Abstract
Familial caregivers of Alzheimer’s disease (AD) patients experience an emotional and physical burden which characterizes a chronic stress condition. The resulting hypothalamic-pituitary-adrenal axis dysfunction favors an imbalance of neurotoxic/neuroprotective factors and causes cognitive impairments, increasing the caregivers’ risk for cognitive decline and compromising their ability to provide adequate care of the patient. Therefore, the present study aimed to investigate the reversibility of the cognitive impairments of familial caregivers of AD patients during their caregiving-related chronic stress condition. Thirty-three caregivers (61.42 + 2.68 years; 27 women) and thirty-four controls (57.91 ± 2.16 years, 20 women) were evaluated for their cognitive functioning (attention, executive function, processing speed and memory) with a neuropsychological battery (Digit-span, Trail Making, Stroop and the Logical Memory tests). Subjects’ cortisol/dehydroepiandrosterone (DHEA) ratios were determined by radioimmunoassay, and their brain-derived neurotrophic factor (BDNF) levels were analyzed by ELISA. An incidental contextual memory task, with or without an associative encoding instruction, was used to investigate if caregivers have a cognitive reserve prone to rehabilitation. The contextual memory impairment of caregivers was associated with prefrontal and hippocampal cognitive dysfunctions, alterations of the cortisol/DHEA ratio and lower BDNF levels. Even so, the contextual memory impairment could be improved by the associative encoding condition. This study suggests that the cognitive impairments of caregivers are not necessarily irreversible, as indicated by the results obtained for contextual memory, which could be improved despite the ongoing chronic stress and associated hormonal and neurotrophin dysfunctions.
Lay summary
The support of a relative with Alzheimer’s Disease submits the familial caregivers to a chronic stress condition that increases their own risk of cognitive decline. This study suggests that, irrespective to their alterations on cortisol/DHEA ratio and BDNF levels, caregivers have a cognitive reserve that could probably be engaged to limit the negative effects of chronic stress on cognition.
Introduction
Alzheimer’s disease (AD) is the leading cause of disability and dependence among older people (Alzheimer’s Disease Association, Citation2014). Most AD patients are cared by family members, which are exposed to greater strain than do caregivers of older adults with other conditions (Dassel, Carr, & Vitaliano, Citation2017). The “ironic tragedy,” as put by Vitaliano (Citation2010), is that the chronic stress condition associated to the patient’s support increases the risk of familial caregivers for cognitive decline and dementia, besides other negative health outcomes (Corrêa et al., Citation2015b; Norton et al., 2010; Richardson, Lee, Berg-Weger, & Grossberg, Citation2013; Vitaliano, Murphy, Young, Echeverria, & Borson, Citation2011).
Chronic stress is associated with the hypothalamic-pituitary-adrenal (HPA) axis hyperactivity (McEwen, Citation2004) and besides the potential neurotoxic effects of high cortisol levels (Lupien, McEwen, Gunnar, & Heim, Citation2009), it is also accompanied by decreases in two neuroprotective substances, dehydroepiandrosterone (DHEA) and brain-derived neurotrophic factor (BDNF) (Cattaneo, Cattane, Begni, Pariante, & Riva, Citation2016; Kowiański et al., Citation2017; Maninger, Wolkowitz, Reus, Epel, & Mellon, Citation2009). Alterations of cortisol, DHEA and BDNF levels seem to play a key role on prefrontal and hippocampal dysfunctions, leading to the impairment of cognitive components such as attention, executive function and different memory types (Kim, Pellman, & Kim, Citation2015; Lu, Nagappan, & Lu, Citation2014; Pluchino et al., Citation2015; Shansky & Lipps, Citation2013). The caregiver’s impairment in these cognitive domains are well established (Allen et al., Citation2017) and an association with alterations on cortisol/DHEA ratios and BDNF levels has been shown by ours (Corrêa et al., Citation2015a, Citation2016) and other research groups (Vedhara et al., Citation2002).
Caregivers’ cognitive impairments can compromise their quality of life and hinder the adequate care of the patient (Vitaliano et al., Citation2011). Therefore, we propose to investigate if the cognitive impairments of familial caregivers are reversible even during their caregiving-related chronic stress condition and associated hormonal and neutrophin dysfunctions. For doing so, we choose an incidental contextual memory paradigm.
Contextual memory is the where, when and how component of episodic, or autobiographical, memories (Johnson, Hashtroudi, & Lindsay, Citation1993). It is dependent on prefrontal and hippocampal functions (Mitchell & Johnson, Citation2009) and essential for coherent episodic memory representations (Hollingworth & Rasmussen, Citation2010). Using a cross-sectional case-control design, we evaluated the effect of high and low cognitive support at encoding on contextual memory recognition of AD familial caregivers. The incidental nature of our memory task, combined with the different encoding conditions, allows the identification of latent process-based components (such as neural reserve and neural compensation) (Balardin et al., Citation2009; dos Santos et al., Citation2010). If present, these components suggest the existence of a cognitive reserve that is prone to rehabilitation (Barulli & Stern, Citation2013).
Considering the aim of this study, our hypothesis was that AD caregivers would show reversible contextual memory deficits, suggesting the existence of a cognitive reserve that can be engaged during the chronic stress phase, despite the cognitive and physiological alterations associated with this condition.
Materials and methods
Participants
Thirty-three (61.42 ± 2.68 years; 27 women) family caregivers of AD patients were recruited from the assistance group for caregivers of AD patients of the Brazilian Alzheimer Association (ABRAZ, Porto Alegre, Brazil). Additionally, thirty-four (57.91 ± 2.16 years, 20 women) non-caregiver (control) subjects were recruited from the community. Exclusion criteria were: use of medications that could interfere with the HPA axis or cognition, past or current use of psychoactive drugs, unstable medical conditions, neurological trauma or diseases, scores on Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, Citation1975) compatible with dementia and scores on Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI) (Cunha, Citation2001) indicative of severe depressive or anxiety symptoms, as well as visual or hearing impairments. None of the control subjects reported ongoing or previous psychiatric problems. All participants were required to abstain from alcohol use 24 h before testing. The body mass index (BMI) of all participants was also evaluated. The Research Ethics Committee of the Pontifical Catholic University of Rio Grande do Sul (Porto Alegre, Brazil) approved this study. All participants gave their written informed consent.
Stress symptoms evaluation
Psychological and physical stress symptoms were evaluated according to the Lipp Inventory of Stress Symptoms for Adults (ISSL) (Lipp & Guevara, Citation1994) a questionnaire that classifies subjects as non-stressed or stressed and, in the latter case, defines the phase of stress.
