Citation2008). In our study, we pretreated INS-1 cells with SIRT1 agonist RSV or inhibitor EX527 respectively to interrupt the activity of SIRT1. RSV, a natural polyphenol plant antitoxin presented in grapes, is a specific SIRT1 agonist that can alleviate LPS-induced oxidative stress. EX527 is a potent selective SIRT1 inhibitor, which inhibits specificity by 200 times more than SIRT2 and SIRT3. Our results showed that exposure of INS-1 cells to 1 mg/L LPS for 24 h induced a significant increase in ROS and superoxide production. But RSV pretreatment could inhibit LPS-induced ROS and superoxide production, promote cell proliferation, and reverse insulin secretion reduction. Inhibiting the activity of SIRT1 by EX527 decreased INS-1 cells viability and SOD activity, aggravated insulin secretion dysfunction, increased MDA and ROS production compared with control group. After stimulating INS-1 cells with 16.7 mmol/L glucose, pretreated INS-1 cells with RSV increased insulin secretion compared with LPS group. This is in accordance with Yu WC’s study (Yu, Chen, Hwang, Chen, & Chou, Citation2017) that pancreatic β-cell death and impaired insulin synthesis was relevant with the expression of SIRT1. Other study also demonstrated that SIRT1 up-regulation might promote an anti-oxidative effect which in turn attenuate oxidative damage including increasing SOD activity and decreasing MDA levels, alleviate the levels of inflammatory cytokines (IL-6 and TNF-α) (da Cunha and Arruda, Citation2017). But Park SJ’s study (Park et al., Citation2017) was contrary to current belief, the metabolic effects in glucose homeostasis may be activated independently of SIRT1. We concluded from our study that LPS-induced oxidative stress in INS-1 cells might be alleviated through activating SIRT1 by pretreating with RSV, but cells might suffer from oxidative stress accompanied with the effect of endoplasmic reticulum stress and different inflammation responses in early or late inflammation stage.
FoxO1 is a key transcription factor, which is regulated by activating Insulin/Insulin-like growth factor (IGF-1)/phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway to prompt FoxO1 phosphorylation and translocation, leads to lose its transcriptional activity. The shuttle of FoxO1 from cytoplasm to nucleus where its activity increases takes an important role in regulating insulin secretion (Yin et al., Citation2016). FoxO1 as an important substrate of SIRT1, could be deacetylated by SIRT1, which further regulate the formation of free radical scavengers and participate in suppressing gene transcription by epigenetic mechanisms (Mendes, Lelis, & Santos, Citation2017; Zhang et al., Citation2017). In this study, INS-1 cells were incubated with 1 mg/L LPS for 24 h pretreated with or without 10 µmol/L RSV or 20 µmol/L EX527 for 1 h. We found that the acetylation levels of FoxO1 reduced with RSV pretreatment. SIRT1 and FoxO1 translocated into the cytoplasm in response to LPS stimulation. This is identified with Cochran BJ’s research (Cochran et al., Citation2014) that transfection of Ins-1E cells with a mutated FoxO1, which is restricted to the nucleus confirmed the requirement for FoxO1 nuclear exclusion by blocking insulin secretion in apolipoprotein A-I (apoA-I)-treated INS-1E cells. In response to nitric oxide, FoxO1 translocates from cytoplasm to nucleus and stimulates the expression of the DNA repair gene GADD45α, resulting in FoxO1-dependent DNA repair. Kulebyakin, Penkov, Blasi, Akopyan, and Tkachuk (Citation2016) clarified the relation between nuclear content of FoxO1 and the dynamics of its translocation after insulin addition, they found that due to insulin action, nuclear FoxO1 rapidly degrades in the cytoplasm. When phosphorylated FoxO1 (P-FoxO1) binds to 14-3-3 dimers and occurs nuclear export. P-FoxO1 does not induce pro-apoptotic genes, may rather exert beneficial effects on β cells instead (Gerst et al., Citation2015). Research showed that FoxO1 translocation in response to high glucose stimulation (Kim et al., Citation2012). Nevertheless, RSV could abrogate palmitate-stimulated FoxO1 nuclear translocation (Wu et al., Citation2012). So the role of FoxO1 in regulating cell fate in different stress situation is so complicated. In our study, we pretreated RSV to up-regulate SIRT1 activity for 1 h, then intervened with LPS for 24 h, FoxO1 may degrade in the cytoplasm and transfer to nuclear to activate related DNA repair genes to resist damages. Meanwhile, SIRT1 may de-acetylate FoxO1, reduce its transcriptional activity and induce FoxO1 retention in the nucleus. However, this protection may also be accompanied by other chemical modifications such as FoxO1 phosphorylation and ubiquitination degradation, further in control of its transcriptional activity to target cells.