The stress phases of the ISSL were based on Hans Selye’s three-stage Stress Response Model (alarm, resistance and exhaustion) (Selye, Citation1956). However, during the ISSL validation, two distinct moments (characterized by different amounts and intensities of the same symptoms) were identified in Selye’s resistance phase. The new identified stage was called pre-exhaustion phase by the ISSL authors. In short, the four ISSL stress phases can be described as follows: alarm, considered the positive phase of stress, is characterized by the fight-or-flight reactions, preserves survival and frequently promotes a wellbeing sensation; resistance, wherein the body automatically tries to adapt to the stressful stimulus and regain homeostasis; pre-exhaustion, when the body defenses begin to subside to the stressor and homeostasis cannot be maintained, characterized by oscillations between feelings of wellbeing and discomfort and by the appearance of some diseases; exhaustion, characterized by a total resistance breakdown, presence of some symptoms that are similar to the alarm phase (but much more intense) and development of more serious diseases (Lipp, Citation2003).
Contextual memory task
The materials and procedures for the assessment of recognition memory for objects and contexts have been described elsewhere (Balardin et al., Citation2009; dos Santos et al., Citation2010). Participants were unaware that a test session for recognition of objects and contexts would follow the training session. To allow the evaluation of the cognitive reserve, participants were randomly assigned to one of two encoding (training) conditions: with (associative condition) or without (non-associative condition) an incidental binding cue to facilitate the association of the object to its context. Performance increases from the non-associative to the associative encoding condition are considered an indicative of cognitive reserve (Balardin et al., Citation2009; Corrêa et al., Citation2015b; dos Santos et al., Citation2010).
During the training session, participants viewed eighteen photographs of objects from different semantic categories (household appliances, tools, toys, and clothing) placed in three locations: a living room, a kitchen and an office. For each photograph, participants had to respond to one of two questions: (a) how much the object is used in their daily activities (non-associative encoding condition) or (b) how well the object fits in the room (associative encoding condition). After a 5-Minute interval, in which participants were engaged in a distracting activity, the memory test was given. Thirty-six photographs were shown during this phase: each of the 18 objects previously presented (9 in the same room as previously presented, and 9 in a different room) and 18 distractor objects not previously presented (6 presented in the living room, 6 in the kitchen and 6 in the office). Participants responded verbally as to whether the object was presented during the encoding phase of the study, or not. If the participant indicated that the object was previously presented, three consecutive photographs of the same object in each location (living room, kitchen and office) were depicted on a computer screen and participants were asked to indicate in which of the three locations the object had appeared previously. The order of photograph presentation was randomized for each subject.
Scores of contextual recognition memory are expressed as the proportion of objects attributed to the correct context, considering the number of objects accurately identified as old.
Neuropsychological measures
All participants completed a neuropsychological battery to evaluate different cognitive components that could affect the performance on the contextual memory task. Working memory was evaluated with the Digit-span tests (Nascimento, Citation2004; Wechsler, Citation1997), attention and processing speed were analyzed with the Trail Making A and B tests (Strauss et al., Citation2006), inhibitory response capacity was assessed with the word/color (III) version of the Stroop test (Strauss et al., Citation2006), and delayed recall of declarative memory was investigated with the Logical memory test version II (Wechsler, Citation1987). All procedures followed the recommended guidelines for each specific task and have been briefly described elsewhere (Corrêa et al., Citation2015a).
Coping strategy evaluation
The Brazilian version of the Coping Strategies Inventory (Savóia, Santana, & Mejias, Citation1996) comprises 66 items that encompass thoughts and actions used to deal with internal or external demands of a specific stressful event. In this manner, the questionnaire evaluates if the individual’s coping strategy is problem-focused or emotion-focused.
Cortisol and DHEA analysis
Participants were asked to collect saliva samples at home at 8:00 and 22:00 h on the day of the memory assessment. Subjects were instructed to write down at which time they woke up and at which time they effectively collected their first saliva sample. Only samples collected in the first hour after awakening were included in the cortisol and DHEA analysis. The samples were stored between 0 °C and 4 °C by the subjects and were delivered to the laboratory within three days, where they were frozen at −80 °C until further analysis. After thawing, each sample was divided for cortisol and DHEA assessment. Samples for cortisol analysis were centrifuged for 3 min at 1500 rpm and then analyzed by radioimmunoassay (Beckman Coulter kit, Immunotech) using a gamma counter. The assay sensitivity was estimated at 0.09 nmol/L. Samples for the DHEA analysis were centrifuged for 3 min at 2500 rpm and then analyzed by radioimmunoassay (Beckman Coulter kit, Immunotech). The sensitivity of the DHEA assay was estimated at 0.06 nmol/L. All samples for both cortisol and DHEA were analyzed in duplicate, and the results from each of the sampling times were expressed in nmol/L.
BDNF
Blood was collected from each volunteer via venipuncture into an anticoagulant-free vacuum tube. The clotted blood samples were then centrifuged at 4000 rpm for 10 minutes, and the serum was kept frozen at –80 °C until further analysis (Corrêa et al., Citation2016). The serum BDNF analysis was performed using an ELISA kit following the manufacturer’s instructions (Millipore, USA). In short, microtiter plates (96-well flat-bottom) were coated for 24 h at 4 °C with the samples diluted 1:100 in sample diluent and the standard curve ranging from 7.8 to 500 ng/ml of BNDF. The plates were then washed four times with wash buffer followed by the addition of biotinylated mouse anti-human BNDF monoclonal antibody (diluted 1:1000 in sample diluent), which was incubated for 3 h at room temperature. After washing, a second incubation was carried out with streptavidin-horseradish peroxidase conjugate solution (diluted 1:1000) for 1 h at room temperature. After addition of the substrate and stop solution, the amount of BDNF was determined (absorbance set at 450 nm). The standard curve demonstrated a direct relationship between optical density and BDNF concentration.
Statistical analysis
Sample sizes were calculated whit the Biostat 5.0 software (Ayres, Ayres, Ayres, & Santos, Citation2007), based on the results obtained in former studies of our research group (Barlardin et al., 2009; Corrêa et al., Citation2015a), and considering a β = 0.80, a two-tailed α = 0.05 and ANOVA (four treatments) as the primary statistical analysis to be used in this study. The sample sizes were calculated for all variables and the results indicated that a minimum of 16 subjects/experimental group would encompass the needs of all variables and be adequate for the purposes of this study.
Differences between groups on demographic, clinical and neuropsychological characteristics were analyzed with independent samples t tests and chi-squared statistics. Results of the contextual memory task were analyzed with two-way ANOVAs (with encoding condition and experimental group as main variables) and confirmed with one-way ANOVAs and Bonferroni’s post hoc test whenever necessary. Since the Shapiro-Wilk test rejected the normality of data distribution for cortisol/DHEA ratios, raw data were log-transformed for analysis with a mixed design ANOVA (with sampling time as the within variable and experimental group as the between variable) and confirmed with dependent and independent measures ANOVAs. The raw data for cortisol/DHEA are reported in order to be physiologically meaningful. BDNF levels were analyzed with a one-way ANOVA. To strengthen the internal validity and generalizability of our results, we also run covariance analysis (ANCOVAs) for all variables (neuropsychological tests, contextual memory performance, BDNF levels and cortisol/DHEA ratios), including age, depressive and anxiety symptoms as possible confounding variables. The rationale for doing so is described in the results section. Finally, linear regressions were used to evaluate the associations between: (I) the neuropsychological tests and the physiological parameters (cortisol/DHEA ratio and BDNF levels); (II) the contextual memory performance and the other neuropsychological tests; (III) the contextual memory scores and the physiological variables (cortisol/DHEA ratio and BDNF levels). Effect sizes for were expressed as Cohen’s ds (Cohen, Citation1988) for independent samples t tests, eta squared (η2p) for analysis of variance (Lakens, Citation2013) and Pearson correlation coefficient (R) for linear regressions (Maher, Markey, & Ebert-May, Citation2013). Results are expressed as mean ± standard error and the statistical significance was set at p < .05.
Results
Demographic and clinical characteristics
shows the demographic and psychiatric characteristics of caregivers and controls. Groups did not differ in age [t = 1.021, df =65, p = .311, Cohen’s ds= 0.249], gender [Pearson Chi-Square =0.003, p = .954], years of education [t = 0.960, df =65, p = .340, Cohen’s ds= 0.234], MMSE [t = –0.099, df =65, p = .921, Cohen’s ds= 0.024] and BMI [t = –0.813, df =65, p = .420, Cohen’s ds= 0.198]. However, caregivers showed significantly higher scores on depression [BDI, t = 10.418, df =65, p < .001, Cohen’s ds = 2.443] and anxiety [BAI, t = 6.692, df =65, p < .001, Cohen’s ds= 1.63] symptoms. As explained in the methods section, only controls who were classified as non-stressed were included in the study. Thus, no symptoms of chronic stress were reported for controls and they were also not evaluated for coping strategies. As can be seen in , most caregivers where in the resistance phase (49% of the sample) of stress, but a significant portion was also in the near exhaustion (24% of the sample) and exhaustion (27% of the sample) phases. Moreover, caregivers showed more psychological than physical stress symptoms. As expected from our recruitment strategy, caregivers had a high weekly assistance schedule (117.93 ± 10.56), were already committed to their patients for some years (4,5 in average) and used mostly problem-focused coping strategies.
Table 1. Demographic and psychiatric measures (mean ± standard error) of controls and caregivers.
The significant group differences on BDI and BAI scores, the quite large range of age (31–84 years) and the evidences on the effects of these variables on neuropsychological, cortisol, DHEA and BDNF outcomes (Byers & Yaffe, Citation2011; Ferrari & Magri, Citation2008; Lommatzch et al., 2005; Samson & Barnes, Citation2013) led us to test the effects of age, depression and anxiety symptoms as possible confounding factors in the interpretation of our cognitive and physiological data. Thus, covariance analyses were run (as described below) in order to strengthen internal validity and generalizability of results.
Cortisol/DHEA ratios
The raw values of cortisol and DHEA that originated the cortisol/DHEA ratios can be seen in . shows the cortisol/DHEA ratios of controls and caregivers. The two-way ANOVA identified significant time [F(1,65) = 596.064, η2p = 0.895 p < .001] and group effects [F(1,65) = 4.146, η2p = 0.06, p = .046], but no significant interaction between them [F(1,65) = 3.049, η2p = 0.085, p = .045] on cortisol/DHEA ratios. As indicated by repeated measures ANOVAs, cortisol/DHEA ratios were higher at 8:00 than at 22:00 h in controls [F(1,32) = 331.659, η2p = 0.910, p < .001] and caregivers [F(1,32) = 230.551, η2p = 0.878, p < .001]. Moreover, one-way ANOVAs indicated significant group differences at 22:00 h, when caregivers showed higher cortisol/DHEA ratios than controls [F(1,65) = 19.164, η2p = 0.228, p < .001], but not at 8 AM [F(1,65) = 0.153, η2p = 0.002, p = .697]. Depressive and anxiety symptoms showed no significant effects as covariates on cortisol/DHEA ratios (all p > .05). Age, on the other hand, was a significant covariate for cortisol/DHEA ratios at 8:00 [p = .037] and 22:00 h [p < .001]. Even so, the resulting adjustment of means was not able to modify the outcomes described above for sampling time effects [F(1,65) = 83.011, η2p = 0.565, p < .001] and group differences on cortisol/DHEA ratios at 22:00 h [F(1,65) = 3.687, η2p = 0.068 p < .034].
Figure 1. Cortisol/DHEA ratios (mean ± standard error) of caregivers and controls at 8:00 and 22:00h. *p < .05 between groups.
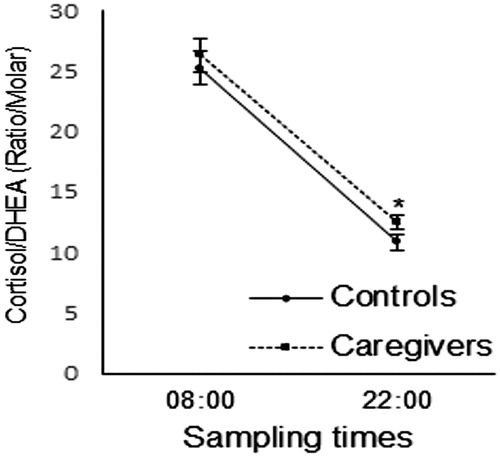
Table 2. Raw saliva cortisol and DHEA levels (mean ± standard error) of controls and caregivers.
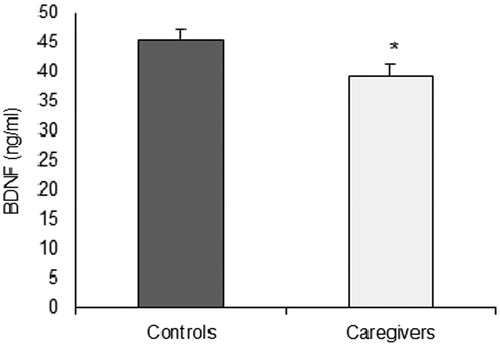
BDNF levels
Serum BDNF levels of controls and caregivers are shown in . The one-way ANOVA indicated significantly lower levels of BDNF in caregivers compared with controls [F (1,67) = 5.198, η2p = 0.074, p = .026]. Age, depressive and anxiety symptoms were not significant covariates of BDNF levels (all p > .05).