TLR4 is a common kind of pathogen pattern recognition molecule, which exists in most cells in the islet (Li, Song, Gao, Chang, & Qin, Citation2012). Research showed that LPS could decrease insulin secretion in a TLR4-dependent manner (Amyot, Semache, Ferdaoussi, Fontes, & Poitout, Citation2012). TLR4 is associated with insulin resistance in type 2 diabetes, Zhang et al. (Citation2016) found that insulin suppresses LPS/TLR4 signals in leukocytes through the mTORC2-Akt-FoxO signaling axis. In our previous study, we observed that LPS induced insulin secretion dysfunction accompanied by upregulating the expression of TLR4 (Du et al., Citation2012; Ge et al., Citation2011). In this study, our data demonstrated that LPS increased TLR4 protein expression and ROS production in INS-1 cells, which was reversed by RSV. This result was supported by the research which clarified the TLR4 effects on LPS-induced cellular proliferation (Che et al., Citation2017). After LPS stimulation, the levels of TLR4, MyD88, and NF-κB p65 increased, meanwhile LPS induced NF-κB p65 protein translocation to the nucleus. Thus TLR4/MyD88/NF-κB signaling pathway may be accounted for anti-inflammation effect on LPS-induced dysfunction in MS1 cells (Lu et al., Citation2017). Additionally, through modulating expression of TLR4, bacteria induced nuclear localization of FoxO1 was dependent upon TLR4 and was significantly reduced by inhibiting ROS generation and de-acetylated (SIRT1 and histone deacetylases) (Dong et al., Citation2017). Ohtsu et al. (Citation2017) demonstrated that LPS stimulated IL-6 secretion via the TLR4-NF-κB-ROS pathways and resveratrol attenuated these inflammatory responses in mouse macrophages. Taken together, we speculate that LPS might induce a large amount of ROS production in INS-1 cells, activate TLR4 and NF-κB, and further amplify the inflammatory response. RSV and EX527 are involved in regulating oxidative stress-induced inflammation damage by activating or inhibiting SIRT1 activity, induced de-acetylated or nuclear-cytoplasm shuttle of FoxO1. However, it may also be affected by different inflammation levels at acute or chronic inflammation stages, and responses from immune system.
Mitochondria are important multifunctional organelles that regulate energy production, apoptosis, and calcium homeostasis. Many pathways and disorders are involved in mitochondrial dysfunction and oxidative stress (Han et al., Citation2017). There is a mutual influence between glucose metabolism and mitochondrial function in pancreatic islets (Shi et al., Citation2017). Remaining β-cells contain reduced mitochondrial DNA copy number in hypertrophic pancreatic islets diabetic rats, which may be related to decrease mitochondrial biogenesis or enhanced mitophagy (Spacek et al., Citation2017). As the main site of ROS production, mitochondria are susceptible to oxidative damage or directly suffer from toxic metabolites accumulated in certain metabolic diseases. In our study, 1 mg/L LPS could induce oxidative stress in INS-1 cells after 24 h incubation, which made a negative effect on the structure and function of mitochondria. Fragmented mitochondrial membrane and changed osmotic pressure difference led to mitochondria swelling and disordered ridges. Furthermore, LPS decreased mitochondrial membrane potential in INS-1 cells. RSV pretreatment reversed the effect of LPS and alleviated swelling. Whereas pretreated with EX527 had an opposite effect to RSV. Mitochondrial membrane potential reduction is a feature of early apoptosis. LPS could induce apoptosis which was alleviated by RSV pretreatment, otherwise EX527 increased the apoptosis rate compared with control group. We speculated that RSV could partially protect mitochondrial function by SIRT1 activation. The process of normal mitochondrial functions maintenance is so complex, which also involves the regulation of mitochondria regeneration and mitophagy.
Conclusions
LPS could induce oxidative stress by activating TLR4, further amplify inflammation signal and induce mitochondrial dysfunction, ultimately lead to apoptosis in INS-1 cells. RSV, an activator of SIRT1, deacetylaed its downstream factor FoxO1 and decreased its transcriptional activity by boosting its nuclear protein expression, improved cellular viability and insulin secretion, mitigated apoptosis. Interestingly, EX527 did not evidently aggravate LPS-induced dysfunction. There might have another family member activation of SIRTs to compensate SITR1 dysfunction.
Taken together, Islet β cells injury is an important reason for stress hyperglycemia after infection. Here our study may shed light on the role of SIRT1/FoxO1 in preventing INS-1 cells from oxidative stress injury and apoptosis, providing some useful information on the treatment of sepsis-induced stress hyperglycemia.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Amyot, J., Semache, M., Ferdaoussi, M., Fontes, G., & Poitout, V. (2012). Lipopolysaccharides impair insulin gene expression in iso lated islets of langerhans via toll-like receptor-4 and NF-kappaB signalling. PLoS One, 7, e36200. doi:10.1371/journal.pone.0036200
- An, R., Zhao, L., Xu, J., Xi, C., Li, H., Shen, G., … Sun, L. (2016). Resveratrol alleviates sepsisinduced myocardial injury in rats by suppressing neutrophil accumulation, the induction of TNFalpha and myocardial apoptosis via activation of Sirt1. Molecular Medicine Reports, 14, 5297–5303. doi:10.3892/mmr.2016.5861
- Arruda, M.O., Mendes, S.J.F., Teixeira, S.A., de Mesquita, L.S.S., de Sousa Ribeiro, M.N., Galvão, S. D S L., … Monteiro-Neto, V. (2017). The hydroalcoholic extract obtained from mentha piperita l. leaves attenuates oxidative stress and improves survival in lipopolysaccharide-treated macrophages. Journal of Immunologic Research, 2017, 1. doi:10.1155/2017/2078794
- Balloni, A., Lari, F., & Giostra, F. (2017). Evaluation and treatment of hyperglycemia in critically ill patients. Acta Bio-Medica : Atenei Parmensis, 87, 329–333.
- Cao, Y., Jiang, X., Ma, H., Wang, Y., Xue, P., & Liu, Y. (2016). SIRT1 and insulin resistance. Journal of Diabetes Complications, 30, 178–183. doi:10.1016/j.jdiacomp.2015.08.022
- Che, F., Yin, J., Quan, Y., Xie, X., Heng, X., Du, Y., & Wang, L. (2017). TLR4 interaction with LPS in glioma CD133+ cancer stem cells induces cell proliferation, resistance to chemotherapy and evasion from cytotoxic T lymphocyte-induced cytolysis. Oncotarget, 8, 53495–53507. doi:10.18632/oncotarget.18586
- Cochran, B.J., Bisoendial, R.J., Hou, L., Glaros, E.N., Rossy, J., Thomas, S.R., … Rye, K.-A. (2014). Apolipoprotein A-I increases insulin secretion and production from pancreatic beta-cells via a G-protein-cAMP-PKA-FoxO1-dependent mechanism. Arteriosclerosis, Thrombosis, and Vascular Biology, 34, 2261–2267. doi:10.1161/ATVBAHA.114.304131
- Da Cunha, M.S.B., & Arruda, S.F. (2017). Tucum-do-Cerrado (Bactris setosa Mart.) May promote anti-aging effect by upregulating sirt1-nrf2 pathway and attenuating oxidative stress and inflammation. Nutrients, 9, 1243. doi:10.3390/nu9111243
- Dong, G., Song, L., Tian, C., Wang, Y., Miao, F., Zheng, J., … Graves, D.T. (2017). FOXO1 regulates bacteria-induced neutrophil activity. Frontiers in Immunology, 8, 1088. doi:10.3389/fimmu.2017.01088
- Du, S.C., Ge, Q.M., Lin, N., Dong, Y., & Su, Q. (2012). ROS-mediated lipopolysaccharide-induced apoptosis in INS-1 cells by modulation of Bcl-2 and Bax. Cellular and Molecular Biology (Noisy-Le-Grand), 58, OL1654–OL1659.