Neuropsychological data
The performance of caregivers was worse than that of controls at all neuropsychological tests, as shown by the scores obtained for Forward [F(1,65) = 64.031, η2p = 0.496, p < .001] and Backward [F(1,65) = 98.435, η2p = 0.602 p < .001] digit span, Trail Making A [F(1,65) = 13.635, η2p = 0.173, p < .001] and B [F(1,65) = 14.000, η2p = 0.177, p < .001], Stroop III [F(1,65) = 16.001, η2p = 0.198, p < .001] and Logical Memory II [F(1,65) = 67.677, η2p = 0.510, p < .001]. Depressive and anxiety symptoms had no significant effects as covariates on cognitive performance (all p > .05). Age, on the other hand, had a significant effect on all neuropsychological tests (all p < .05). Even so, the resulting adjustment of the means did not eliminate differences in cognitive performance between caregivers and controls (all p < .01, η2p = 0.199 – 0.606). The only exceptions were Trail Making A (p = .174) and B (p = .061), in which adjustment of the means eliminated the differences of performance between groups ().
Table 3. Neuropsychological measures (mean ± standard error) of controls and caregivers.
Linear regressions () showed that performance on all neuropsychological tests (except for Trail Making A and B) was negatively associated with the 22:00 h cortisol/DHEA ratio (all p < .05). BDNF, on the other hand, was positively associated with the performance on Backward digit span and Logical Memory II (all p < .05).
Table 4. Linear regressions between physiological parameters and the neuropsychological tests.
Contextual memory test
As explained before, the evaluation of the cognitive reserve with the contextual memory task demanded the subdivision of each experimental group into two subgroups with different encoding instructions. Thus, four experimental groups emerged: controls in the associative encoding condition (n = 17), controls in the non-associative encoding condition (n = 17), caregivers in the associative encoding condition (n = 15) and caregivers in the non-associative encoding condition (n = 18). A one-way ANOVA was run to compare the demographic, clinical, neuropsychological and physiological characteristics of the four experimental subgroups. Physical and emotional stress symptoms were not significantly different between the caregivers’ subgroups (all p > .05). However, the previously described () differences between controls and caregivers on BAI and BDI scores, cortisol/DHEA ratios and BDNF levels were maintained (all p < .001). Performance on object recognition was also compared among the four subgroups (), since contextual memory scores were calculated only for objects correctly recognized as old. No main effects of group [F(1,67) = 1.297, η2p = 0.020, p = .259] and encoding condition [F(1,67) = 3.506, η2p = 0.053, p = .066] where seen on object recognition. These results where further confirmed with an one-way ANOVA, which showed no significant differences between the four subgroups on object recognition [F (1,67) = 1.757, η2p = 0.070, p = .165], and remained unchanged with the introduction of age, depression and anxiety symptoms as covariates in the statistical model (all p > .05).
Figure 3. Object recognition (A) and context recognition (B) performances (mean ± SEM) of controls and caregivers under two encoding conditions. *p < .05 compared to the context recognition of the other subgroups.
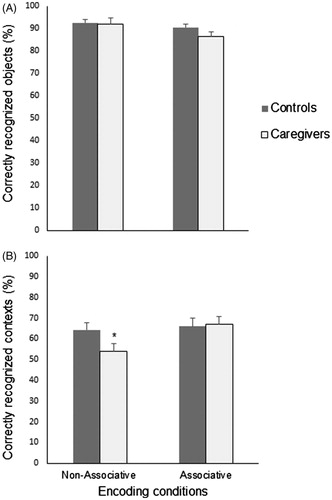
The performance of caregivers and controls on the contextual memory task is shown in . The two-way ANOVA revealed a main effect of group [F(1,67) = 7.285, η2p = 0.104, p = .009], indicating reliable performance differences between caregivers and controls, and a main effect of encoding instruction [F(1,67) = 7.204, η2p = 0.103, p = .009], suggesting that the task with the associative encoding instruction resulted in a higher performance than the task without the incidental binding cue. Interaction between group and encoding condition reached borderline values of significance [F(1,67) = 3.690, η2p = 0.055, p = .059]. A one-way ANOVA [F(3,67) = 6.398, η2p = 0.234, p = .001] followed by Bonferroni post hoc tests showed that caregivers in the encoding condition without the associative instruction had the worst contextual recognition scores among the experimental subgroups (all p < .05). However, the introduction of the associative instruction was able to improve the performance of caregivers (p = .012), matching their contextual recognition scores with those of the control group (p = 1.00). Age, depressive and anxiety symptoms had significant effects on contextual memory as covariates (all p < .05). However, the between group differences [F(3, 67) = 3.255, η2p = 0.140, p = .028] and the improving effect of the associative encoding condition on the contextual memory of caregivers [p = .034] remained even with the resulting adjustment of means.
Contextual memory, cognitive, and physiological parameters
As described earlier, besides showing a worse performance than controls on most neuropsychological tests, caregivers had higher cortisol/DHEA ratios at 22:00 h and lower BDNF levels than controls. Therefore, linear regression analyses were carried out to explore the possible associations of these outcomes with the contextual memory deficits shown by the caregivers. Thus, data from controls and caregivers in the non-associative encoding condition were entered in the linear regressions. As can be seen in , significant positive associations were found for the performance on contextual memory and all neuropsychological tests that showed significant differences between controls and caregivers (Forward & Backward digit span, Stroop III and Logical Memory II tasks) [all p < .05]. Among the physiological parameters, only cortisol/DHEA ratio at 10PM showed a significant negative relation with contextual memory performance [p < .001].
Table 5. Results of the linear regressions between the performance on contextual memory and the other neuropsychological and physiological parameters.
also shows that, when performed with the associative encoding instruction (a condition in which the contextual memory of caregivers and controls was no longer different), no significant associations were found between contextual memory and the other neuropsychological tasks (all p > .05), nor between contextual memory and cortisol/DHEA ratios at 22:00 h (all p > .05).
Discussion
This study aimed to evaluate if the cognitive dysfunctions of familial caregivers are reversible, since their caregiving functions are associated with chronic stress and an imbalance between neurotoxic and neuroprotective substances, two potentially hindering factors for cognitive processing. Thus, in a first attempt to investigate this issue we used an incidental contextual memory task with different encoding conditions. As expected, contextual memory of AD caregivers was impaired. This impairment was: (I) associated with prefrontal and temporal cognitive dysfunctions; (II) directly correlated with an imbalance of the cortisol/DHEA ratio at 22:00 h and indirectly associated with lower BDNF levels; (III) not seen in the subjects that performed the associative encoding condition of the memory task. This last result suggests that there is a cognitive reserve that can be engaged to ameliorate impairments of contextual memory in caregivers of dementia patients.