- Fritz, R.D., & Radziwill, G. (2011). CNK1 and other scaffolds for Akt/FoxO signaling. Biochimica et Biophysica Acta, 1813, 1971–1977. doi:10.1016/j.bbamcr.2011.02.008
- Ge, Q.M., Du, S.C., Bian, F., Lin, N., & Su, Q. (2011). Effects of lipopolysaccharides on TLR4 expression in INS-1 rat insulinoma cells. Cellular and Molecular Biology (Noisy-Le-Grand, France), 57, OL1513–OL1519.
- Gerst, F., Kaiser, G., Panse, M., Sartorius, T., Pujol, A., Hennige, A.M., … Ullrich, S. (2015). Protein kinase Cdelta regulates nuclear export of FOXO1 through phosphorylation of the chaperone 14-3-3zeta. Diabetologia, 58, 2819–2831. doi:10.1007/s00125-015-3744-z
- Ghosh, C.C., Thamm, K., Berghelli, A.V., Schrimpf, C., Maski, M.R., Abid, T., … Parikh, S.M. (2015). Drug repurposing screen identifies foxo1-dependent angiopoietin-2 regulation in sepsis. Critical Care Medicine, 43, e230–e240. doi:10.1097/CCM.0000000000000993
- Han, R., Hu, M., Zhong, Q., Wan, C., Liu, L., Li, F., … Ding, W. (2017). Perfluorooctane sulphonate induces oxidative hepatic damage via mitochondria-dependent and NF-kappaB/TNF-alpha-mediated pathway. Chemosphere, 191, 1056–1064. doi:10.1016/j.chemosphere.2017.08.070
- Kim, M., Chung, H., Yoon, C., Lee, E., Kim, T., Kim, T., … Park, J. (2012). Increase of INS-1 cell apoptosis under glucose fluctuation and the involvement of FOXO-SIRT pathway. Diabetes Research and Clinical Practice, 98, 132–139. doi:10.1016/j.diabres.2012.04.013
- Kim, M-K., Shin, H.M., Jung, H.S., Lee, EJu., Kim, T.K., Kim, T.N., … Park, J.H. (2017). Comparison of pancreatic beta cells and alpha cells under hyperglycemia: Inverse coupling in pAkt-FoxO1. Diabetes Research and Clinical Practice, 131, 1–11. doi:10.1016/j.diabres.2017.05.017
- Kobayashi, M., Kikuchi, O., Sasaki, T., Kim, H.-J., Yokota-Hashimoto, H., Lee, Y.-S., … Kitamura, T. (2012). FoxO1 as a double-edged sword in the pancreas: Analysis of pancreas- and beta-cell-specific FoxO1 knockout mice. American Journal of Physiology: Endocrinology and Metabolism, 302, E603–E613. doi:10.1152/ajpendo.00469.2011
- Kulebyakin, K., Penkov, D., Blasi, F., Akopyan, Z., & Tkachuk, V. (2016). The transcription factor Prep1 controls hepatic insulin sensitivity and gluconeogenesis by targeting nuclear localization of FOXO1. Biochemical and Biophysical Research Communications, 481, 182–188. doi:10.1016/j.bbrc.2016.10.146
- Lee, Y., Jeong, G.S., Kim, K.M., Lee, W., & Bae, J.S. (2017). Cudratricusxanthone A attenuates sepsis-induced liver injury via SIRT1 signaling. Journal of Cellular Physiology, 233, 5441–5446. doi:10.1002/jcp.26390
- Lee, Y.S., Lee, E.K., Oh, H.H., Choi, C.S., Kim, S., & Jun, H.S. (2014). Sodium meta-arsenite ameliorates hyperglycemia in obese diabetic db/db mice by inhibition of hepatic gluconeogenesis. J Diabetes Research, 2014, 1. doi:10.1155/2014/961732
- Lenzen, S., Drinkgern, J., & Tiedge, M. (1996). Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Biology and Medicine, 20, 463–466. doi:10.1016/0891-5849(96)02051-5
- Li, Z., He, Q., Zhai, X., You, Y., Li, L., Hou, Y., … Zhao, J. (2016). Foxo1-mediated inflammatory response after cerebral hemorrhage in rats. Neuroscience Letters, 629, 131–136. doi:10.1016/j.neulet.2016.06.013
- Li, M., Song, L., Gao, X., Chang, W., & Qin, X. (2012). Toll-like receptor 4 on islet beta cells senses expression changes in high-mobility group box 1 and contributes to the initiation of type 1 diabetes. Experimental and Molecular Medicine, 44, 260–267. doi:10.3858/emm.2012.44.4.021
- Lu, M., Zhang, Q., Chen, K., Xu, W., Xiang, X., & Xia, S. (2017). The regulatory effect of oxymatrine on the TLR4/MyD88/NF-kappaB signaling pathway in lipopolysaccharide-induced MS1 cells. Phytomedicine, 36, 153–159. doi:10.1016/j.phymed.2017.10.001
- Mantzarlis, K., Tsolaki, V., & Zakynthinos, E. (2017). Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxidative Medicine and Cellular Longevity, 2017, 1–10. doi:1155/2017/5985209
- Mendes, K.L., Lelis, D.F., & Santos, S.H.S. (2017). Nuclear sirtuins and inflammatory signaling pathways. Cytokine and Growth Factor Reviews, 38, 98–105. doi:10.1016/j.cytogfr.2017.11.001
- Nugent, K., Edriss, H., & Selvan, K. (2016). Hyperglycemia and outcomes in patients with sepsis. Journal of Thoracic Disease, 8, E575–E577. doi:10.21037/jtd.2016.05.63
- Ohtsu, A., Shibutani, Y., Seno, K., Iwata, H., Kuwayama, T., & Shirasuna, K. (2017). Advanced glycation end products and lipopolysaccharides stimulate interleukin-6 secretion via the RAGE/TLR4-NF-kappaB-ROS pathways and resveratrol attenuates these inflammatory responses in mouse macrophages. Experimental and Therapeutic Medicine, 14, 4363–4370. doi:10.3892/etm.2017.5045
- Ou, X., Lee, M.R., Huang, X., Messina-Graham, S., & Broxmeyer, H.E. (2014). SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells, 32, 1183–1194. doi:10.1002/stem.1641
- Park, S.-J., Ahmad, F., Um, J.-H., Brown, A.L., Xu, X., Kang, H., … Chung, J.H. (2017). Specific Sirt1 activator-mediated improvement in glucose homeostasis requires sirt1-independent activation of AMPK. EBioMedicine, 18, 128–138. doi:10.1016/j.ebiom.2017.03.019
- Rada, P., Pardo, V., Mobasher, M., Garcia, I., Ruiz, L., Gonzalez Rodriguez, A., … Valverde, A. (2017). SIRT1 controls acetaminophen hepatotoxicity by modulating inflammation and oxidative stress. Antioxidants and Redox Signaling, 28, 1187–1208. doi:10.1089/ars.2017.7373
- Ramsey, K.M., Mills, K.F., Satoh, A., & Imai, S. (2008). Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell, 7, 78–88. doi:10.1111/j.1474-9726.2007.00355.x
- Shankar-Hari, M., Phillips, G.S., Levy, M.L., Seymour, C.W., Liu, V.X., Deutschman, C.S., … Singer, M. (2016). Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA, 315, 775–787. doi:10.1001/jama.2016.0289
- Shi, T.T., Yang, F.Y., Liu, C., Cao, X., Lu, J., Zhang, X.L., … Yang, J.K. (2017). Angiotensin-converting enzyme 2 regulates mitochondrial function in pancreatic beta-cells. Biochemical and Biophysical Research Communications, 495, 860–866. doi:10.1016/j.bbrc.2017.11.055