The caregiver sample of this study was composed of middle-aged and older adults, mostly daughters and wives of the demented patients. Most caregivers reported four or more years of full time caregiving (50%) and used a problem-focused coping strategy (85%). Thus, they had already gone through the early stress phases and assumed a coping style expected to allow a better management of the situation (Gilhooly et al., Citation2016). These characteristics of our caregivers are not surprising. They were recruited from an assistance group for caregivers of AD patients (ABRAZ), suggesting their attempt to manage the situation by seeking for information about the disease and guidance to avoid stressful situations, besides looking for social support (Kneebone & Martin, Citation2003). However, it is worth noting that the caregivers reported a high weekly assistance schedule and half of them (51%) were already on the near-exhaustion and exhaustion phases of stress, in which the body’s defenses begin to subside to the stressful stimuli and diseases develop (Lipp Citation2003; Lipp & Guevara, Citation1994). Psychological stress symptoms prevailed over physical symptoms, and BDI and BAI scores indicated that depression and anxiety symptoms were also present. These characteristics are common among familial caregivers (Roth, Fredman, & Haley, Citation2015; Vitaliano et al., Citation2011) and can be explained by the physical and emotional overburden, this last caused mainly by the affective connection between the caregiver and the patient (Dassel et al., Citation2017). The caregiver’s chronic stress was further confirmed by their higher cortisol/DHEA ratio, which was previously shown to be a physiological marker of chronic stress in caregivers of demented patients (Corrêa Citation2015b, Citation2016; Vedhara et al., Citation2002) and other conditions (Kamin & Kertes, Citation2017).
Compromise of caregiver’s mental health was also indicated by the results of prefrontal and temporal lobe-dependent tasks. Executive function (inhibitory response capacity), attention, working memory and declarative memory were impaired, corroborating previous results from ours (Corrêa et al., Citation2015a, Citation2016; Palma et al.,Citation2011) and other research groups (see Allen et al., Citation2017 for a review). Higher nadir cortisol/DHEA ratios and lower BDNF levels accompanied these cognitive dysfunctions. It is important to note that these cognitive and physiological results were maintained even with the introduction of age, depression and anxiety symptoms as covariates in the statistical analysis. Moreover, significant negative correlations were found between the cortisol/DHEA ratio at 22:00 h and executive function, attention, working memory and declarative memory. On the other hand, positive associations were found between BDNF levels and performance on attention, working memory and declarative memory. These results agree with the notion that the prefrontal cortex and the temporal lobe structures are responsive to these hormones and neurotrophin, which are capable to modulate processes such as neurogenesis, neuroplasticity and neuronal survival (Jeanneteau & Chao, Citation2013; Maninger et al., Citation2009; McEwen, Citation2012). Alterations in the levels of cortisol, DHEA and BDNF are expected to occur in chronic stress conditions (Lupien et al., Citation2009; Maninger et al., Citation2009; Pluchino et al., Citation2013;), and interfere on prefrontal and temporal lobe-dependent cognitive functions (Kim et al., Citation2015; Lu, Nagappan, & Lu, Citation2014; Pluchino et al., Citation2015; Shansky & Lipps, Citation2013).
Since brain BDNF crosses the blood–brain barrier (Pan, Banks, Fasold, Bluth, & Kastin, Citation1998), is seems reasonable to assume that blood BDNF protein levels resemble those in the brain. This assumption is reinforced by several evidences: serum BDNF is positively associated to cortical levels of N-acetyl aspartate, a marker of neuronal integrity (Lang, Hellweg, Seifert, Schubert, & Gallinat, Citation2007); brain pathologies are associated to a decrease in BDNF levels in brain and blood, whereas successful therapeutic interventions can increase BDNF levels in these two compartments (Autry & Monteggia, Citation2012; Diniz & Teixeira, Citation2011; Gezen-Ak et al., Citation2013); the severity of neurological symptoms, such as the degree of cognitive impairment, is correlated to serum BDNF levels (Corrêa et al., Citation2016; Küster et al., Citation2017; Zhang et al., Citation2012). Based on these evidences, we assume that central and peripheral levels of BDNF are associated. However, the primary source of the serum BDNF alterations (if central, peripheral or both) is still unknown. BDNF is also produced in several peripheral organs, such as heart, gut, thymus, spleen and skeletal muscles (Lommatzsch et al., Citation1999, Citation2005). However, studies focused on mental health investigate mainly the BDNF produced in the brain and, even so, they are just beginning to unravel the complex interplay between stress and BDNF availability (McEwen et al., Citation2015) and realize the importance of central BDNF to regulate not only the brain, but also peripheral organ functions (Marosi & Mattson, Citation2014). Despite these limitations, serum BDNF has repeatedly been suggested as a biomarker for brain pathologies and therapeutic efficacy (Polyakova et al., Citation2015). As suggested by the results obtained in this study, it could be a biomarker of the negative effects of chronic stress of caregiving on cognition.
As previously mentioned, we chose the contextual memory task to investigate the reversibility of the caregiver’s cognitive dysfunctions based on the importance of this type of memory in everyday life (Eichenbaum, Yonelinas & Ranganath, Citation2007), and because our experimental paradigm offers the possibility to explore the cognitive reserve of caregivers (Balardin et al., Citation2009; dos Santos et al., Citation2010). As shown in the results section, caregivers had a worse contextual memory performance than controls in the encoding condition without an associative strategy. Literature suggests that contextual memory processing depends largely on executive and monitoring functions of the prefrontal cortex (Logan, Sanders, Snyder, Morris, & Buckner, Citation2002) and on the integration of central and contextual features by the temporal lobe (mainly hippocampus) (Eichenbaum et al., Citation2007). As explained before, these structures are also vulnerable to the adverse effects of chronic stress (Lupien et al., Citation2009; McEwen, Citation2004, Citation2012), cortisol/DHEA imbalance (Maninger et al., Citation2009; McEwen, Citation2012) and decreased BDNF levels (Erickson et al., Citation2010; Lu et al., Citation2014). Accordingly, a positive association was found between the performance on contextual memory and on prefrontal (executive function, attention, working memory) and temporal (declarative memory) dependent tasks. This means that the disadvantage of caregivers on the contextual memory task was associated with the impairment shown on their prefrontal and temporal cognitive functions. Caregivers’ alterations on BDNF levels and cortisol/DHEA ratios seem to be related to their contextual memory dysfunctions. Our study showed that caregivers had lower BDNF levels, that lower BDNF levels were associated with functional impairments of prefrontal cortex and hippocampus, and that these impairments were associated with the contextual memory deficit. Thus, these results suggest an indirect association between the lower BDNF levels of caregivers and their contextual memory impairment, despite the absence of a significant linear regression between contextual memory and this neurotrophin. Moreover, contextual memory performance was negatively associated with cortisol/DHEA ratio at 10PM, as shown by the linear regression results. Thus, the contextual memory impairments of caregivers were associated with their higher cortisol/DHEA ratios. Although the experimental design of our study does not allow the establishment of a cause-effect relationship, the associations described above give rise to the possibility that chronic stress suffered by the caregivers induces an imbalance of the cortisol/DHEA ratio and a decrease in BDNF levels, causing dysfunctions on brain structures involved with contextual memory processing.