- Spacek, T., Pavluch, V., Alán, L., Capková, N., Engstová, H., Dlasková, A., … Ježek, P. (2017). Nkx6.1 decline accompanies mitochondrial DNA reduction but subtle nucleoid size decrease in pancreatic islet beta-cells of diabetic Goto Kakizaki rats. Scientific Reports, 7, 15674. doi:10.1038/s41598-017-15958-6
- Tao, S., Yuan, Q., Mao, L., Chen, F.L., Ji, F., & Cui, Z.H. (2017). Vitamin D deficiency causes insulin resistance by provoking oxidative stress in hepatocytes. Oncotarget, 8, 67605–67613. doi:10.18632/oncotarget.18754
- Wang, X., Ge, Q.M., Bian, F., Dong, Y., & Huang, C.M. (2017). Inhibition of TLR4 protects rat islets against lipopolysaccharide-induced dysfunction. Molecular Medicine Reports, 15, 805–812. doi:10.3892/mmr.2016.6097
- Wang, K., Tang, Z., Wang, J., Cao, P., Li, Q., Shui, W., … Zhang, Y. (2017). Retraction notice to Polysaccharide from Angelica sinensis ameliorates high-fat diet and STZ-induced hepatic oxidative stress and inflammation in diabetic mice by activating Sirt1-AMPK pathway [JNB 43 (2017) 88-97]. Journal of Nutritional Biochemistry, 47, 133. doi:10.1016/j.jnutbio.2017.07.007
- Wang, S., Wang, J., Zhao, A., & Li, J. (2017). SIRT1 activation inhibits hyperglycemia-induced apoptosis by reducing oxidative stress and mitochondrial dysfunction in human endothelial cells. Molecular Medicine Reports, 16, 3331–3338. doi:10.3892/mmr.2017.7027
- Wu, L., Zhou, L., Lu, Y., Zhang, J., Jian, F., Liu, Y., … Li, G. (2012). Activation of SIRT1 protects pancreatic beta-cells against palmitate-induced dysfunction. Biochimica et Biophysica Acta, 1822, 1815–1825. doi:10.1016/j.bbadis.2012.08.009
- Yin, Y., Yong, W., Yu, J., Zhang, X., Lin, H., Zhu, Y., & Han, X. (2016). Pdcd2l promotes palmitate-induced pancreatic beta-cell apoptosis as a foxo1 target Gene. PLoS One, 11, e0166692. doi:10.1371/journal.pone.0166692
- Yu, W.C., Chen, Y.L., Hwang, P.A., Chen, T.H., & Chou, T.C. (2017). Fucoidan ameliorates pancreatic beta-cell death and impaired insulin synthesis in streptozotocin-treated beta cells and mice via a Sirt-1-dependent manner. Molecular Nutrition and Food Research, 61, 136. doi:10.1002/mnfr.201700136.
- Zhang, M., Zhang, Q., Hu, Y., Xu, L., Jiang, Y., Zhang, C., … Yan, G. (2017). miR-181a increases FoxO1 acetylation and promotes granulosa cell apoptosis via SIRT1 downregulation. Cell Death and Disease, 8, e3088. doi:10.1038/cddis.2017.467
- Zhang, Z., Amorosa, L.F., Coyle, S.M., Macor, M.A., Birnbaum, M.J., Lee, L.Y., & Haimovich, B. (2016). Insulin-dependent regulation of mtorc2-akt-foxo suppresses tlr4 signaling in human leukocytes: Relevance to type 2 diabetes. Diabetes, 65, 2224–2234. doi:10.2337/db16-0027
- Zhao, L., An, R., Yang, Y., Yang, X., Liu, H., Yue, L., … Qu, Y. (2015). Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: The role of SIRT1 signaling. Journal of Pineal Research, 59, 230–239. doi:10.1111/jpi.12254
- Zhou, X., Zeng, X.-Y., Wang, H., Li, S., Jo, E., Xue, C.C.L., … Ye, J.-M. (2014). Hepatic FoxO1 acetylation is involved in oleanolic acid-induced memory of glycemic control: Novel findings from study 2. PLoS One, 9, e107231. doi:10.1371/journal.pone.0107231