The introduction of an incidental associative encoding instruction on the contextual memory task was able to abolish any significant performance difference between caregivers and controls. The incidental nature of our contextual memory task prevents the use of conscious strategies and efforts to memorize information. Thus, the performance improvement of caregivers from the contextual memory task without associative encoding instruction to the task with associative encoding instruction unravels cognitive resources that, although not automatically engaged by the subjects, are prone to rehabilitation. In this sense, these cognitive resources can be considered as representative of the caregiver’s cognitive reserve (Barulli & Stern, Citation2013). It must also be highlighted that with the introduction of the associative encoding instruction, the correlation between the contextual memory performance and the other cognitive tasks could no longer be seen. The same is true for the association between contextual memory and cortisol/DHEA ratio at 22:00h, which also vanished with the introduction of the associative encoding strategy. Thus, the associative encoding instruction was able to circumvent the executive, attention, working memory and declarative memory dysfunctions of the caregivers, as well as their cortisol/DHEA imbalance.
Among the limitations of this study there’s the issue of sample size. A larger sample may have been more accommodating for drawing stronger conclusions about the relations between BDNF and contextual memory. Thus, future studies with larger samples should reevaluate this issue. Moreover, although we were able to show that caregivers have a cognitive reserve that allows cognitive rehabilitation, our results cannot simply be generalized. There are different actual and lifelong factors (e.g. lifestyle and its interactions with genetic and physiologic factors) that can affect cognitive reserve (Cheng, Citation2016). Thus, future studies should carefully evaluate the impact of these aspects on the success of caregiver’s cognitive rehabilitation. Additionally, in contrast to most other chronic stress studies, we analyzed cortisol secretion only at 8:00 and 22:00h on one single day. Although not optimal, this method was chosen because it allowed caregivers to collect saliva samples at home (increasing study adherence), without the complications associated with multiple samplings throughout the day (Adam & Kumari, Citation2009) or the risk of compromising the strict standardization and timing required by other techniques, such as the cortisol awaking response (Vugt et al., Citation2005; Wahbeh, Kishiyama, Zajdel, & Oken, Citation2008). An interesting option for future studies would be the analysis of hair cortisol and DHEA, a highly promising complementary technique for the retrospective assessment of chronic stress (Qiao et al., Citation2017; Russell, Koren, Rieder, & Van Uum, Citation2012).
In conclusion, the results obtained in this study support the notion that familial caregivers of AD patients are subjected to negative effects of chronic stress on mental health. We also expand the knowledge on the field by showing that the cognitive impairments of caregivers are not necessarily irreversible, as indicated by the results obtained for contextual memory. More importantly, our experimental paradigm indicates that caregivers have a cognitive reserve prone to rehabilitation and, probably, with broader effects on cognitive functions than those shown in this study. As AD is characterized by a negative prognosis, and the patient is supported by the caregiver throughout the disease progression, it can be assumed that the cognitive deficit of the caregiver would increase over the years, unless preventive strategies are implemented. Therefore, the possibility of having a significant cognitive reserve brings an alternative to be explored in this population. Future studies should carefully investigate the extent of this cognitive reserve and how it could be used to improve the cognitive functions of caregivers, promoting a better life quality for them and their patients.
Acknowledgments
We thank Dr. Flavio Kapczinski for help with ELISA assays and Mrs. Iara Portugal for her support in recruiting the caregivers at the ABRAZ–Porto Alegre.
Disclosure statement
I.I.L. Argimon and E. Bromberg are CNPq research fellows. M.S. Corrêa was funded by a FAPERGS/CAPES fellowship. K. Vedovelli and B.L. Giacobbo had a CAPES fellowship. The remaining author has no conflict of interest to declare.
Additional information
Funding
References
- Adam, E.K., & Kumari, M. (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436. doi:10.1016/j.psyneuen.2009.06.011
- Allen, A.P., Curran, E.A., Duggan, Á., Cryan, J.F., Chorcoráin, A.N., Dinan, T.G., … Clarke, G. (2017). A systematic review of the psychobiological burden of informal caregiving for patients with dementia: Focus on cognitive and biological markers of chronic stress. Neuroscience & Biobehavioral Reviews, 73, 123–164. doi:10.1016/j.neubiorev.2016. 12.006
- Alzheimer’s Association. (2014). 2014 Alzheimer’s disease facts and figures. Alzheimer’s Dementia, 10, e47–e92. doi:org/10.1016/j.jalz.2014.02.001
- Autry, A.E., & Monteggia, L.M. (2012). Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological Reviews, 64, 238–258. doi:10.1124/pr.111.005108
- Ayres, M., Ayres, M., Jr, Ayres, D.L., & Santos, A.A.S. (2007). Bioestat 5.0 aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém: IDSM.
- Barulli, D., & Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17, 502. doi:10.1016/j.tics.2013.08.012
- Balardin, J. B., Vedana, G., Ludwig, A., Lima, D. B. D., Argimon, I., Schneider, R. … Bromberg, E. (2009). Contextual memory and encoding strategies in young and older adults with and without depressive symptoms. Aging and Mental Health, 13, 313–318. doi:10.1080/13607860802534583
- Byers, A.L., & Yaffe, K. (2011). Depression and risk of developing dementia. Nature Reviews Neurology, 7, 323–331. doi:10.1038/nrneurol.2011.60
- Cattaneo, A., Cattane, N., Begni, V., Pariante, C.M., & Riva, M.A. (2016). The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Translational Psychiatry, 6, e958. doi:10.1038/tp.2016.214
- Cheng, S.T. (2016). Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Current Psychiatry Reports, 18, 85. doi:10.1007/s11920-016-0721-2
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic.
- Corrêa, M.S., da Silveira, E.M., de Lima, D.B., Balardin, J.B., Walz, J.C., Kapczinski, F., … Bromberg, E. (2015a). The role of encoding strategies in contextual memory deficits in patients with bipolar disorder. Neuropsychological Rehabilitation, 25, 122–136. doi:10.1080/09602011.2014.969281
- Corrêa, M.S., Giacobbo, B.L., Vedovelli, K., Lima, D. B D., Ferrari, P., Argimon, I. I D L., … Bromberg, E. (2016). Age effects on cognitive and physiological parameters in familial caregivers of Alzheimer’s disease patients. PLoS One, 11, e0162619. doi:10.1371/journal.pone.0162619
- Corrêa, M.S., Vedovelli, K., Giacobbo, B.L., de Souza, C.E.B., Ferrari, P., de Lima Argimon, I.I., … Bromberg, E. (2015b). Psychophysiological correlates of cognitive deficits in family caregivers of patients with Alzheimer disease. Neuroscience, 286, 371–382. doi:10.1016/j.neuroscience.2014.11.052
- Cunha, J. (2001). Beck scales. São Paulo: Casa do Psicólogo.
- Dassel, K.B., Carr, D.C., & Vitaliano, P. (2017). Does caring for a spouse with dementia accelerate cognitive decline? Findings from the health and retirement study. The Gerontologist, 57, 319–328. doi:10.1093/geront/gnv148
- Diniz, B.S., & Teixeira, A.L. (2011). Brain-derived neurotrophic factor and Alzheimer's disease: Physiopathology and beyond. Neuromolecular Medicine, 13, 217–222. doi:10.1007/s12017-011-8154-x
- dos Santos, C.M., Balardin, J.B., Irigaray, T.Q., Schröder, N., Rieder, C.R.M., & Bromberg, E. (2010). Incidental encoding strategies did not improve contextual memory in Parkinson’s disease patients. Neurorehabilitation and Neural Repair, 24, 450–456. doi:10.1177/1545968309355987
- Eichenbaum, H., Yonelinas, A.P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152. doi:10.1146/annurev.neuro.30.051606.094328
- Erickson, K.I., Prakash, R.S., Voss, M.W., Chaddock, L., Heo, S., McLaren, M., … Kramer, A.F. (2010). Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. Journal of Neuroscience, 30, 5368–5375. doi:10.1523/jneurosci.6251-09.2010
- Ferrari, E., & Magri, F. (2008). Role of neuroendocrine pathways in cognitive decline during aging. Ageing Research Reviews, 7, 225–233. doi:10.1016/j.arr.2008.07.001
- Folstein, M.F., Folstein, S.E., & McHugh, P.R. (1975). ‘‘Mini-Mental State’’: A practical 21 method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6
- Gezen-Ak, D., Dursun, E., Hanağası, H., Bilgiç, B., Lohman, E., Araz, Ö.S., … Yılmazer, S. (2013). BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. Journal of Alzheimer's Disease, 37, 185–195. doi:10.3233/JAD-130497
- Gilhooly, K.J., Gilhooly, M.L.M., Sullivan, M.P., McIntyre, A., Wilson, L., Harding, E., … Crutch, S. (2016). A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatrics, 16, 106. doi:10.1186/s12877-016-0280-8
- Hollingworth, A., & Rasmussen, I.P. (2010). Binding objects to locations: The relationship between object files and visual working memory. Journal of Experimental Psychology Human Perception & Performance, 36, 543–564. doi:10.1037/a0017836
- Jeanneteau, F., & Chao, M.V. (2013). Are BDNF and glucocorticoid activities calibrated?. Neuroscience, 239, 173–195. doi:10.1016/j.neuroscience.2012.09.017
- Johnson, M.K., Hashtroudi, S., & Lindsay, D.S. (1993). Source monitoring. Psychological Bulletin, 114, 3–28. doi:10.1037/0033-2909.114.1.3
- Kamin, H.S., & Kertes, D.A. (2017). Cortisol and DHEA in development and psychopathology. Hormones and Behavior, 89, 69–85. doi:10.1016/j.yhbeh.2016.11.018
- Kim, E.J., Pellman, B., & Kim, J.J. (2015). Stress effects on the hippocampus: A critical review. Learning & Memory, 22, 411–416. doi:10.1101/lm.037291.114
- Kneebone, I.I., & Martin, P.R. (2003). Coping and caregivers of people with dementia. British Journal of Health Psychology, 8, 1–17. doi:10.1348/135910703762879174
- Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., & Moryś, J. (2017). BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology, 38, 579–593. doi:10.1007/s10571-017-0510-4
- Küster, O.C., Laptinskaya, D., Fissler, P., Schnack, C., Zügel, M., Nold, V., … von Arnim, C.A.F. (2017). Novel blood-based biomarkers of cognition, stress, and physical or cognitive training in older adults at risk of dementia: Preliminary evidence for a role of BDNF, Irisin, and the Kynurenine pathway. Journal of Alzheimer's Disease, 59, 1097–1111. doi:10.3233/JAD-170447
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. doi:10.3389/fpsyg.2013.00863
- Lang, U.E., Hellweg, R., Seifert, F., Schubert, F., & Gallinat, J. (2007). Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biological Psychiatry, 62, 530–535. doi:10.1016/j.biopsych.2007.01.002
- Lipp, M.E.M. (2003). Mecanismos neuropsicofisiológicos do Stress: Teoria e Aplicacações Clínicas (vol. 3). São Paulo: Casa do Psicólogo.
- Lipp, M.E.N., & Guevara, A.J.H. (1994). Validacão empírica do Inventário de Sintomas de Stress. Estudos de Psicologia, 11, 43–49. doi:10.1590/S0102-79721999000100005
- Logan, J.M., Sanders, A.L., Snyder, A.Z., Morris, J.C., & Buckner, R.L. (2002). Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron, 33, 827–840. doi:10.1016/S0896-6273(02)00612-8
- Lommatzsch, M., Zingler, D., Schuhbaeck, K., Schloetcke, K., Zingle, C., & Schuff-Werner, P. (2005). The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging, 26, 115–123. doi:10.1016/j.neurobiolaging.2004.03.002
- Lommatzsch, M., Braun, A., Mannsfeldt, A., Botchkarev, V.A., Botchkareva, N.V., Paus, R., … Renz, H. (1999). Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. The American Journal of Pathology, 155, 1183–1193. doi:10.1016/S0002-9440(10)65221-2
- Lommatzsch, M., Quarcoo, D., Schulte-Herbrüggen, O., Weber, H., Virchow, J.C., Renz, H., & Braun, A. (2005). Neurotrophins in murine viscera: A dynamic pattern from birth to adulthood. International Journal of Developmental Neuroscience, 23, 495–500. doi:10.1016/j.ijdevneu.2005.05.009
- Lu, B., Nagappan, G., & Lu, Y. (2014). BDNF and synaptic plasticity, cognitive function, and dysfunction. Handbook of Experimental Pharmacology, 220, 223–250. doi:10.1007/978-3-642-45106-5_9
- Lupien, S.J., McEwen, B.S., Gunnar, M.R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. doi:10.1038/nrn2639
- Maher, J.M., Markey, J.C., & Ebert-May, D. (2013). The other half of the story: Effect size analysis in quantitative research. CBE—Life Sciences Education, 12, 345–351. doi:org/10.1187/cbe.13-04-0082
- Maninger, N., Wolkowitz, O.M., Reus, V.I., Epel, E.S., & Mellon, S.H. (2009). Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in Neuroendocrinology, 30, 65–91. doi:10.1016/j.yfrne.2008.11.002
- Marosi, K., & Mattson, M.P. (2014). BDNF mediates adaptive brain and body responses to energetic challenges. Trends in Endocrinology and Metabolism, 25, 89–98. doi:10.1016/j.tem.2013.10.006
- McEwen, B.S. (2004). Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032, 1–7. doi:10.1196/annals.1314.001
- McEwen, B.S. (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America, 109, 17180–17185. doi:10.1073/pnas.1121254109
- McEwen, B.S., Bowles, N.P., Gray, J.D., Hill, M.N., Hunter, R.G., Karatsoreos, I.N., & Nasca, C. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18, 1353–1363. doi:10.1038/nn.4086
- Mitchell, K.J., & Johnson, M.K. (2009). Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory?. Psychological Bulletin, 135, 638–677. doi:10.1037/a0015849
- Nascimento, E. (2004). WAIS III – Escala de inteligência Wechsler para adultos - Manual/David Wechsler – Adaptação e Padronização de uma Amostra Brasileira (Vol. 1). São Paulo: Casa do Psicólogo.
- Norton, M.C., Smith, K.R., Østbye, T., Tschanz, J. T., Corcoran, C., Schwartz, S., … Welsh-Bohmer, K.A.; Cache County Investigators. (2010). Greater risk of dementia when spouse has dementia? The Cache County study. Journal of the American Geriatrics Society, 58, 895–900. doi:10.1111/j.1532-5415.2010.02806.x
- Palma, K.A.X.A., Balardin, J.B., Vedana, G., de Lima Argimon, I.I., Luz, C., Schröder, N., … Bromberg, E. (2011). Emotional memory deficit and its psychophysiological correlate in family caregivers of patients with dementia. Alzheimer Disease and Associated Disorders, 25, 262–268. doi:10.1097/WAD.0b013e318209e453
- Pan, W., Banks, W.A., Fasold, M.B., Bluth, J., & Kastin, A.J. (1998). Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology, 37, 1553–1561. doi:10.1016/S0028-3908(98)00141-5
- Pluchino, N., Drakopoulos, P., Bianchi-Demicheli, F., Wenger, J.M., Petignat, P., & Genazzani, A.R. (2015). Neurobiology of DHEA and effects on sexuality, mood and cognition N. The Journal of Steroid Biochemistry and Molecular Biology, 145, 273–280. doi:0.1016/j.jsbmb.2014.04.012
- Pluchino, N., Russo, M., Litta, P., Cela, V., Genazzani, A.R., & Santoro, N.A. (2013). Steroid hormones and BDNF. Neuroscience, 239, 271–279. doi:10.1016/j.neuroscience.2013.01.025
- Polyakova, M., Stuke, K., Schuemberg, K., Mueller, K., Schoenknecht, P., & Schroeter, M.L. (2015). BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. Journal of Affective Disorders, 174, 432–440. doi:10.1016/j.jad.2014.11.044
- Qiao, S., Li, X., Zilioli, S., Chen, Z., Deng, H., Pan, J., & Guo, W. (2017). Hair measurements of Cortisol, DHEA, and DHEA to Cortisol Ratio as biomarkers of chronic stress among people living with HIV in China: Known-group validation. PLoS One, 12, e0169827. doi:10.1371/journal.pone.0169827
- Richardson, T.J., Lee, S.J., Berg-Weger, M., & Grossberg, G.T. (2013). Caregiver health: Health of caregivers of Alzheimer’s and other dementia patients. Current Psychiatry Reports, 15, 367. doi:10.1007/s11920-013-0367-2
- Roth, D.L., Fredman, L., & Haley, W.E. (2015). Informal caregiving and its impact on health: A reappraisal from population-based studies. The Gerontologist, 55, 309–319. doi:10.1093/geront/gnu177
- Russell, E., Koren, G., Rieder, M., & Van Uum, S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37, 589–601. doi:10.1016/j.psyneuen.2011.09.009
- Samson, R.D., & Barnes, C. (2013). Impact of aging brain circuits on cognition. The European Journal of Neuroscience, 37, 1903–1915. doi:10.1111/ejn.12183
- Savóia, M.G., Santana, P.R., & Mejias, N.P. (1996). Adaptação do Inventário de Estratégias de Coping de Folkman e Lazarus para o Português. Psicologia USP, 7, 183–201.
- Selye, H. (1956). The stress of life. NY: McGraw-Hill.
- Shansky, R.M., & Lipps, J. (2013). Stress-induced cognitive dysfunction: Hormone-neurotransmitter interactions in the prefrontal cortex. Frontiers in Human Neuroscience, 7, 123. doi:10.3389/fnhum.2013.00123
- Strauss, E., Sherman E. & Spreen, O. (2006). A compendium of neuropsychological tests: administration, norms, and commentary (3rd ed., 1216 p.). New York, NY; Oxford University Press.
- Vedhara, K., McDermott, M.P., Evans, T.G., Treanor, J.J., Plummer, S., Tallon, D., … Schifitto, G. (2002). Chronic stress in nonelderly caregivers: Psychological, endocrine and immune implications. Journal of Psychosomatic Research, 53, 1153–1161. doi:10.1016/s0022-3999(02)00343-4
- Vitaliano, P.P. (2010). An ironic tragedy: Are spouses of persons with dementia at higher risk for dementia than spouses of persons without dementia?. Journal of the American Geriatrics Society, 58, 976–978. doi:10.1111/j.1532-5415.2010.02843.x
- Vitaliano, P.P., Murphy, M., Young, H.M., Echeverria, D., & Borson, S. (2011). Does caring for a spouse with dementia promote cognitive decline? A hypothesis and proposed mechanisms. Journal of the American Geriatrics Society, 59, 900–908. doi:10.1111/j.1532-5415.2011.03368.x
- Vugt, M.E., Nicolson, N.A., Aalten, P., Lousberg, R., Jolle, J., & Verhey, F.R.J. (2005). Behavioral problems in dementia patients and salivary cortisol patterns in caregivers. The Journal of Neuropsychiatry and Clinical Neurosciences, 17, 201–207. doi:10.1176/jnp.17.2.201
- Wahbeh, H., Kishiyama, S.S., Zajdel, D., & Oken, B.S. (2008). Salivary cortisol awakening response in mild Alzheimer disease, caregivers, and noncaregivers. Alzheimer Disease and Associated Disorders, 22, 181–183. doi:10.1097/WAD.0b013e31815a9dff
- Wechsler, D. (1987). Manual for the Weschsler Memory Scale - Revised. San Antonio, TX: Corporation TP.
- Wechsler, D. (1997). WAIS III: Administration and Scoring Manual. San Antonio, TX: Harcourt.
- Zhang, X.Y., Chen, D.C., Xiu, M.H., Haile, C.N., Luo, X., Xu, K., … Kosten, T.R. (2012). Cognitive and serum BDNF correlates of BDNF Val66Met gene polymorphism in patients with schizophrenia and normal controls. Human Genetics, 131, 1187–1195. doi:10.1007/s00439-012-1150-x